Abstract
Objectives
We assessed healthcare resource utilization (HCRU) and costs of cardiovascular (CV) events in patients with a history of atherosclerotic cardiovascular disease (ASCVD) in Germany.
Methods
We conducted a retrospective matched case-control study based on German claims data from 1 January 2012 to 31 December 2017 using the “Institute for Applied Health Research Berlin” (InGef) Research Database. Cases who had a myocardial infarction (MI), stroke and angina pectoris identified by ICD-10-GM codes between 1 January 2014 and 31 December 2016 were matched to event-free controls by an exact matching approach without replacement at a ratio of 1:2. Costs and HCRU were assessed in individual 1-year follow-up periods after the index event for the overall cohort and subgroups of MI cases and stroke cases.
Results
The overall cohort consisted of a total of 14,169 cases with a CV index event matched to 28,338 controls. The mean age of the overall cohort was 73.3 years, 34.1% of the patients were female, 3,717 (26.2%) had an MI, and 3,752 (26.5%) had stroke. Following the index events, 12.2% of cases in the overall cohort, 12.6% of MI cases, and 8.7% of stroke cases experienced a recurrent CV event. CV cases had on average 1.7 more all-cause hospitalizations (p <0.001) and 6.1 more outpatient visits (p <0.001) during the 1-year follow-up period than did controls. In the MI and stroke subgroups, cases had on average 1.8 and 1.6 more all-cause hospitalizations and 7.0 and 4.0 more outpatient visits, respectively (differences were statistically significant). Compared to controls, cases incurred on average higher total healthcare costs: by €11,898 for overall cases, by €16,349 for MI, and by €14,360 in stroke cases (overall: p <0.001; MI: p <0.001; stroke: p <0.001).
Conclusion
CV events in ASCVD patients pose a considerable clinical burden on patients and cause significant costs for the German statutory healthcare system.
Introduction
Cardiovascular disease is the number one cause of death in Germany, accounting for around 40% of deaths annuallyCitation1,Citation2. Of the ten most frequent diagnoses leading to death in 2019, 50% were related to heart disease, with chronic ischemic heart disease (8.0%), acute myocardial infarction (MI) (4.8%), and heart failure (3.9%) among the top fiveCitation1. The burden of atherosclerotic cardiovascular disease (ASCVD) is rapidly growing due to the increasing prevalence of major risk factors such as obesity, hypertension, hypercholesterolemia, and type 2 diabetes, as well as other lifestyle factors such as unhealthy diet and physical inactivityCitation3–6. These factors also directly influence the risk for cardiovascular (CV) events and, according to the 2019 European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) guidelines, established ASCVD is associated with a very high risk for CV eventsCitation7. Elevated low-density lipoprotein cholesterol (LDL-C) is one of the main causal risk factors for ASCVD. Reduction of LDL-C can be achieved with standard statin regimens in most individuals, resulting in a reduced risk for subsequent CV events. A meta-analysis found that – irrespective of baseline LDL-C concentration – the extent of the proportional reduction in CV events is directly proportional to the absolute LDL reductionCitation8. According to the 2019 ESC/EAS guidelinesCitation7 for the management of dyslipidaemias, the recommended pharmacological treatments for very high risk patients are high-intensity lipid-lowering therapies (LLTs) aiming at reduction of LDL-C levels to <1.4 mmol/L and at least 50% from baseline LDL-C. With the launch of PCSK9 inhibitors in 2015, a new class of highly potent LLTs became available for the treatment of patients with ASCVDCitation7,Citation9. CV events such as MI or stroke are associated with significant costs due to related hospitalizations, emergency care, or outpatient physician visitsCitation10. Therefore, ASCVD represents a major economic burden on healthcare systems in terms of direct and indirect costs associated with morbidity and mortalityCitation11. Cardiovascular disease accounted for roughly 14% (€46.4 billion) of the €338.2 billion overall medical expenses in Germany in 2015, representing the disease area with the highest economic burden on the German statutory health insurance systemCitation12. It is expected that total costs (i.e. direct plus indirect costs) related to MI and stroke will increase by an estimated €4.6 billion in Germany by 2030, resulting in an impact of up to €21.5 billion in annual healthcare costsCitation13.
Most data informing CV-specific event rates in patients with existing cardiovascular disease are derived from clinical trials. While such data have a high internal validity, clinical trials may underestimate real-world event rates due to the idealistic setting required to determine the safety and efficacy of interventions. Inclusion criteria are often more strict, excluding patients with multiple comorbidities, and participants are monitored more frequently with more rigorous examinations compared to the population treated in a real-life settingCitation14. Since ASCVD constitutes such a high and potentially underestimated burden, the aim of this study was to quantify healthcare resource utilization (HCRU) and healthcare costs associated with CV events in a very high-risk population with a history of ASCVD. We also evaluated LLT intensity, recurrent CV event rates, and cardiovascular mortality. Given the large number of patients who are living with ASCVD, there is, furthermore, an imperative to evaluate the epidemiology of this patient group outside of large clinical trials. Claims data in Germany provide a comprehensive picture of the population, including diagnoses, events, costs, and HCRU, as the majority of the population (87.2% by end of 2017) is covered by the statutory health insurance with almost full coverage (co-payments of €5–€10 per prescription and €10 per hospital day).
Methods
Study design
A retrospective matched case-control study based on German claims data using the “Institute for Applied Health Research Berlin” (InGef) Research Database was conducted. All available years in the database at the time of the analysis were included for the study period spanning from 1 January 2012 to 31 December 2017. The timeframe of 1 January 2012 to 31 December 2013 was used to identify patients with a history of ASCVD and LLT prescriptions, as well as the exclusion of specific comorbid conditions for the overall study population. From this study population, cases were identified by CV events in the 3-year period from 1 January 2014 to 31 December 2016. The first identified CV event constituted the index event. Remaining patients without a CV event in this period were selected as potential controls. Outcomes were assessed in individual 1-year follow-up periods after the index event (Supplementary Material, Appendix 1) including data up to 31 December 2017.
Data source
The InGef Research Database includes verified claims data of the participating insurance companies. Approximately 75 different statutory health insurances were part of the InGef Research Database, corresponding to two-thirds of the overall number of health insurances in Germany. The pooled claims data of the participating health insurances in the InGef Research Database consisted of about 7 million covered people, of which an adjusted sample of approximately 4 million people was drawn, structured to represent the German population in terms of age and sex. This adjusted sample represented 4.8% of the German population and 5.5% of the German statutory health insurance population as of 2018 and has been shown to have good external validityCitation15–17. The InGef Research Database complies with all data protection regulations in accordance with German Social LawCitation18,Citation19; personal information of patients, physicians, and other healthcare providers is anonymized before the data are made available for research.
Study population
The study population included patients with a history of ASCVD from 1 January 2012 to 31 December 2013. The overall case cohort included patients who had an index event between 1 January 2014 and 31 December 2016 identified by diagnosis codes of the International Classification of Diseases 10th revision, German Modification (ICD-10-GM)Citation20 in the inpatient setting. Hospitalizations with a primary diagnosis of MI (ICD-10-GM: I21.–Acute myocardial infarction, I22.–Subsequent myocardial infarction), angina pectoris (ICD-10-GM: I20.–Angina pectoris) or stroke (ICD-10-GM: I63.–Cerebral infarction) were included and defined as index events. Only inpatient diagnoses were considered for the identification of the index events, however, in the German healthcare setting patients with MI, angina pectoris, and stroke are almost invariably hospitalized even if the event is first diagnosed in the outpatient setting. The remaining patients were considered as potential controls. The follow-up period was 365 days, including the index event. Patients were required to be continuously observable from 1 January 2012 until the end of their individual follow-up period, except for patients who died during their follow-up period. Only adult patients (aged ≥18 years at index event) with a history of ASCVD and LLT prescriptions from 1 January 2012 to 31 December 2013 were considered for inclusion. Evidence of ASCVD was identified by using ICD-10-GM codes (including MI, hospitalization for angina pectoris, stroke, transient ischemic attack, peripheral arterial disease, coronary revascularization, and other ASCVD). Use of LLT was determined by record of at least two LLT prescriptions in this period. Exclusion criteria were diagnoses of human immunodeficiency virus (HIV), acquired immunodeficiency syndrome (AIDS), cancer, end-stage renal disease, or transplantation (Supplementary Material, Appendix 2).
Matching
ASCVD patients with an index event were matched in a ratio of 1:2 to controls. All patients who fulfilled the selection criteria had a history of ASCVD, at least two LLT prescriptions, and no comorbidities of exclusion but had no index event were considered as potential controls. An exact matching approach without replacement based on age, sex, type of ASCVD event history and number of additional risk factors (heart failure, smoking, hypertension, diabetes mellitus, lipid disorders, obesity, and chronic kidney disease [Supplementary Material, Appendix 4]) was applied. This approach was deemed the most appropriate to balance the study cohorts due to the limited number of matching parameters that were included. To keep the population of potential controls as large as possible and facilitate matching, we tried to limit the number of matching parameters to a minimum. For example, we chose to match on number of cardiovascular risk factors rather than include each risk factor as a matching variable. Because we wanted to investigate the impact of an additional CV event in a population with an existing history of ASCVD, matched controls were assigned the same index date as the index CV event of the case.
Analysis
Demographic characteristics and LLT use were assessed at baseline (1 January 2012 to 31 December 2013). Intensity of LLT therapy and Charlson comorbidity index were analyzed in an individual 1-year pre-index period. Since the prescribed dosage is not available in the German data set, the intensity of LLT use was estimated based on the prescription patterns of the patient, including milligrams per prescription and number of days until the next fill. The treatment intensity was examined as a categorical variable with three levels (low, moderate, and high) based on the calculated medication dose per day for each agent (Supplementary Material, Appendix 5). Recurrent CV events, mortality, costs, and HCRU were assessed during the 1-year follow-up period. Recurrent CV events were assessed as CV event rates in the follow-up period associated with hospitalizations for the defined CV events of interest. Only hospitalizations were considered to capture recurrent events, as CV event diagnoses may frequently be used for follow-up care in the outpatient setting. Mortality was assessed as overall mortality and CV-related mortality in the follow-up period. Since German claims data do not contain information on the cause of death, CV death was defined as any death that occurred related to hospitalizations with CV events within a 30-day time period following a hospital admission for MI, angina pectoris, or stroke or with discharge reason “death” for such hospitalizations with a length of stay longer than 30 days. Over a 1-year post-index period, hospitalizations of all causes were considered. Healthcare cost were retrieved from a payor perspective and included direct medical cost as well as cost for sick leave reimbursed by the statutory health insurance. Costs were measured in current prices (no inflation adjustments were applied) over the 1-year post-index period without further adaptation. HCRU and costs were analyzed by summary statistics using mean, standard deviation, median, Q1, and Q3 for continuous variables. Categorical variables were summarized as numbers and proportions. Index event costs included the costs of the respective hospitalization. All outcome analyses were performed for the overall cohort and subgroups of MI cases and stroke cases. Statistical analyses were carried out using Microsoft R Open 3.5.0.
Results
Study population and characteristics
Of the eligible patients fulfilling the inclusion criteria, 16,541 cases with an index event of MI, angina pectoris or stroke were identified during the period from 1 January 2014 to 31 December 2017. In total, 111,445 potential controls were identified. As a result of the matching process, a total of 14,169 cases with a CV index event could be matched to 28,338 controls for the overall cohort. The index event distribution for cases in the overall cohort was 3,717 (26.2%) MI cases; 3,752 (26.5%) stroke cases; and 6,700 (47.3%) angina pectoris cases. The mean age of the overall cohort was 73.3 years and 34.1% of the patients were female (). These characteristics were relatively consistent across the subgroups; the mean age of the MI subgroup was 73.8 years and 31.0% of the patients were female, and the mean age of the stroke subgroup was 76.7 years and 43.5% of the patients were female ().
Table 1. Baseline characteristics of the overall matched study cohorts.
Table 2. Baseline characteristics of the matched subgroups.
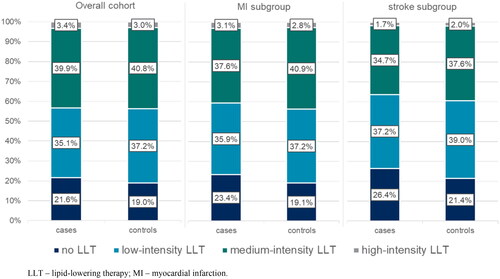
More than 20% of cases in the overall cohort did not receive any LLT in the 1-year period preceding the index event. Most cases (75%) in the overall cohort received low- or medium-intensity LLT, and only 3.4% of cases received high-intensity LLT. Results were relatively consistent across the subgroups ().
Recurrent CV events
Following the index events, 12.2% of cases in the overall cohort had a recurrent CV event of any type within a mean of 158.2 days. The proportion of cases with a recurrent CV event was similar in the MI subgroup but considerably lower in the stroke subgroup; 12.6% of MI cases had a recurrent CV event of any type within a mean of 148.9 days and 8.7% of stroke cases experienced a recurrent CV event within a mean of 163.9 days. The majority of recurrent events for cases in the overall cohort were angina pectoris (58.8%), followed by MI (22.9%) and stroke (18.3%) (). Regarding the subgroups, for MI cases 42.0% of the recurrent events within 1 year of their index event were recurrent MI and for stroke cases 69.8% of recurrent events were recurrent stroke, respectively (). The mean observation time until the occurrence of a recurrent event overall was 158.8 days.
Mortality
The mortality rate among cases in the overall cohort was 13.6% (n = 1,927). By our CV death definition, almost half of the deaths (n = 934) could be associated with CV events, which represented a 6.6% CV-related mortality rate. In the MI subgroup, the mortality rate among cases was 21.7% and the CV-related mortality rate among cases was 12.8%. In the stroke subgroup, the all-cause mortality rate among cases was 22.4% and the CV-related mortality rate among cases was 10.0% within the 1-year follow-up period.
Healthcare resource utilization
All cases in the study cohort had at least one hospitalization (related to the CV index event), whereas 24.9% of controls in the overall cohort were hospitalized at least once during the 1-year follow-up. On average, cases in the overall cohort and the MI and stroke subgroups were admitted to the hospital more frequently than their respective controls, with 2.1, 2.2, and 2.0 hospitalizations for overall, MI, and stroke cases, respectively, compared to 0.4, 0.4, and 0.4 mean hospitalizations for overall, MI, and stroke controls. Inpatient stays of CV event cases were also associated with longer durations; the mean length of stay per hospitalization for the overall, MI, and stroke cases was 10.1, 11.6, and 13.5 days, respectively, compared to 8.6 days for the overall cases controls, 9.0 days for the MI cases controls, and 8.8 days for the stroke cases controls (overall: p <0.001; MI: p <0.001; stroke: p <0.001) ().
Table 3. Summary statistics of the HCRU.
The mean number of outpatient provider visits was higher for cases in the overall cohort with 31.7 visits and in both subgroups (MI cases 30.6 visits, stroke cases 28.5 visits). Respective controls in the overall cohort had an average of 25.6 visits, controls in the MI subgroup an average of 23.6 visits, and controls in the stroke subgroup an average of 24.5 visits during the 1-year follow-up period. The observed differences between cases and controls were significant overall and for both subgroups (overall: p <0.001; MI: p <0.001; stroke: p <0.001).
Overall, 7.3% of cases with a CV event took sick leave at least once; in the controls 5.6% took sick leave (p <0.001). While these proportions were similar in the MI subgroup (7.0% of cases vs 4.9% of controls; p <0.001), the stroke subgroup showed lower proportions of patients who took sick leave (2.9% of cases vs 2.5% of controls), which were nevertheless significantly higher for the cases (p =0.006). In addition to taking sick leave more often, cases in all cohorts took significantly more days of sick leave than their respective controls. Cases in the overall cohort took on average 6.0 days of sick leave, respective controls 2.3 days (p <0.001), cases in the MI subgroup took on average 8.0 days, respective controls 2.0 days (p <0.001), and in the stroke subgroup cases took 3.7 days of sick leave, whereas their respective controls took 1.2 days of sick leave on average (p <0.001). HCRU across the cohorts is summarized in .
Healthcare costs
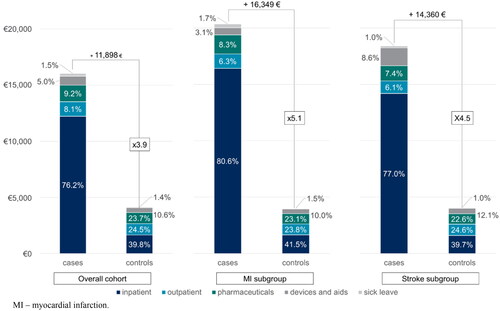
The mean total healthcare costs for cases in the overall cohort were €16,045 during the 1-year follow-up, which was €11,898 higher than healthcare costs for controls with €4,147 (p <0.001). This difference in mean healthcare costs was even higher within the subgroups, with average costs of €20,360 and €18,415 for MI and stroke cases compared to €4,011 and €4,055 for MI and stroke controls, respectively (MI: p <0.001; stroke: p <0.001) ().
Table 4. Total healthcare costs in cases and controls for the overall cohort and by subgroups.
Focusing on direct healthcare costs by excluding the costs for sick leave, the mean for the overall cohorts amounted to €15,804 and €4,087 for cases and controls, respectively. Inpatient costs were the primary cost driver, with a mean cost of €12,220 for cases in the overall cohort constituting 76.2% of total costs (). Inpatient costs in controls comprised only 39.8% of the overall cohort. A substantial portion of these costs was due to the hospitalization of the index CV event, which accounted for 42.2% of the total inpatient costs in the 1-year follow-up period. In relation to the overall total costs in the follow-up, the index CV event hospitalization accounted for 32.2% of the costs. Although pharmaceuticals ranked as the second highest cost category for cases in the overall cohort (mean cost: €1,471), the second highest cost category for cases in the MI cohort (mean cost: €1,689), and the third highest cost category for cases in the stroke cohort (mean cost: €1,354), LLTs only accounted for 3.7%, 3.3%, and 3.1% of the costs in each category, respectively.
Figure 2. Healthcare costs distribution by sectors of the overall cohort and controls.
Abbreviation: MI, myocardial infarction.
The healthcare costs for all cost categories were significantly higher in comparison to the controls in the overall study population as well as in the MI subgroup and the stroke subgroup (p <0.001 for each category; Supplementary Material, Appendix 6).
Discussion
The goal of this retrospective claims data analysis was to estimate HCRU and healthcare costs associated with CV events in a very high-risk population with a history of ASCVD. In addition to the costs of hospitalizations related to CV events, the costs for follow-up care in the outpatient setting (such as monitoring and pharmaceutical treatment) and the increased burden caused by potential recurrent events within 1 year were considered. To the best of our knowledge, there is no analysis evaluating HCRU and healthcare costs associated with CV events in German patients with a history of ASCVD. The results of our study show that patients with ASCVD who experience CV events have a substantial clinical burden, a high risk for death, and use many healthcare resources, resulting in significantly increased costs in comparison to ASCVD patients without CV events. Patients with CV events are at high risk for recurrent events. Recurrent events were one potential cause of the increased average cost in that period. Although total pharmaceutical costs increased after a CV event, LLT prescriptions were not the primary driver of increased pharmaceutical costs in the follow-up period.
Recurrent event rates and mortality rates were higher when compared to results from other analyses examining recurrent event rates in MI and stroke populations without further specific risk factors for CV events. A claims data study in Germany determined a slightly higher recurrence rate for stroke (7.4%) and a comparable all-cause mortality rate (17.0%) within 1 year after the index stroke eventCitation21. A hospital-based registry in Norway showed that patients hospitalized with stroke or transient ischemic attack exhibited a recurrence rate similar to the one observed in our study (5.4%) but a lower risk of all-cause death (10.7%) within the first year of follow-upCitation22. For MI, a registry study from Japan identified an in-hospital mortality rate of 8.5% and a recurrent event rate of 4.5% in MI patients during a median follow-up of 3.9 yearsCitation23.
Like our study, several studies have indicated that substantial proportions of patients do not receive high-intensity LLT as it is recommended by the 2016 and 2019 ESC/EAS guidelines for management of dyslipidemiaCitation2,Citation24–27.
Our results were aligned with other analyses investigating costs related to cardiovascular disease and CV events, considering that most studies did not focus on high-risk populations as our study didCitation10,Citation28–32.
In general, claims data offer several strengths for analyses in the healthcare system. Since claims data are recorded independently of any study purposes or clinical research recruiting participants, they constitute an appropriate tool for analyzing epidemiological measures, HCRU, and costs. German claims data show almost a complete picture of healthcare costs, as patients covered by the German statutory health insurance pay very little out of pocket. By using a matching approach, a comprehensive picture of the additional burden of CV events could be captured since not all additional costs and HCRU are directly attributable to the coded diagnoses and events.
German health insurance claims data have certain limitations. No additional clinical information, such as LDL-C levels or smoking status, is available in the database. Therefore, identification of additional risk factors and history of ASCVD is solely dependent on ICD-10-GM codes. The dosage/intake of LLTs and over-the-counter medications are also not available in the data. Likewise, the medications dispensed to patients during hospital stays are not included in the claims data; therefore, LLTs administered in this context are not captured. Furthermore, the CV related mortality was analyzed by an approach assuming that death occurring within 30 days of CV hospital admissions is attributable to the CV events, because no cause of death is recorded. Regarding the calculated costs, no inflation adjustment was applied. Cost data in our study reflect the costs per healthcare utilization at the time of reimbursement. Statistical matching techniques are also not without limitations. The exact matching techniques that we used in this study helped us to achieve good comparability of the resulting cohorts and allowed us to draw subgroups from the overall cohorts with their respective controls. However, since increasing the number of matching variables results in more potential controls becomes ineligible, we had to limit the number of matching variables to the minimum. In an attempt to achieve balance between adequate control of confounding and the sample size of the study, we chose to match on number of cardiovascular events rather than considering each of them as a matching variable. With our matching approach, we have lost 14.3% of our study population, which the study team considered acceptable.
Conclusions
The study shows that CV events are associated with additional CV risk, mortality, and significantly increased HCRU and costs in the first year following the event in German ASCVD patients covered by the statutory health insurance system. Very high-risk patients are rarely treated with high-intensity LLT in contrast to the ESC/EAS guideline recommendations. More intensive LDL-C management might lead to prevention of CV events, thus improving clinical outcomes and alleviating economic burden in the German healthcare system and overall economy.
Transparency
Declaration of funding
This work was supported by Amgen Inc, Thousand Oaks, CA, USA.
Declaration of financial/other relationships
ED was an employee of Amgen GmbH at the time of the study and holds stock of Amgen Inc. ES is an employee of Amgen (Europe) GmbH and holds stock of Amgen Inc. LP was an employee of Amgen Inc at the time of the study and holds stock of Amgen Inc. CJ and CM are employees of Xcenda GmbH, who received funds from Amgen Inc to conduct the study. RL reports consultancy fees from Amgen. The authors declare that no further conflict of interest exists. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
Conception, design or planning of the study: Eduard Sidelnikov, Eugen Dornstauder, Christian Jacob, Christopher Maas, Lionel Pinto. Acquisition of the data: Christian Jacob, Christopher Maas. Analysis of the data: Eduard Sidelnikov, Eugen Dornstauder, Christian Jacob, Christopher Maas. Interpretation of the results: Eduard Sidelnikov, Eugen Dornstauder, Christian Jacob, Christopher Maas, Lionel Pinto, Reiner Leidl, Ingo Ahrens. Drafting of the manuscript: Eduard Sidelnikov, Christian Jacob, Christopher Maas. Critically reviewing or revising the manuscript for important intellectual content: Eugen Dornstauder, Lionel Pinto, Reiner Leidl, Ingo Ahrens.
Supplemental Material
Download MS Word (139 KB)Acknowledgements
The analyses were performed in collaboration with Prof. Dr. Wolfgang Greiner and the Institute for Applied Health Research Berlin (InGef).
Data availability statement
The data used in this study cannot be made available in the manuscript, the supplemental files, or in a public repository due to German data protection laws (Bundesdatenschutzgesetz). To facilitate the replication of results, anonymized data used for this study are stored on a secure drive at the Institute for Applied Health Research Berlin (InGef). Access to the data used in this study can only be provided to external parties under the conditions of the cooperation contract of this research project and can be assessed upon request, after written approval ([email protected]), if required.
References
- Deutsche Herzstiftung e.V. Deutscher Herzbericht 2019. 2019.
- März W, Dippel F-W, Theobald K, et al. Utilization of lipid-modifying therapy and low-density lipoprotein cholesterol goal attainment in patients at high and very-high cardiovascular risk: real-world evidence from Germany. Atherosclerosis. 2018;268:99–107.
- Lechner K, von Schacky C, McKenzie AL, et al. Lifestyle factors and high-risk atherosclerosis: pathways and mechanisms beyond traditional risk factors. Eur J Prev Cardiol. 2020;27(4):394–406.
- Fuchs FD, Whelton PK. High blood pressure and cardiovascular disease. Hypertension. 2020;75(2):285–292.
- Lacruz ME, Kluttig A, Hartwig S, et al. Prevalence and incidence of hypertension in the general adult population. Medicine. 2015;94(22):e952.
- Gatwood J, Bailey JE. Improving medication adherence in hypercholesterolemia: challenges and solutions. Vasc Health Risk Manag. 2014;10:615–625.
- Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41(1):111–188.
- Baigent C, Blackwell L, Emberson J, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670–1681.
- Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376(18):1713–1722.
- Chapman RH, Liu LZ, Girase PG, et al. Determining initial and follow-up costs of cardiovascular events in a US managed care population. BMC Cardiovasc Disord. 2011;11:11.
- Tarride J-E, Lim M, DesMeules M, et al. A review of the cost of cardiovascular disease. Can J Cardio. 2009;25(6):195–202.
- Statistisches Bundesamt DESTATIS. Herz-Kreislauf-Erkrankungen verursachen die höchsten Kosten. 2017; [cited 2021 Feb 04]. Available from: https://www.destatis.de/DE/Themen/Gesellschaft-Umwelt/Gesundheit/Krankheitskosten/_inhalt.html#sprg232688.
- Dornstauder E, Sidelnikov E, Minartz C, et al. Burden of Disease and Social IMPACT of Myocardial Infarction and Ischemic Stroke in Germany from 2018 until 2030. ISPOR Europe 2020. 2020.
- Akobeng AK. Assessing the validity of clinical trials. J Pediatr Gastroenterol Nutr. 2008;47(3):277–282.
- Andersohn F, Walker J. Characteristics and external validity of the German Health Risk Institute (HRI) database. Pharmacoepidemiol Drug Saf. 2016;25(1):106–109.
- Bundesministerium für Gesundheit. Kennzahlen der Gesetzlichen Krankenversicherung 2007 bis. 2018. Kennzahlen und Faustformeln. Available from: https://www.bundesgesundheitsministerium.de/fileadmin/Dateien/5_Publikationen/Gesundheit/Broschueren/KF2019Bund_Maerz_2019.pdf.
- Statistisches Bundesamt DESTATIS. Ergebnisse der Bevölkerungsfortschreibung auf Grundlage des Zensus. 2011. Available from: https://www.destatis.de/DE/ZahlenFakten/GesellschaftStaat/Bevoelkerung/Bevoelkerungsstand/Tabellen/Zensus_Geschlecht_Staatsangehoerigkeit.html.
- Bundesministerium der Justiz und für Verbraucherschutz. § 287 SGB V. Available from: https://www.gesetze-im-internet.de/sgb_5/__287.html.
- Bundesministerium der Justiz und für Verbraucherschutz. § 75 SGB X. Available from: https://www.gesetze-im-internet.de/sgb_10/__75.html.
- Deutschen Institut für Medizinische Dokumentation und Information. ICD-10-GM Version 2020 Systematisches Verzeichnis. 2019.
- Stahmeyer JT, Stubenrauch S, Geyer S, et al. The frequency and timing of recurrent stroke. Dtsch Arztebl Int. 2019;116(42):711–717.
- Khanevski AN, Bjerkreim AT, Novotny V, et al. Recurrent ischemic stroke: incidence, predictors, and impact on mortality. Acta Neurol Scand. 2019;140(1):3–8.
- Nakatani D, Sakata Y, Suna S, et al. Incidence, predictors, and subsequent mortality risk of recurrent myocardial infarction in patients following discharge for acute myocardial infarction. Circ J. 2013;77(2):439–446.
- Breuckmann F, Hochadel M, Darius H, et al. Guideline-adherence and perspectives in the acute management of unstable angina – initial results from the German chest pain unit registry. J Cardiol. 2015;66(2):108–113.
- Gitt A, Rieber J, Hambrecht R, et al. Do acute coronary events affect lipid management and cholesterol goal attainment in Germany? Results from the dyslipidemia international study II. Wien Klin Wochenschr. 2018;130(23–24):707–715.
- Huang Q, Grabner M, Sanchez R, et al. Clinical characteristics and unmet need Among patients with atherosclerotic cardiovascular disease stratified by statin use. Am Health Drug Benefits. 2016;9(8):434–444.
- Waßmuth S, Rohe K, Noack F, et al. Adherence to lipid-lowering therapy in patients with coronary heart disease From The state Of Saxony-Anhalt, Germany. Vasc Health Risk Manag. 2019;15:477–483.
- Schmid T. Costs of treating cardiovascular events in Germany: a systematic literature review. Health Econ Rev. 2015;5(1):27.
- Reinhold T, Lindig C, Willich SN, et al. The costs of myocardial infarction – a longitudinal analysis using data from a large German health insurance company. J Public Health. 2011;19(6):579–586.
- GKV Spitzenverband. GKV-Kennzahlen. Available from: https://www.gkv-spitzenverband.de/service/zahlen_und_grafiken/gkv_kennzahlen/gkv_kennzahlen.jsp.
- Davis KL, Meyers J, Zhao Z, et al. High-risk atherosclerotic cardiovascular disease in a real-world employed Japanese population, cardiovascular event rates, and costs. J Atheroscler Thromb. 2015;22(12):1287–1304.
- Kirsch F, Becker C, Schramm A, et al. Effects of continuous enrollment in a structured disease management program in patients with coronary artery disease after acute myocardial infarction on adherence to guideline recommended medication, health care expenditures, and survival. Eur J Health Econ. 2020;21(4):607–619.