Abstract
Background
Targeted germline testing is recommended for those with or at risk of breast, ovarian, or colorectal cancer. The affordability of genetic sequencing has improved over the past decade, therefore the cost-effectiveness of testing for these cancers is worthy of reassessment.
Objective
To systematically review economic evaluations on cost-effectiveness of germline testing in breast, ovarian, or colorectal cancer.
Methods
A search of PubMed and Embase databases for cost-effectiveness studies on germline testing in breast, ovarian, or colorectal cancer, published between 1999 and May 2022. Synthesis of methodology, cost-effectiveness, and reporting (CHEERS checklist) was performed.
Results
The incremental cost-effectiveness ratios (ICERs; in 2021-adjusted US$) for germline testing versus the standard care option in hereditary breast or ovarian cancer (HBOC) across target settings were as follows: (1) population-wide testing: 344–2.5 million/QALY; (2) women with high-risk: dominant = 78,118/QALY, 8,337–59,708/LYG; (3) existing breast or ovarian cancer: 3,012–72,566/QALY, 39,835/LYG; and (4) metastatic breast cancer: 158,630/QALY. Likewise, ICERs of germline testing for colorectal cancer across settings were: (1) population-wide testing: 132,200/QALY, 1.1 million/LYG; (2) people with high-risk: 32,322–76,750/QALY, dominant = 353/LYG; and (3) patients with existing colorectal cancer: dominant = 54,122/QALY, 98,790–6.3 million/LYG. Key areas of underreporting were the inclusion of a health economic analysis plan (100% of HBOC and colorectal studies), engagement of patients and stakeholders (95.4% of HBOC, 100% of colorectal studies) and measurement of outcomes (18.2% HBOC, 38.9% of colorectal studies).
Conclusion
Germline testing for HBOC was likely to be cost-effective across most settings, except when used as a co-dependent technology with the PARP inhibitor, olaparib in metastatic breast cancer. In colorectal cancer studies, testing was cost-effective in those with high-risk, but inconclusive in other settings. Cost-effectiveness was sensitive to the prevalence of tested variants, cost of testing, uptake, and benefits of prophylactic measures. Policy advice on germline testing should emphasize the importance of these factors in their recommendations.
PLAIN LANGUAGE SUMMARY
Breast, ovarian, prostate, and colorectal cancers are among the top causes of cancer related deaths. A substantial proportion of people with these cancers have inherited mutations. The identification of these gene abnormalities could provide people with opportunities to utilize preventive risk reduction surgeries or undertake frequent routine testing for these cancers. However, genetic testing requires healthcare resources and money. Previous reviews on the cost-effectiveness of genetic testing in familial cancers have concluded that targeted screening i.e., selective assessment of people at high-risk could justify the costs of testing. Our evaluation of economic studies in breast and ovarian cancer, however, suggests that genetic testing is cost-effective across a wide variety of situations starting from the screening of all healthy women above 30 years to the testing of women with existing breast or ovarian cancer. Testing in metastatic breast cancer to inform treatment with Olaparib, a drug known to selectively improve survival in people with genetic mutations, was the sole exception where testing was not cost-effective. Contrary to findings for breast or ovarian cancer, testing for colorectal cancer was cost-effective in people with high-risk i.e., family history but inconclusive in other situations. Evidence on the cost-effectiveness of testing in prostate cancer is lacking and as a result we were not able to provide advice in this cancer group.
Systematic review registration:
Introduction
Genetic testing is recommended for the risk assessment and management of hereditary cancer, including breast, ovarian, and colorectal cancersCitation1–3. Between 10% and 20% of these cancers have been linked with pathogenic germline variants (breast: 10%; ovarian: 20%; colorectal: 10%)Citation4–7. The underlying aetiological mechanisms for these cancers comprise a failure of homologous recombination repair (HRR) or DNA mismatch repair (dMMR). Carriers of pathogenic variants (BRCA and lynch syndrome variants) tend to present with cancer at an early ageCitation8,Citation9 and have an aggressive disease profile with high mortalityCitation10. Identifying patients at increased hereditary risk may provide opportunities for early management, such as intensive surveillance with routine screening (e.g. colonoscopy) in colorectal cancerCitation11 or pro-active treatments like prophylactic risk-reduction surgeries in women predisposed to breast or ovarian cancerCitation12.
Previous reviewsCitation13–15 on the economics of genetic testing in breast, ovarian, and colorectal cancer indicate that testing is likely to be cost-effective when targeted towards populations with a higher-than-average risk of being a carrier or if multigene assays were utilized in the analysis. Findings from the review by Koldekoff et al.Citation14 on breast and ovarian cancer studies, however, may be limited due to the authors criteria to selectively assess studies that examined germline testing in healthy women with high-risk and in women with existing breast and ovarian cancer with a cascade testing component. Studies on population-wide testing of women within the general population, testing of racial/ethnic groups with high-risk (Ashkenazi Jewish women) and studies that failed to include cascade testing were excluded. On the contrary, DiMarco et al.Citation13 examined the cost-effectiveness of genetic testing for colorectal cancer over a wider range of populations and concluded that universal or targeted testing may be cost-effective when performed before 70 years of age.
There have been several additional studies on the cost-effectiveness of germline testing since the previous reviewsCitation16–26. Some of them assessed population-wide testingCitation17,Citation20, while others evaluated the cost-utility of germline testing guided treatments in cancer patients with mutationsCitation26. Next-generation sequencing (NGS) has also become affordableCitation27. Therefore, it would be interesting to see if the reduction in testing costs expands the utility of germline testing to populations where testing was previously known to be beneficial but cost-prohibitive. In this context, the aim of the current systematic review was to undertake a contemporary and comprehensive assessment of economic evaluations that studied the cost-effectiveness of germline testing for breast, ovarian, prostate, and colorectal cancer.
Materials and methods
Inclusion criteria and search strategy
A protocol (CRD42020161967) for this systematic review was registered at the International Prospective Register of Systematic Reviews (PROSPERO). A comprehensive search of electronic databases at Medline (PubMed) and Embase (Elsevier) was performed in accordance with the Preferred Reporting System for Systematic Reviews and Meta-Analysis (PRISMA) guidelinesCitation28. A checklist of the PRISMA items has been provided in Supplementary Table S1. We examined full economic evaluations (studies that had both costs and effectiveness measures) that assessed germline testing for breast, ovarian, prostate, and colorectal cancer in the databases between 1 January 1999 and 6 May 2022. The following search terms were used: genetic testing, germline testing, breast cancer, prostate cancer, colorectal cancer, lynch syndrome, cost-effectiveness analysis, cost-benefit analysis, economics, Markov, and decision support techniques. Details about the search strategy with MESH terms, PICO, inclusion, and exclusion criteria have been provided in Supplementary Table S2. To be brief, studies were included if they met the following inclusion criteria:
Patients: (1) All adults in the general population, at risk of breast, ovarian, prostate, or colorectal cancer, or (2) patients with a pre-established diagnosis of breast, ovarian, prostate, or colorectal cancer.
Intervention: Germline testing followed by personalized management including (1) intensive surveillance (colonoscopy, faecal immunohistochemistry, mammograms, etc.), (2) risk-reduction surgeries, or (3) specific pharmacotherapy, selective for cancer pathways (PARP inhibitors, EGFR inhibitors, etc.).
Comparator: standard clinical care (1) without germline testing, (2) selective testing of individuals at high-risk, or (3) reflex germline testing after immunohistochemistry
Outcome: costs per life year gained (LYG) or costs per quality-adjusted life year (QALY).
Data abstraction, synthesis, and quality assessment
Parameters relevant to germline testing and cost-effectiveness, including details about the country/setting, type of cancer, population for germline testing, referent population, pathogenic variants assessed, probability of being variant positive, cost of testing, cascade testing, type of prophylaxis in test population versus referent, compliance rates of prophylaxis or frequency of intensive surveillance, model used in the analysis, study perspective, discounting, incremental cost-effectiveness ratio (ICER), willingness-to-pay (WTP) threshold, and factors affecting cost-effectiveness (sensitivity analysis) were extracted from each study. We grouped results for hereditary breast and ovarian cancer (HBOC) together for reasons of sample size, on aetiological grounds (association with defects in homologous recombination repair mechanics)Citation29 and similarities in prophylactic measures (risk-reduction mastectomy, i.e. RRM; risk-reduction salpingo-oophorectomy, i.e. RRSO; or both). Studies in colorectal cancer evaluated several diagnostic strategies including no testing, reflex testing, as well as direct germline testing. Reflex testing begins with tumour-based screening that requires a tissue biopsy sample and is followed by germline testing only after a positive IHC test for MLH1 or BRAF. On the contrary, direct germline testing is less invasive and is typically performed on peripheral blood or saliva samples. Therefore, when reporting the ICERs for colorectal cancer studies we have strived to compare germline testing versus no testing, as the two strategies are more in line with the criteria for the review. Reflex testing was used as the referent, only in studies where it was the sole comparator for germline testing. All reported costs have been standardized from values reported in the original article to 2021 US$ using the CCEMG-EPPI-Centre Cost ConverterCitation30. A meta-analysis was not possible as there was substantial heterogeneity among included studies in the target population for testing.
Reporting quality of the studies was assessed by two authors (ST and BH) using the recently updated Consolidated Health Economic Evaluation Reporting Standards (CHEERS) checklistCitation31. These guidelines received a major overhaul in 2022 and, compared to the previous iteration, include new items on patient engagement and the use of a health economic analysis plan (HEAP). The goal of the CHEERS assessment was to report the essential information needed for interpretation of health economic evaluations, check for reliability of study findings, efforts made for patient or community engagement, and offer clarity to readers by providing the location of the 28 assessed items within each article.
Results
Search strategy and the final sample of studies
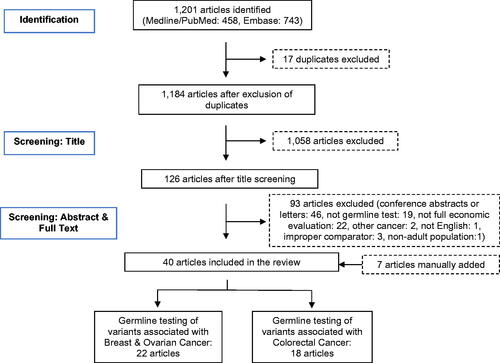
An initial search of the databases using a pre-defined set of keywords (Supplementary Table S2) resulted in the identification of 1,201 potential articles (). Removal of duplicates and title screening brought this sample down to 126 articles. Further screening of abstracts and full texts led to the exclusion of an additional 93 articles (46 conference abstracts or letters, 19 not germline testing, 22 not full economic evaluations, two studies based on other cancers, one study not in English language, three studies using non-relevant comparators, one study in non-adult population). At this point of screening, we manually added seven articles that were not identified through the database search, five of which were articles published within the last year and two other studies that were present in a previous reviewCitation15. The final set of articles included in the review was 40 (22 HBOC, 18 colorectal cancer). There were no studies on the cost-effectiveness of germline testing in prostate cancer. and present a detailed overview of the characteristics of all the articles included in the review.
Table 1. Characteristics of economic studies on germline testing for breast and ovarian cancer.
Table 2. Characteristics of economic studies on germline testing for colorectal cancer.
Germline testing in HBOC: Cost-effective across most settings ()
The bulk of breast or ovarian cancer studies were on targeted testing of healthy women with higher-than-average risk (40.9%, n = 9 studies) and patients with pre-established breast or ovarian cancer diagnosis (36.4%, n = 8 studies). The analyses were typically performed from a health payer perspective (68.2%, n = 15 studies), included cascade testing of relatives of probands (40.9%, n = 9 studies), tested exclusively for BRCA variants (72.7%, n = 16 studies), had testing costs ≤ US$500 (59.1%, n = 13 studies), used Markov modelling (45.4%, n = 10 studies), and appraised the cost-effectiveness of testing against willingness-to-pay thresholds of US$30,000–100,000/QALY or LYG (90.9%%, n = 20 studies).
Germline testing was cost-effective or cost-saving in the vast majority of studies (90.9%, n = 20 studies, ICERs in US$: dominant = 91,392/QALY; 8,337–59,708/LYG). The two exceptions where testing was not cost-effective included the study by Tengs and BerryCitation49, that assessed population-wide testing in the general population (BRCA prevalence: 0.8%, ICER: US$2.5million/QALY), and the study by Saito et al.Citation26 in women with metastatic breast cancer, where testing was performed to inform treatment with the PARP inhibitor, olaparib (ICER: US$158,630/QALY).
Evaluating the cost-effectiveness of germline testing for HBOC across specific settings, the ICERs of the testing strategy compared to the referent (no germline testing or testing limited to those with family history/clinical risk) for (1) population-wide testing of healthy women was US$344 − 2.5 million/QALY; (2) healthy women at high-risk was US$dominant = 78,118/QALY; 8,337/LYG; (3) healthy women with previous benign breast biopsy was US$59,708/LYG; (4) women with existing breast or ovarian cancer was US$3,012–72,566/QALY, 39,835/LYG; and (5) women with metastatic breast cancer was US$158,630/QALY.
Sensitivity analyses revealed that the uptake of risk-reduction surgeries (45.5%, n = 10 studies), cost of germline testing (36.4%, n = 8 studies), the proportion of test positive cases (18.2%, n = 4 studies), and the assumed survival benefits or utility of testing (13.6%, n = 3 studies) were some of the major factors influencing cost-effectiveness.
Germline testing for colorectal cancer: cost-effective in people with high-risk ()
Studies in the colorectal cancer group assessed the cost-effectiveness of germline testing in people with high-risk (38.9%, n = 7 studies) and those with established colorectal cancer (50.0%, n = 9 studies). Few studies evaluated the economics of population-wide testing (11.1%, n = 2 studies). Most studies were designed from a health payer perspective (72.2%, n = 13 studies), included a cascade testing component (66.7%, n = 12 studies), tested for lynch syndrome variants, i.e. MLH1, MSH2, MSH6, PMS2 (88.9%, n = 16 studies), had testing costs > US$1000 (55.6%, n = 10 studies), used Markov models (50.0%%, n = 9 studies), and had WTP thresholds between US$30,000 and 100,000/QALY or LYG (77.8%, n = 14 studies).
Overall, the results for cost-effectiveness in colorectal cancer studies were mixed. While germline testing was cost-effective in healthy people with high-risk (ICERs: US$32,322–76,750/QALY; dominant-353/LYG), there was widespread variation in findings for colorectal cancer patients (ICERs: US$dominant = 54,122/QALY; 98,790–6.3 million/LYG) and in population-wide testing scenarios (ICERs: US$132,200/QALY; 1.1 million/LYG).
Among studies that were not cost-effective, fourCitation52,Citation54,Citation56,Citation60 out of five studiesCitation18,Citation52,Citation54,Citation56,Citation60 had testing costs above US$1,000. In the 2015 study by Barzi et al.Citation56, the authors recommended against universal germline testing and expressed that the low prevalence of variants associated with colorectal cancer in the general population (0.2%) and substantial costs of testing (US$3,520) explain their observed findings. Meanwhile the more recent, 2022 study by Guzauskas et al.Citation17 saw a substantial drop in testing costs (US$200) and the ICER for population-wide testing versus testing those with high-risk was US$132,200/QALY. The authors concluded that universal germline testing was likely to be cost-effective after using a WTP cut-off of US$150,000/QALY. Overall, cost-effectiveness of germline testing in colorectal cancer was sensitive to the price of testing (38.9%, n = 7 studies), compliance rates of surveillance (27.8%, n = 5 studies), probability of being variant positive (27.8%, n = 5 studies), the inclusion of cascade testing (15.8%, n = 3 studies), health benefits or utility of testing (11.1%, n = 2 studies), WTP thresholds (11.1%, n = 2 studies), and discounting (11.1%, n = 2 studies).
Summary of reporting quality (Supplementary Tables S3 and S4)
More than 77% of HBOC studies (n = 17 studies) provided information on at least 24 of the 28 CHEERS items. None of the studies had a health economic analysis plan (HEAP) with 58-core items as advised by CHEERSCitation31. Engagement with patients and others was also another area that was grossly under-reported, with 95.4% of the studies failing to include information on the two items assessing this component. Other areas with under-reporting included title (4.6%, n = 1 study), abstract (4.6%, n = 1 study), study perspective (4.6%, n = 1 study), measurement of outcomes (18.2%, n = 4 studies), valuation of resources and costs (4.6%, n = 1 studies), currency and price conversion (4.6%, n = 1 study), characterization of heterogeneity (9.1%, n = 2 studies), study parameters (4.6%, n = 1 study), source of funding (10.0%, n = 2 studies), and details on conflict of interest (27.3%, n = 6 studies).
Similarly, 83.3% of colorectal cancer studies (n = 15 studies) reported on at least 24 of the 28 CHEERS items. Information on HEAP (100%, n = 19 studies), approach to engagement with patients and others (100%, n = 19 studies), effect of engagement with patients and others (100%, n = 19 studies), measurement of outcomes (38.9%, n = 7 studies), valuation of resources and costs (16.7%, n = 3 studies), characterizing heterogeneity (11.1%, n = 2 studies), source of funding (11.1%, n = 2 studies), and statement on conflict of interest (5.6%, n = 1 study) were under-reported.
Discussion
In the current systematic review, we examined previous economic evaluations on the cost-effectiveness of testing for germline variants associated with breast, ovarian, or colorectal cancer. Germline testing was cost-effective in the majority of hereditary breast or ovarian cancer (HBOC) studies (20 of 22 studies) and approximately three-quarters of colorectal cancer studies (13 of 18 studies). Specifically, in the HBOC group, germline testing was likely to be cost-effective in (1) population-wide testing of all healthy women (variant prevalence of 0.5–0.7%); (2) women with higher-than-average risk (variant prevalence ≥ 2.5%); and (3) women with existing breast or ovarian cancer (variant prevalence of 5.5–17.2%), preferably with cascade testing of relatives of probands. The situation is more complex in colorectal cancer where testing was cost-effective when performed in people with higher-than-average risk (lynch-syndrome variant prevalence ≥ 1.5%) but displayed mixed results with widespread variation when performed in patients with existing colorectal cancer. Incidentally we also explored the economics of germline testing in prostate cancer, another cancer group associated with a high prevalence of heritable mutationsCitation64,Citation65. The National Comprehensive Cancer Network (NCCN) recommends germline testing based on family history or clinical stage/risk group of prostate cancer (high risk localized, very-high localized, regional node positive, and metastatic prostate cancer)Citation66. It is anticipated that these broad recommendations would impose a significant workload on the health care systemCitation67. The benefits of germline testing in such a wide audience is unclearCitation68,Citation69, except for the niche segment of men with metastatic castration resistant prostate cancer (mCRPC), where testing was used to guide personalized treatment with PARP inhibitors and improved survivalCitation70,Citation71. The economics of adopting such an approach, however, is not clear. Our search strategy led us to two studiesCitation19,Citation24 on the cost-effectiveness of genetic test-directed treatment with the PARP inhibitor, Olaparib in men with mCRPC. However, the setting for both studies were men who tested positive for three or 15 pre-specified gene alterations, i.e. both the intervention group (olaparib therapy) and the comparator (standard care) were men who were variant positive. It was not possible to examine the cost-benefits of a germline testing versus no testing scenario and both studiesCitation19,Citation24 were excluded from the review. Overall, the evidence on cost-effectiveness of germline testing in prostate cancer is limited, therefore policy advice for testing in this cancer group is not yet possible.
Sensitivity analyses performed within the final sample of studies included in the review showed that the cost-effectiveness of germline testing could be influenced by the prevalence of tested variants, cost of testing, uptake of prophylactic measures (risk reduction surgeries, intensive surveillance), the survival benefits associated with prophylaxis, WTP thresholds, and cost inflation. Differences in real-world data or assumptions made about these parameters may have contributed to the observed heterogeneity in ICERs across studies.
Examining each of the influential parameters, 36–37% of studies in both HBOC and colorectal cancer reported their results as being sensitive to cost of germline testing. Pricing was on average higher in colorectal cancer relative to HBOC. Around 36.8% of colorectal cancer studies reported testing costs over US$2,000, a stark contrast to 18.2% of studies with similar pricing in HBOC. A few recent studiesCitation17,Citation20,Citation21,Citation25,Citation32,Citation34 saw a reduction in price of testing, yet for the most part costs remained highly variable across settingsCitation27,Citation72.
Assumptions on uptake of prophylactic risk reduction surgeries also influenced results and was the most predominant factor affecting cost-effectiveness in HBOC studies. Uptake rates for RRM were usually lower than those for RRSO. The variation in adoption of RRM (9–100%) and RRSO (25–100%) rates across studies could be attributed to cultural differences in perceived ceilings for childbearing age, stigmas associated with the surgeries, costs of surgery, and patient perceptions on long-term survival post-surgeryCitation73. In colorectal cancer where preventive surgeries have no clear benefitsCitation74, elective risk reduction surgeries (e.g. colectomy) were not the mainstay of management and test positive cases were usually followed-up with intensive surveillance (colonoscopy or sigmoidoscopy every 1–3 years). The study by Breheny et al.Citation63 was the sole exception where intensive surveillance was followed by proactive colorectal surgery (at 45 years for Lynch syndrome carriers; at 21 years for familial adenomatous polyposis). Elective colorectal surgery could reduce the incidence of metachronous colorectal cancer in younger patients with high-riskCitation75,Citation76; however, the strategy is not widely employed and may need further investigationCitation77.
Apart from the differences in costs of testing and assumptions on prophylaxis, studies also differed in terms of the modelling techniques used for their analysis. Cohort-based state-transitions models such as the Markov were the most prominent design used in both HBOC (45% of studies) and colorectal cancer studies (52.6% of studies). Decision-tress (HBOC: 22.7%; colorectal: 31.6%) and patient-level microsimulation models (HBOC: 27.3%, colorectal: 10.5%) were the other two designs used. Markov models may have been used more frequently owing to their less stringent computation and individual patient-level data requirements. Unlike the memory-less Markov models, microsimulation models have the advantage of being able to account for patient-level characteristics and their effect on future events (such as uptake of prophylaxis, etc.) and are, therefore, a better design for screening type interventionsCitation78. However, they do require more extensive individual patient data and are also computationally more intensive. Finally, decision trees are logic-based designs lacking the ability to account for time-dependent variables or recurring events and are, therefore, the simpler of the three modelling techniquesCitation79.
Germline testing is also useful to inform treatment with drugs that act on underlying pathogenic mechanisms for hereditary cancer. The cost-effectiveness of germline testing guided therapy was assessed in two studiesCitation26,Citation45. Saito et al.Citation26 examined the cost-effectiveness of the PARP inhibitor, olaparib, in BRCA positive women with metastatic breast cancer versus standard chemotherapy in those without mutation profiling. Testing-guided treatment was not cost-effective (ICER: US$158,630 QALY) and the authors commented that cost-effectiveness could improve with a reduction in costs of BRCA profiling and olaparib therapy. Meanwhile, Green et al.Citation45 examined the utility of germline testing to determine eligibility for tamoxifen therapy in women with previous benign breast biopsy (i.e. high-risk of future breast cancer) and demonstrated cost-effectiveness (ICER: 59,708/QALY).
Our findings for the cost-effectiveness of germline testing in HBOC and colorectal cancer concur with previous reviewsCitation13–15 in the utility of targeted screening of people with higher-than-average risk. However, unlike the previous work by Koldekoff et al.Citation14 where studies on population-wide genetic testing for HBOC were excluded, we made no such omission. Our decision to include these studies holds merit, as recent studiesCitation17,Citation20,Citation32,Citation34 suggest that population-wide testing could be cost-effective in HBOC as well as colorectal cancer. These findings could be explained by reduction in germline testing costs and the choice of WTP limits. All four of the recent studiesCitation17,Citation20,Citation32,Citation34 had testing costs ≤ US$200, a reduction from the typical range in the review ( and ). Regarding WTP thresholds the three HBOC studiesCitation20,Citation32,Citation34 used the more commonly used cut-off of US$100,000/QALY or LYG, while the colorectal cancer studyCitation17 based its conclusion at moderately high WTP of $150,000 per QALY. Other notable observations from the current review were the heterogeneity among studies both in modelling techniques (Markov, microsimulation, decision-tree, etc.), uptake of prophylactic measures, compliance with intensive surveillance, probability of being variant positive, discounting, and WTP thresholds. Differences in most of these variables may, however, be an inherent tenet of the population or setting, induced by cultural perceptions towards the perceived value of testing or dependent on the GDP of countries, etc. Extending the discussion on variation in findings across country or setting, Manchanda et al.Citation32 assessed the cost-effectiveness of BRCA testing across multiple countries. Population-wide BRCA testing was cost-saving in high-income countries (UK, US, and Netherlands, ICERs: dominant, WTP: US$42,656–57,589/QALY), cost-effective in upper-middle-income countries (China and Brazil, ICERs: US$13,579–18,066/QALY; WTP: US$15,181–15,531/QALY), and cost-prohibitive in lower-middle income countries (India, ICER: US$23,031/QALY, WTP: US$6,574/QALY). Overall, due to the heavy influence of individual-level variables, patient level-microsimulation may be a more appropriate design to evaluate cost-effectiveness of germline testing than the widely used cohort level Markov models.
Table 3. Summary of characteristics of economic studies on germline testing for breast or ovarian cancer.
Table 4. Summary of characteristics of economic studies on germline testing for colorectal cancer.
Our assessment of reporting quality using CHEERSCitation31 suggested that studies need to do a better job at reporting measurement of outcomes. Patient or stakeholder engagement was another glaring omission in most studies. Items requiring reporting in these areas have been introduced in the recently updated CHEERS criteriaCitation31. This is an important area of research, especially in interventions for cancer patients, where there are uncertainties about WTP limitsCitation80–83. Future studies could provide valuable insight by weighing patient and stakeholder preferences for cost-utility of germline testing compared to other healthcare needs.
The limitations of this review are noteworthy. First, we assessed the cost-utility of germline testing and did not include evaluations on genomic or somatic testing. Both testing modalities fall under the purview of genetic testing, yet there are clear distinctions between them. While somatic testing looks for genetic changes that develop over the individual’s lifetime, germline testing is focused on heritable mutations and our findings are limited to the latter. Second, the heterogeneity of included studies regarding the target group for testing and the small number of articles within each of these strata prevented us from performing a metanalysis. Also, our findings are not comprehensive as we did not include studies published in non-English languages. Third, information on prognostic markers such as HER2 or triple negative status (additionally provides information about estrogen or progesterone receptors on cancer cells) was not provided in most HBOC studies. Patients with BRCA mutations have a higher likelihood of being triple negativeCitation84, which may further reduce their long-term survival. Information on triple negative status could, therefore, explain some of the variation in cost-effectiveness across studies.
Germline testing in breast, ovarian, or colorectal cancer has the potential to provide timely and cost-effective personalized healthcareCitation85,Citation86. Previous reviews of HBOCCitation14,Citation87 recommend testing based on family history and for women with pre-existing breast or ovarian cancer. Our evaluation considers recently published studies on population-wide testingCitation20,Citation32,Citation34 and suggests that testing for HBOC is likely to be cost-effective across most scenarios, i.e. population-wide testing of healthy women, targeted testing of those with family history, and testing of women with existing breast or ovarian cancer. In colorectal cancer where genetic sequencing costs were relatively high and pathogenic variant prevalence lower, germline testing demonstrated cost-effectiveness in people with higher-than-average risk but was inconclusive in other settings. Testing is also recommended to inform personalized treatment with PARP inhibitors in advanced stage breast, ovarian, and prostate cancerCitation1,Citation3,Citation66. Several clinical trials have demonstrated the significant survival benefits of PARP over the standard care optionCitation88,Citation89. Yet, studies on the economics of these co-dependent technologies (germline testing followed by PARP therapy)Citation90 have been limited and inconclusive. The approach is currently not financially feasible in HBOCCitation26,Citation91, but is potentially cost-effective in prostate cancer after adopting relatively high WTP cut-offs of US$150,000/QALYCitation24. Despite the gain in QALYs the high price of PARP inhibitors may be the primary driver of the increased costsCitation24,Citation26,Citation89,Citation91. Policy advice on germline testing should recommend weighing the benefits of testing with careful consideration to the prevalence of pathogenic variants in the testing scenario, costs of genetic sequencing and subsequent personalized treatments, and the uptake and benefits of prophylactic measures.
Transparency
Author contributions
ST performed the literature search, data abstraction and synthesis, quality assessment, and prepared the first draft of the manuscript. HT, PS, and SH contributed to the conceptual design of the study and towards revisions to the manuscript. BH reviewed the literature search, methodology, quality assessment, and provided feedback for revisions. All the authors read and agreed to the final version of the article.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplemental Material
Download MS Word (182.1 KB)Acknowledgements
None reported.
Declaration of funding
This research is part of a doctoral thesis by Srinivas Teppala. Dr. Teppala is the recipient of a Griffith University Health Group International Postgraduate Scholarship and a Griffith University Postgraduate Research Scholarship (2019–2022). Haitham Tuffaha holds a Priority Impact Research Award – Future Leader funded by Prostate Cancer Foundation of Australia.
Declaration of financial/other relationships
The authors report no conflicts of interest related to this manuscript.
References
- NCCN. 2022. Clinical practice guidelines in oncology, breast cancer. Version 4.2022. Avaialble from: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf.
- NCCN. 2022. Clinical practice guidelines in oncology, colon cancer. Version 1.2022. Avaialble from: https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf.
- NCCN. 2022. Clinical practice guidelines in oncology, ovarian cancer including fallopian tube cancer and primary peritoneal cancer. Version 4.2022. Avaialble from: https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf
- Ashour M, Ezzat Shafik H. Frequency of germline mutations in BRCA1 and BRCA2 in ovarian cancer patients and their effect on treatment outcome. CMAR. 2019;11:6275–6284.
- Macrae FA. Colorectal cancer: epidemiology, risk factors, and protective factors. In: Goldberg RM, Seres D, editor. UpToDate. Waltham (MA): UpToDate Inc; 2022. Available from: https://www.uptodate.com/contents/colorectal-cancer-epidemiology-risk-factors-and-protective-factors
- Sun J, Meng H, Yao L, et al. Germline mutations in cancer susceptibility genes in a large series of unselected breast cancer patients. Clin Cancer Res. 2017;23(20):6113–6119.
- Yurgelun MB, Kulke MH, Fuchs CS, et al. Cancer susceptibility gene mutations in individuals with colorectal cancer. J Clin Oncol. 2017;35(10):1086–1095.
- Stigliano V, Sanchez-Mete L, Martayan A, et al. Early-onset colorectal cancer: a sporadic or inherited disease? World J Gastroenterol. 2014;20(35):12420–12430.
- Tilanus-Linthorst MM, Obdeijn IM, Hop WC, et al. BRCA1 mutation and young age predict fast breast cancer growth in the Dutch, United Kingdom, and Canadian magnetic resonance imaging screening trials. Clin Cancer Res. 2007;13(24):7357–7362.
- Evans DGR, Ingham SL. Reduced life expectancy seen in hereditary diseases which predispose to early-onset tumors. Appl Clin Genet. 2013;6:53–61.
- Yurgelun MB, Hampel H. Recent advances in lynch syndrome: diagnosis, treatment, and cancer prevention. Am Soc Clin Oncol Educ Book. 2018;38:101–109.
- Jatoi I. Risk-Reducing options for women with a hereditary breast cancer predisposition. Eur J Breast Health. 2018;14(4):189–193.
- Di Marco MED, Panic N, Baccolini V, et al. Which lynch syndrome screening programs could be implemented in the “real world”? A systematic review of economic evaluations. Genet Med. 2018;20(10):1131–1144.
- Koldehoff A, Danner M, Civello D, et al. Cost-effectiveness of targeted genetic testing for breast and ovarian cancer: a systematic review. Value Health. 2021;24(2):303–312.
- Grosse SD. When is genomic testing cost-effective? Testing for lynch syndrome in patients with newly-diagnosed colorectal cancer and their relatives. Healthcare. 2015;3(4):860–878.
- Cenin DR, Naber SK, de Weerdt AC, et al. Cost-effectiveness of personalized screening for colorectal cancer based on polygenic risk and family history. Cancer Epidemiol Biomarkers Prev. 2020;29(1):10–21.
- Guzauskas GF, Jiang S, Garbett S, et al. Cost-effectiveness of population-wide genomic screening for lynch syndrome in the United States. Genet Med. 2022;24(5):1017–1026.
- Kang YJ, Killen J, Caruana M, et al. The predicted impact and cost-effectiveness of systematic testing of people with incident colorectal cancer for lynch syndrome. Med J Aust. 2020;212(2):72–81.
- Li Y, Lin S, Zhong L, et al. Is olaparib cost effective in metastatic castration-resistant prostate cancer patients with at least one favorable gene mutation in BRCA1, BRCA2 or ATM? Pharmacogenomics. 2021;22(13):809–819.
- Michaan N, Leshno M, Safra T, et al. Cost effectiveness of whole population BRCA genetic screening for cancer prevention in Israel. Cancer Prev Res (Phila). 2021;14(4):455–462.
- Pastorino R, Basile M, Tognetto A, et al. Cost-effectiveness analysis of genetic diagnostic strategies for lynch syndrome in Italy. PLoS One. 2020;15(7):e0235038.
- Ramdzan AR, Manaf MRA, Aizuddin AN, et al. Cost-effectiveness of colorectal cancer genetic testing. IJERPH. 2021;18(16):8330.
- Salikhanov I, Heinimann K, Chappuis P, et al. Swiss cost-effectiveness analysis of universal screening for lynch syndrome of patients with colorectal cancer followed by Cascade genetic testing of relatives. J Med Genet. 2022;59(9):924–930.
- Su D, Wu B, Shi L. Cost-effectiveness of genomic test-directed olaparib for metastatic castration-resistant prostate cancer. Front Pharmacol. 2020;11:610601.
- Sun L, Cui B, Wei X, et al. Cost-effectiveness of genetic testing for all women diagnosed with breast cancer in China. Cancers. 2022;14(7):1839.
- Saito S, Nakazawa K, Nagahashi M, et al. Cost-effectiveness of BRCA1/2 mutation profiling to target olaparib use in patients with metastatic breast cancer. Per Med. 2019;16(6):439–448.
- Desai K, Hooker G, Gilbert K, et al. Real-world trends in costs of next generation sequencing (NGS) testing in U.S. setting. J Clin Oncol. 2021;39(15_suppl):e18824–e18824.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535.
- Petrucelli N, Daly MB, Pal T. 1993. BRCA1- and BRCA2-Associated hereditary breast and ovarian cancer. In: Adam MP, Mirzaa GM, Pagon RA, Wallace SE, Bean LJH, Gripp KW, Amemiya A, editors. GeneReviews(®). Seattle: University of Washington. (Copyright © 1993-2022, University of Washington, Seattle. GeneReviews is a registered trademark of the University of Washington, Seattle. All rights reserved.).
- CCEMG-EPPI-Centre. 2022. Campbell and cochrane economics methods group and the evidence for policy and practice information and coordinating centre. CCEMG–EPPI-Centre cost converter. Available from: https://eppi.ioe.ac.uk/costconversion/
- Husereau D, Drummond M, Augustovski F, et al. Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. BMJ. 2022;376:e067975.
- Manchanda R, Sun L, Patel S, et al. Economic evaluation of population-based brca1/brca2 mutation testing across multiple countries and health systems. Cancers. 2020;12(7):1929–1938.
- Hurry M, Eccleston A, Dyer M, et al. Canadian cost-effectiveness model of BRCA-driven surgical prevention of breast/ovarian cancers compared to treatment if cancer develops. Int J Technol Assess Health Care. 2020;36(2):104–112.
- Guzauskas GF, Garbett S, Zhou Z, et al. Cost-effectiveness of population-wide genomic screening for hereditary breast and ovarian cancer in the United States. JAMA Netw Open. 2020;3(10):e2022874.
- Sun L, Brentnall A, Patel S, et al. A cost-effectiveness analysis of multigene testing for all patients with breast cancer. JAMA Oncol. 2019;5(12):1718.
- Moya-Alarcón C, González-Domínguez A, Simon S, et al. Cost-utility analysis of germline BRCA1/2 testing in women with high-grade epithelial ovarian cancer in Spain. Clin Transl Oncol. 2019;21(8):1076–1084.
- Tuffaha HW, Mitchell A, Ward RL, et al. Cost-effectiveness analysis of germ-line BRCA testing in women with breast cancer and Cascade testing in family members of mutation carriers. Genet Med. 2018;20(9):985–994.
- Patel S, Legood R, Evans DG, et al. Cost effectiveness of population based BRCA1 founder mutation testing in Sephardi Jewish women. Am J Obstet Gynecol. 2018;218(4):431.e431–431.e412.
- Manchanda R, Patel S, Gordeev VS, et al. Cost-effectiveness of population-based BRCA1, BRCA2, RAD51C, RAD51D, BRIP1, PALB2 mutation testing in unselected general population women. J Natl Cancer Inst. 2018;110(7):714–725.
- Lim KK, Yoon SY, Mohd Taib NA, et al. Is BRCA mutation testing cost effective for early stage breast cancer patients compared to routine clinical surveillance? The case of an upper middle-income country in Asia. Appl Health Econ Health Policy. 2018;16(3):395–406.
- Manchanda R, Patel S, Antoniou AC, et al. Cost-effectiveness of population based BRCA testing with varying Ashkenazi Jewish ancestry. Am J Obstetr Gynecol. 2017;217(5):578.e571–578.e512.
- Li Y, Arellano AR, Bare LA, et al. A multigene test could cost-effectively help extend life expectancy for women at risk of hereditary breast cancer. Value Health. 2017;20(4):547–555.
- Eccleston A, Bentley A, Dyer M, et al. A cost-effectiveness evaluation of germline BRCA1 and BRCA2 testing in UK women with ovarian cancer. Value Health. 2017;20(4):567–576.
- Manchanda R, Legood R, Burnell M, et al. Cost-effectiveness of population screening for BRCA mutations in Ashkenazi jewish women compared with family history-based testing. J Natl Cancer Inst. 2015;107(1):380.
- Green LE, Dinh TA, Hinds DA, et al. Economic evaluation of using a genetic test to direct breast cancer chemoprevention in white women with a previous breast biopsy. Appl Health Econ Health Policy. 2014;12(2):203–217.
- Kwon JS, Daniels MS, Sun CC, et al. Preventing future cancers by testing women with ovarian cancer for BRCA mutations. J Clin Oncol. 2010;28(4):675–682.
- Holland ML, Huston A, Noyes K. Cost-effectiveness of testing for breast cancer susceptibility genes. Value Health. 2009;12(2):207–216.
- Balmaña J, Sanz J, Bonfill X, et al. Genetic counseling program in familial breast cancer: analysis of its effectiveness, cost and cost-effectiveness ratio. Int J Cancer. 2004;112(4):647–652.
- Tengs TO, Berry DA. The cost effectiveness of testing for the BRCA1 and BRCA2 breast-ovarian cancer susceptibility genes. Dis Manag Clin Outcomes. 2000;2:15–24.
- Grann VR, Whang W, Jacobson JS, et al. Benefits and costs of screening Ashkenazi Jewish women for BRCA1 and BRCA2. J Clin Oncol. 1999;17(2):494–500.
- Pereira C, Areia M, Dinis-Ribeiro M. Cost-utility analysis of genetic polymorphism universal screening in colorectal cancer prevention by detection of high-risk individuals. Dig Liver Dis. 2019;51(12):1731–1737.
- Chen YE, Kao SS, Chung RH. Cost-effectiveness analysis of different genetic testing strategies for lynch syndrome in Taiwan. PLoS One. 2016;11(8):e0160599.
- Snowsill T, Huxley N, Hoyle M, et al. A model-based assessment of the cost–utility of strategies to identify lynch syndrome in early-onset colorectal cancer patients. BMC Cancer. 2015;15(1):313.
- Severin F, Stollenwerk B, Holinski-Feder E, et al. Economic evaluation of genetic screening for lynch syndrome in Germany. Genet Med. 2015;17(10):765–773.
- Gallego CJ, Shirts BH, Bennette CS, et al. Next-generation sequencing panels for the diagnosis of colorectal cancer and polyposis syndromes: a cost-effectiveness analysis. J Clin Oncol. 2015;33(18):2084–2091.
- Barzi A, Sadeghi S, Kattan MW, et al. Comparative effectiveness of screening strategies for lynch syndrome. J Natl Cancer Inst. 2015;107:djv005.
- Wang VW, Koh PK, Chow WL, et al. Predictive genetic testing of first degree relatives of mutation carriers is a cost-effective strategy in preventing hereditary non-polyposis colorectal cancer in Singapore. Fam Cancer. 2012;11(2):279–289.
- Ladabaum U, Wang G, Terdiman J, et al. Strategies to identify the lynch syndrome among patients with colorectal cancer: a cost-effectiveness analysis. Ann Intern Med. 2011;155(2):69–79.
- Dinh TA, Rosner BI, Atwood JC, et al. Health benefits and cost-effectiveness of primary genetic screening for lynch syndrome in the general population. Cancer Prev Res (Phila). 2011;4(1):9–22.
- Mvundura M, Grosse SD, Hampel H, et al. The cost-effectiveness of genetic testing strategies for lynch syndrome among newly diagnosed patients with colorectal cancer. Genet Med. 2010;12(2):93–104.
- Olsen KR, Bojesen SE, Gerdes AM, et al. Cost-effectiveness of surveillance programs for families at high and moderate risk of hereditary non-polyposis colorectal cancer. Int J Technol Assess Health Care. 2007;23(1):89–95.
- Nielsen M, Hes FJ, Vasen HF, et al. Cost-utility analysis of genetic screening in families of patients with germline MUTYH mutations. BMC Med Genet. 2007;8(1):42.
- Breheny N, Geelhoed E, Goldblatt J, et al. Economic evaluation of the familial cancer programme in Western Australia: predictive genetic testing for familial adenomatous polyposis and hereditary non-polyposis colorectal carcinoma. Community Genet. 2006;9(2):98–106.
- Nicolosi P, Ledet E, Yang S, et al. Prevalence of germline variants in prostate cancer and implications for current genetic testing guidelines. JAMA Oncol. 2019;5(4):523.
- Pritchard CC, Mateo J, Walsh MF, et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N Engl J Med. 2016;375(5):443–453.
- NCCN 2022. Clinical practice guidelines in oncology, prostate cancer. version 1.2023. Avaialble from: https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf.
- Sokolova A, Cheng H. Germline testing in prostate cancer: when and who to test. Oncology (Williston Park). 2021;35(10):645–653.
- Szymaniak BM, Facchini LA, Giri VN, et al. Practical considerations and challenges for germline genetic testing in patients with prostate cancer: recommendations from the germline genetics working group of the PCCTC. J Clin Oncol Oncol Pract. 2020;16(12):811–819.
- Giri VN, Knudsen KE, Kelly WK, et al. Implementation of germline testing for prostate cancer: Philadelphia prostate cancer consensus conference 2019. J Clin Oncol. 2020;38(24):2798–2811.
- de Bono J, Mateo J, Fizazi K, et al. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med. 2020;382(22):2091–2102.
- Abida W, Patnaik A, Campbell D, et al. Rucaparib in men with metastatic castration-resistant prostate cancer harboring a BRCA1 or BRCA2 gene alteration. J Clin Oncol. 2020;38(32):3763–3772.
- Meadows RJ, Padamsee TJ. Financial constraints on genetic counseling and further risk-management decisions among U.S. women at elevated breast cancer risk. J Genet Couns. 2021;30(5):1452–1467.
- Salhab M, Bismohun S, Mokbel K. Risk-reducing strategies for women carrying BRCA1/2 mutations with a focus on prophylactic surgery. BMC Womens Health. 2010;10:28.
- Giardiello FM, Allen JI, Axilbund JE, et al. Guidelines on genetic evaluation and management of lynch syndrome: a consensus statement by the US Multi-society Task Force on colorectal cancer. Am J Gastroenterol. 2014;109(8):1159–1179.
- Møller P, Seppälä T, Bernstein I, et al. Incidence of and survival after subsequent cancers in carriers of pathogenic MMR variants with previous cancer: a report from the prospective lynch syndrome database. Gut. 2017;66(9):1657–1664.
- Dueñas N, Navarro M, Teulé À, et al. Assessing effectiveness of colonic and gynecological risk reducing surgery in lynch syndrome individuals. Cancers. 2020;12(11):3419.
- Stjepanovic N, Moreira L, Carneiro F, et al. Hereditary gastrointestinal cancers: ESMO clinical practice guidelines for diagnosis, treatment and follow-up†. Ann Oncol. 2019;30(10):1558–1571.
- Graves J, Garbett S, Zhou Z, et al. Comparison of decision modeling approaches for health technology and policy evaluation. Med Decis Making. 2021;41(4):453–464.
- Petrou S, Gray A. Economic evaluation using decision analytical modelling: design, conduct, analysis, and reporting. BMJ. 2011;342:d1766.
- Hunt TL, Luce BR, Page MJ, et al. Willingness to pay for cancer prevention. Pharmacoeconomics. 2009;27(4):299–312.
- Yong ASJ, Lim YH, Cheong MWL, et al. Willingness-to-pay for cancer treatment and outcome: a systematic review. Eur J Health Econ. 2022;23(6):1037–1057.
- Collins M, Latimer N. NICE’s end of life decision making scheme: impact on population health. BMJ. 2013;346:f1363.
- Seghers P, Wiersma A, Festen S, et al. Patient preferences for treatment outcomes in oncology with a focus on the older patient-A systematic review. Cancers (Basel). 2022;14(5):1147.
- Baranova A, Krasnoselskyi M, Starikov V, et al. Triple-negative breast cancer: current treatment strategies and factors of negative prognosis. J Med Life. 2022;15(2):153–161.
- Ginsburg GS, Phillips KA. Precision medicine: from science to value. Health Aff (Millwood). 2018;37(5):694–701.
- Vicente AM, Ballensiefen W, Jönsson J-I. How personalised medicine will transform healthcare by 2030: the ICPerMed vision. J Transl Med. 2020;18(1):180.
- D'Andrea E, Marzuillo C, De Vito C, et al. Which BRCA genetic testing programs are ready for implementation in health care? A systematic review of economic evaluations. Genet Med. 2016;18(12):1171–1180.
- Cortesi L, Rugo HS, Jackisch C. An overview of PARP inhibitors for the treatment of breast cancer. Target Oncol. 2021;16(3):255–282.
- Antonarakis ES, Gomella LG, Petrylak DP. When and how to use PARP inhibitors in prostate cancer: a systematic review of the literature with an update on on-going trials. Eur Urol Oncol. 2020;3(5):594–611.
- PBAC 2016. Pharmaceutical Benefits Advisory Committee: Codependent technogies. Available from: https://pbac.pbs.gov.au/product-type-4-codependent-technologies.html
- Gonzalez R, Havrilesky LJ, Myers ER, et al. Cost-effectiveness analysis comparing “PARP inhibitors-for-all” to the biomarker-directed use of PARP inhibitor maintenance therapy for newly diagnosed advanced stage ovarian cancer. Gynecol Oncol. 2020;159(2):483–490.