Abstract
Background
A new 20-valent pneumococcal conjugate vaccine (PCV20) provides protection against 20 pneumococcal serotypes. The vaccine has the potential to decrease the impact of pneumococcal diseases in society and to increase health among vulnerable persons.
Aim
This study investigates the cost-effectiveness of vaccinating Danish adults in different age groups and risk of pneumococcal disease with PCV20 compared to the 23-valent pneumococcal polysaccharide vaccine (PPV23) – either as PCV20 compared to PPV23 or as PPV23 followed by PCV20 compared to PPV23.
Methods
A Markov model adapted to the Danish setting was developed to estimate clinical outcomes and costs of vaccinating the Danish population in specific age and risk groups. The model used a restricted societal perspective and estimated outcomes and costs using a lifetime time horizon. To estimate the clinical outcomes and costs, inputs on vaccine effectiveness and waning were retrieved from other studies whereas data on risk groups, coverage and costs were based on real-world data.
Results
The results showed that in all scenarios the incidence and mortality of pneumococcal disease were reduced when vaccinating with PCV20, resulting in lower costs. For the vaccine target group of adults aged ≥18 years at moderate or high risk and all adults aged ≥65 years both in the case of PPV23+PCV20 compared to PPV23 and in case of PCV20 compared to PPV23 vaccination with PCV20 was found to be a dominant strategy gaining 1,350 or 5,821 quality-adjusted life years (QALYs), respectively, and reducing total costs by 60 or 396 million EUR, respectively, as compared to PPV23 vaccination alone. Similar results of dominant PCV20 strategy were found for other age and risk group comparisons. Both deterministic and probabilistic sensitivity analyses confirmed the results being robust to changes in input parameters and applied assumptions.
Limitations
Like other modelling studies, this analysis has limitations such as lack of detailed data for some inputs.
Conclusion
Vaccination with PCV20 reduced the incidence and mortality of pneumococcal diseases in Danish adults compared to PPV23. This reduction has the potential to reduce the financial burden related to managing diseases while also increasing public health.
Introduction
S. pneumoniae causes invasive and non-invasive infections such as pneumococcal meningitis and sepsis, collectively referred to as invasive pneumococcal disease (IPD) and community-acquired pneumonia (CAP)Citation1,Citation2. There are more than 100 serotypes of S. pneumoniaeCitation3 which differ in relation to invasiveness and pathogenicityCitation1.
In Europe, CAP is the most frequent cause of death by infectionCitation1, particularly among the elderlyCitation2. Studies have shown that older age and chronic or immunocompromising conditions increase the risk of pneumococcal disease and mortalityCitation4–8. As a consequence, studies often stratify individuals into subgroups according to risk of pneumococcal disease due to underlying conditions: low risk (no underlying chronic disease), moderate risk (immunocompetent with underlying chronic disease such as chronic heart disease, diabetes, etc.) and high risk (immunocompromised)Citation9. However, risk can vary across and within these groups, and more concurrent underlying conditions results in higher risk of pneumococcal diseasesCitation10.
The 7-valent pneumococcal conjugate vaccine (PCV7) was introduced in the Danish childhood national immunization program (NIP) in 2007 and replaced by the 13-valent pneumococcal conjugate vaccine (PCV13) in 2010Citation9–11. Since the introduction, IPD incidence among children has substantially decreased, and to a lesser extent among unvaccinated elderly through herd protectionCitation12. The IPD incidence caused by serotypes not contained in PCV13 are however still increasing mainly due to serotype replacement, indicating a need for higher-valent vaccinesCitation13. In 2019, serotype 8 was the most prevalent serotype accounting for one quarter of IPD cases in all age groups, followed by serotype 3, 22 F and 12 FCitation14.
Since 2012, vaccination with PCV13 has been reimbursed in Denmark for adults aged ≥65 years at high risk of pneumococcal disease and was replaced in spring 2022 by the approved 20-valent pneumococcal conjugate vaccine (PCV20). From 2015 to October 2020 PCV13 was also reimbursed for adults aged ≥65 years at moderate risk of pneumococcal diseaseCitation15,Citation16. In April 2020 a NIP providing 23-valent pneumococcal polysaccharide vaccine (PPV23) for all adults aged ≥65 was introduced to prevent overloading the health system during the COVID-19 pandemicCitation17,Citation18. Seventy-three percent (73%) of such adults had accepted by the end of 2021Citation10, and this program will cease in January 2023Citation19.
The pneumococcal conjugate vaccines (e.g. PCV7, PCV13, and PCV20) and the pneumococcal polysaccharide vaccine (i.e. PPV23) are based on two different vaccine technologies, thus resulting in different immunologic reactions. The conjugate vaccines induce a T-cell-dependent response resulting in a strengthened antibody response, generation of memory B-cells, and ability to induce mucosal immunity, which is not the case for the plain polysaccharide vaccinesCitation20–23. Results from a randomized controlled trial in which participants were inoculated with S. pneumoniae showed that PCV13 protected against colonization and carriage incidenceCitation24,Citation25. PPV23 has not proved to have similar protection against colonizationCitation26.
The PCV20 vaccine was approved by the European Medicines Agency (EMA) in February 2022Citation27. PCV20 is based on PCV13 and thus, covers the 13 serotypes included in PCV13 (1, 3, 4, 5, 6 A, 6B, 7 F, 9 V, 14, 18 C, 19 A, 19 F, and 23 F) and seven additional serotypes (8, 10 A, 11 A, 12 F, 15B, 22 F, and 33 F)Citation28. The seven additional serotypes covered by PCV20 caused 47% of all IPD cases in adults aged ≥65 years in Denmark in 2018, and in total, PCV20-covered serotypes accounted for 61% of all IPD cases in the populationCitation29. Phase III trials demonstrated that PCV20 induces a non-inferior immune response compared to PCV13 for all shared serotypes, and a robust immune response to all 20 included serotypes, in adults at different risk for pneumococcal disease (healthy or with stable underlying chronic conditions), in different age groups, and pneumococcal vaccination history (vaccine-naïve or were previously vaccinated with PPV23, PCV13, or both)Citation30–32.
The CAPiTA (Community-Acquired Pneumonia Immunization Trial in Adults) study demonstrated the efficacy of PCV13 to protect against vaccine-type (VT) CAP and IPD in a large cohort of older adults in the Netherlands. The effect of PCV13 occurred shortly after vaccination and was documented to last 5 years with no waning observedCitation33–35. In contrast, meta-analyses have shown evidence for PPV23 protecting against IPD, however, the effect against CAP was inconclusiveCitation36–38. Furthermore, studies have shown a relatively short-term protection against IPD in older adults vaccinated with PPV23Citation39,Citation40. A literature review from 2020 concludes that PPV23 provides limited protection against CAP, with the highest effect in the younger age groups and with waning over timeCitation41. The difference between PCV13 and PPV23 in the prevention of CAP could be related to the ability to induce mucosal immune response, which is only present with conjugate vaccinesCitation23.
In Denmark, publicly funded vaccines can either be part of a NIP wherein the vaccine is given free of charge, or be in a reimbursement scheme, where only part of the vaccine cost is reimbursed with the rest copaid by the citizenCitation42. Given the current Danish adult NIP with PPV23 and the differences between PPV23 and PCV20, the objective of this study was to assess the cost-effectiveness of vaccinating adults in different age and risk groups with PCV20 compared to PPV23 in the following two potential cases:
Vaccination with PCV20 given in addition to the existing PPV23 NIP vaccine (PPV23+PCV20) compared to PPV23 vaccination alone – the case of PCV20 as a reimbursed vaccine
Vaccination with PCV20 instead of PPV23 – the case of PCV20 as a NIP vaccine
In total, seven different scenarios were analyzed related to these two cases in the present study.
Methods
To analyze the costs, effects, and cost-effectiveness of PCV20 a cost-effectiveness model representing a Danish setting was developed.
Populations
Multiple analyses with different study populations according to age and risk of pneumococcal disease were carried out with risk of pneumococcal disease considered as low, moderate, or high according to the existence of chronic and underlying comorbidities. The chronic diseases used to group patients in moderate risk of pneumococcal disease (immunocompetent with underlying chronic disease, i.e. chronic heart diseases, diabetes, pulmonary diseases, chronic kidney diseases and chronic liver diseases) and high risk (immunocompromised, i.e. organ transplants, functional/anatomical asplenia, disorders in immune mechanism, etc.) were based on Danish definitions by Statens Serum Institute. Thus, to determine the share of the Danish population at moderate and high risk, ICD-10 (10th edition of the International Classification of Diseases) codes for the chronic diseases were identified from the Danish National Patient Registry covering all hospital contacts in Denmark. These ICD-10 codes as well as the number of persons in each age and risk group is presented in supplementary material (Table S1 and S2). Persons in low risk had none of the specified chronic diseases.
For PCV20 given in addition to the existing PPV23 NIP vaccine, i.e. PCV20 as a reimbursed vaccine, the following four scenarios were analyzed comparing PPV23+PCV20 with PPV23 alone:
Adults aged 18 years and older at moderate or high risk and all aged ≥65 years
All aged ≥50 years
All aged ≥65 years
All aged ≥65 years at moderate or high risk
In the case of vaccinating with PCV20 instead of PPV23, i.e. PCV20 as a NIP vaccine, the following three scenarios were analyzed comparing PCV20 with PPV23:
Adults aged 18 years and older at moderate or high risk and all Danes aged ≥65 years
All aged ≥50 years
All aged ≥65 years
Model description and inputs
The model developed was a Markov transition model with one-year cycles aiming to capture the lifetime risk of clinical outcomes and costs related to pneumococcal disease in multiple adult populations. The analyses were based on closed cohorts with no new generations entering each cycle. The upper age limit was 99 years, which was used to implement a lifetime time horizon of the analyses. Thus, in the analyses with persons aged 18 years and older the time horizon is 81 years.
In every cycle persons in all risk groups could experience either IPD (sepsis or meningitis) and pneumonia leading to hospitalization (inpatient (IP) pneumonia) or pneumonia not leading to hospitalization (outpatient (OP) pneumonia). Persons with IPD or IP pneumonia could recover or die. Persons with OP pneumonia could recover but would not die of pneumonia. The model allowed for people to change to higher risk groups with increasing age. depicts the risk distribution (i.e. low, moderate and high risk) changes with increasing age. Thus, the changed risk distribution over time is applied in every cycle for the population still alive as the relative number of persons in the moderate and high risk groups increase with increasing age.
Table 1. Clinical inputs to the model: population size, incidence, mortality, utilities, and vaccine serotypes.
illustrates the model states and transitions. Number of cases of sepsis, meningitis, IP pneumonia, and OP pneumonia, case fatality and costs were projected annually for the model population alive each year. Based on the clinical outcomes, mortality (case fatality and general mortality) and effects in terms of life years and quality-adjusted life years (QALYs) were estimated. Economic outcomes included treatment-related costs stratified by disease, risk group, and the sector entailing the costs (primary care sector, hospital sector or municipalities (social care)).
Figure 1. Model stages and transitions. Abbreviations. IP, inpatient; IPD, invasive pneumococcal disease; OP, outpatient.
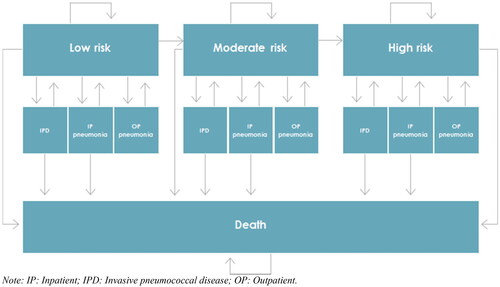
For every analysis (e.g. vaccination of all aged ≥65 years with either PCV20 or PPV23), simulations for a closed cohort of adults vaccinated with PCV20 and simulations for a closed cohort of +65-years old vaccinated with PPV23 were performed and subsequently the difference (incremental) between the two simulations were estimated.
The analyses were conducted using a restricted societal perspective in which vaccination costs, healthcare costs, municipality costs, and costs of patient time and transportation related to receiving treatment were included, but costs due to productivity loss were excluded. Both costs and outcomes were discounted using the official discounting rate set by the Danish Ministry of Finance and differed according to the length of the time horizon. Thus, the discounting rate was 3.5% in years 0–35, 2.5% in years 36–70 and 1.5% after year 70Citation50.
Clinical inputs
The incidence of illness was divided into sepsis, meningitis, IP pneumonia, and OP pneumonia with data for the first three extracted from the Danish National Patient Register from the years 2017 to 2018/2019 and calculated as the average of unique patients during the 2–3 years – see Citation43. The incidence of OP pneumonia was estimated based on a study by Woodhead et al. who found that 78% of all pneumonia cases do not require hospital contactCitation45. Furthermore, it was assumed that persons who are not at increased risk of developing pneumococcal infections experience less severe illness compared to those at increased risk, and therefore are more likely to represent the OP pneumonia cases. Thus, it was assumed that patients at low risk accounted for 90% of all OP pneumonia cases, whereas patients at either moderate risk or high risk only accounted for 5% of the OP pneumonia cases each.
Mortality data were also divided into sepsis, meningitis, and IP pneumonia fatality. With mortality data of sepsis and meningitis derived from the Danish National Patient RegisterCitation43, and data for IP pneumonia derived from Statistics Denmark (the Cause of Death Register)Citation46. For adults aged <65 years meningitis mortality was conservatively set to zero based on the very low number of cases experienced in Denmark. Cases of OP pneumonia were assumed not to be associated with any deaths.
To estimate QALYs gained the time spent in each state was multiplied by the utility of that health state. As Danish utility data covering both age and risk groups are not present, the utility estimates were based on a study by Ara and Brazier, who identified the utility according to age and history of illness in the British populationCitation47. The utility input data for persons at moderate risk were based on a weighted average for persons with a history of diabetes, hypertension, heart attack, and other heart diseases. The utility input data for persons at high risk were based on persons with cancer. The annual disutility associated with sepsis, meningitis, and IP pneumonia was −0.13 and was based on a study by Mangen et al.Citation48. No disutility was assumed for OP pneumonia.
Vaccine coverage, efficacy, and waning
The vaccine coverage assumed for the study population depended on the case investigated. Thus, for comparison of PPV23+PCV20 with PPV23 alone (PCV20 reimbursed vaccine), 73% of the study population in each scenario were vaccinated with PPV23 consistent with the current situation in DenmarkCitation18. In addition, it was assumed that 25% received PCV20 1 year after having received PPV23, based on previous experience from PCV13. In the comparison of PCV20 with PPV23 (PCV20 as a NIP vaccine), the vaccine coverage was assumed to be 73% for both PCV20 and PPV23.
The efficacy of PCV20 against VT IPD and CAP was assumed to be the same as the VT efficacy of PCV13 measured in the CAPiTA trialCitation33, based on non-inferiority of PCV20 to PCV13 for shared vaccine serotypesCitation31. Waning of PCV20 was based on a study by Mangen et al. who identified an annual waning of 0% until year 5, 5% between years 6 and 10, and 10% between years 11 and 16. After year 16, it was assumed that PCV20 had no efficacyCitation51. The vaccine efficacy and waning of PPV23 against IPD was based on surveillance data from Public Health England between 2000 and 2017Citation40. The vaccine efficacy was derived for all age groups by adapting a logarithmic curve to the values for people aged 65–74 years, 75–84 years, and 85–99 years, and then estimating the age-specific values across the three risk groups using relative risks from Djennad et al.Citation40 and the population sizes. It was assumed that PPV23 did not provide any protection against CAP in the base cases due to inconclusive results regarding the vaccine efficacyCitation36–38,Citation52,Citation53. Vaccine waning for PPV23 was based on Djennad et al.Citation40 with a linear decline to 76.2% of initial vaccine efficacy by year 5 followed by a linear decline to no efficacy by year 10. The vaccine effectiveness applied in the analyses are provided in .
Table 2. PCV20 and PPV23 efficacy against vaccine-related diseases.
The vaccine serotype coverage included in the model described the proportion of IPD and pneumococcal pneumonia against which the vaccines provide protection. The serotype coverage of IPD was based on country-specific data from the European Centre for Disease Prevention and Control (ECDC) in the years 2017–2019Citation52. An overview of the IPD vaccine serotype coverage is provided in . As no data was available regarding the CAP serotype distribution, it was assumed to be the same as for IPD. Based on experience by the Danish surveillance authority Statens Serum Institute it was estimated that 20% of all pneumonia was caused by S. pneumoniae.
Using the serotype coverage and vaccine efficacy of both PPV23 and PCV20, it was possible to estimate the joint vaccine efficacy for patients receiving PPV23+PCV20. When individuals received both vaccines, the combined vaccine efficacy was calculated. This calculation applies the vaccine with the higher efficacy of the two after accounting for waning and serotype coverage, assuming no interference or boosting.
Herd immunity
The herd immunity from the childhood NIP was included in the analyses based on the change in the incidence of IPDs over time according to Statens Serum Institute. The mean incidence of IPDs in the Danish population aged ≥65 years was 66 per 100,000 between 2000 and 2007 (prior to inclusion of PCV in the childhood NIP) and by 2018 and 2019 it decreased to 47 and 36 per 100,000, respectively, equal to 41.5 per 100,000 on average in 2018 and 2019Citation15. The herd immunity was estimated based on the mean IPD incidence before introduction of PCV7 in the childhood NIP and the latest data after the introduction, to provide the average reduction in IPD cases. Thus, using a 15-year time difference (year 2003 to year 2018), resulted in an annual reduction of IPD cases due to herd immunity of 2.47%. Data on the herd immunity towards CAP was not available, why the herd immunity found for IPD was assumed to be the case for CAP as well. It was assumed that the herd immunity lasted for the entire time horizon.
Cost inputs
All cost inputs () were presented in Euros (EUR) and 2022 prices. All cost estimates prior to 2022 were inflated to the 2022 price level using the official consumer price index from Statistics DenmarkCitation61. To convert DKK to EUR an exchange rate of 0.1345 was usedCitation62.
Table 3. Cost inputs including treatment-related costs, cost of patient time, transportation time and vaccine cost.
Pharmacy selling prices (including VAT) in September 2022 were used to estimate vaccine costs using a price per dose of PPV23 at EUR 39.7 and a price per dose of PCV20 at EUR 120.7Citation59. To receive a reimbursed vaccine in Denmark, patients need to visit their general practitioner (GP) twice – first to receive a prescription and second to receive the injection. This results in a total administration cost of EUR 40 per vaccinationCitation55. For NIP vaccines only one GP visit is needed.
The treatment costs for patients hospitalized with pneumonia has previously been estimated in Denmark in a national registry study by Brogaard et al.Citation56. Following the findings from Brogaard et al. the costs of treating patients hospitalized with pneumonia were divided between hospitals, primary care sector and municipalities (social care).
The costs related to IPD were based on estimates from a Danish national registry study by Gustafsson et al., investigating the costs associated with treatment of invasive meningococcal diseaseCitation58. In the present analysis, it was assumed that the use of resources associated with treatment of sepsis and meningitis caused by S. pneumoniae corresponds to the treatment of sepsis and meningitis caused by Neisseria meningitidis. In this case the costs of IPD were also divided between hospitals, primary care sector and municipalities. It was conservatively assumed that OP pneumonia patients did not require any treatment.
Patient time and transport costs
The costs associated with patient time and transport were also includedCitation63. It was assumed that patients’ use of time for one GP visit was 30 min with half of it used for transportation. The use of time by patients for hospital admissions was estimated based on the maximum length of stay included in the official Danish DRG tariffs for each disease (sepsis and meningitis: 01MA03–18MA01, and IP pneumonia: 04MA13–04MA16)Citation57. It was assumed that one day of admission results in 16 h spent by the patients plus 30 min of transportation time per admission.
The hourly cost of patient time due to treatment was assumed to be EUR 24.3, which is the average hourly net rate of a Danish employee. The cost of transportation assumes a distance to the nearest hospital of 20 km (40 km per visit)Citation60 and 5 km to the GP (10 km per visit). Using the tax-exempted mileage allowance determined by the Danish state of 0.47 EUR/km, this results in a total round trip transport cost of EUR 18.8 for hospitalization and EUR 4.7 for a GP visitCitation60. An overview of the costs and assumptions for patient time and transport is presented in .
Sensitivity analyses
In addition to the seven scenario analyses conducted in the present study investigating the impact of age and risk groups on the cost-effectiveness of PCV20, sensitivity analyses were performed. Deterministic sensitivity analyses (DSA – one-way sensitivity analyses) were performed to assess the impact of parameter uncertainty, methodological assumptions, and choice of data sources. The following parameters and assumptions were varied in the DSA one at a time: Time horizon (5 and 10 years), cost perspective (health sector perspective), and impact of assuming efficacy of PPV23 against CAP. The last assumption was investigated by changing the source of PPV23 vaccine efficacy to the findings of Lawrence et al. (16–74 years: 25.7%; ≥75 years 4.7%)Citation64. Further to assess this vaccine efficacy estimates for PPV23 from Falkenhorst et al. using the pooled estimates of 73% vaccine efficacy against IPD and a 25% vaccine efficacy against CAP, was analyzed as wellCitation65. Again, vaccine waning was based on Djennad et al.Citation40. Finally, the DSA also included sensitivity analyses on the price of PCV20 and PPV23 (±10%).
Analyses on these parameters and assumptions were chosen as they ex-ante were assessed to have the most impact on the results.
Probabilistic sensitivity analyses (PSA) with 100 model simulations were performed to assess the joint uncertainty of the parameters in each of the seven analyses. A normal distribution with ±10% from the base case parameter value was applied for disease incidences, mortality, and vaccine prices. A gamma distribution was applied to treatment-related costs, cost of patient time and transportation. A beta distribution was used for estimates of utility, disutility, vaccine effectiveness, proportion of pneumonia due to S. pneumoniae, and percentage of IPD due to vaccine serotype.
Results
Comprehensive results for the target group of adults aged ≥18 at moderate or high risk and all aged ≥65 years are presented in and for PCV20 as a reimbursed and NIP vaccine, respectively. Summary cost-effectiveness results – i.e. incremental QALYs and costs, and the incremental cost-effectiveness ratio (ICER) of all scenarios are presented in and . All base case analyses were carried out with a lifetime time horizon and a restricted societal cost perspective.
Table 4. Results – PCV20 as a reimbursed vaccine in the scenario with vaccination of Danish adults aged ≥18 at moderate or high risk as well as all Danes aged ≥65 years.
Table 5. Results – PCV20 as a NIP vaccine in the scenario with vaccination of Danish adults aged ≥18 years at moderate or high risk as well as all Danes aged ≥65 years.
Table 6. Results of deterministic sensitivity analyses – PCV20 as a reimbursed vaccine.
Table 7. Results of base case analyses and deterministic sensitivity analyses – PCV20 as a NIP vaccine.
PCV20 as a reimbursed vaccine: PPV23+PCV20 vs. PPV23
In , the clinical outcomes with the vaccine target group of adults aged ≥18 years at moderate or high risk and all aged ≥65 years showed a reduction in cases of sepsis, meningitis, IP pneumonia and OP pneumonia, when vaccinating with PPV23+PCV20 compared to PPV23 vaccination alone. PPV23+PCV20 also resulted in fewer deaths from both IPD and all-cause pneumonia compared to PPV23 vaccination alone. Consequently, PPV23+PCV20 resulted in a gain of life years and QALYs.
Vaccination with PPV23+PCV20 was associated with a higher vaccine cost and costs to the primary care sector compared to PPV23 vaccination alone. However, PPV23+PCV20 was at the same time associated with lower costs on the remaining parameters; hospital costs, municipality costs, and patient costs, resulting in the total net cost of PPV23+PCV20 vaccination being 60 million EUR lower than PPV23 vaccination alone.
As PPV23+PCV20 resulted in both a QALY gain and a cost reduction, it was identified as the dominant strategy compared to PPV23 alone. The remaining three scenarios of the PCV20 as a reimbursed vaccine situation showed similar results, with PPV23+PCV20 resulting in a higher QALY gain at a lower cost compared to PPV23 alone (). Thus, PPV23+PCV20 was the dominant strategy in all investigated target vaccine populations.
PCV20 as a NIP vaccine: PCV20 vs. PPV23
Results of the clinical outcomes and costs of the comparison of PCV20 with PPV23 in the vaccine target group of adults aged ≥18 years at moderate or high risk and all aged ≥65 years are shown in . Including PCV20 as a NIP vaccine resulted in fewer cases of sepsis, meningitis, IP pneumonia, and OP pneumonia compared to vaccination with PPV23. The mortality due to IPD and all-cause CAP was also reduced in PCV20 compared to PPV23. Consequently, PCV20 resulted in both a gain in life years and QALYs.
The drug cost of vaccinating the target population was higher for PCV20 compared with PPV23 due to the higher cost of PCV20. However, the costs incurred by the hospital sector, primary care sector, and the municipalities were lower for PCV20 compared to PPV23. In addition, the patient costs were also lower with PCV20 compared with PPV23. Thus, resulting in the total net costs being lower for PCV20 compared to PPV23.
The two remaining scenarios in the case of PCV20 as a NIP vaccine instead of PPV23 also resulted in a higher QALY gain at a lower cost for PCV20 compared to PPV23 (). Thus, in the case of having PCV20 as a NIP vaccine instead of PPV23 showed that PCV20 was the dominant strategy in all scenarios.
Sensitivity analyses
The DSA carried out for the case with PCV20 as a reimbursed vaccine () showed that the results were robust to changes in the assumptions around the vaccine efficacy of PPV23 against IPD and CAP, for other cost perspectives, vaccine prices, as well as a time horizon shortened to ten years. Vaccination with PPV23+PCV20 was associated with lower costs and more QALYs gained as compared to PPV23 alone thus, still resulting in a negative ICER showing a dominant strategy with PCV20. For the shorter time horizon of 5 years, only the analysis of ≥65 years at moderate and high risk resulted in PCV20 being the dominant strategy. For the remaining three scenarios the ICER exceeded the normally accepted cost-effectiveness threshold. However, with a time horizon between 5 and 10 years PCV20 as a reimbursed vaccine will be cost-effective in all included scenarios.
Results from the DSA analyses in the situation with PCV20 as a NIP vaccine () showed that the results were robust to changes of the time horizon, vaccine efficacy of PPV23 against IPD and CAP, vaccine prices, and the cost perspective. Thus, PCV20 remained the dominant strategy.
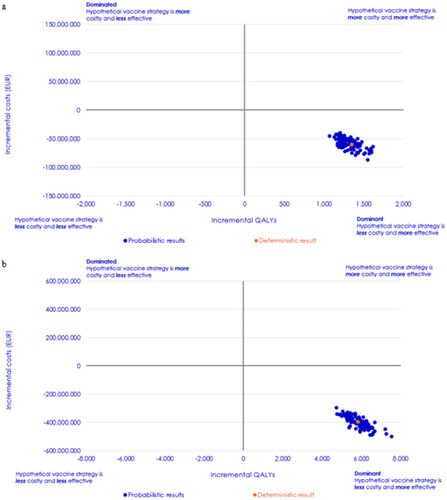
The PSA was only performed in the scenario of vaccinating adults aged ≥18 years at moderate or high risk and all aged ≥65 years. The ICER planes of the PSA are presented in and illustrate that both with PCV20 as a reimbursed vaccine and with PCV20 as a NIP vaccine, PCV20 is the dominant strategy in 100% of the iterations when compared to PPV23. Based on the 100 iterations performed in the PSA the average incremental costs and QALYs gained was estimated to be 59 million EUR and 1,328 QALYs gained with PCV20 as a reimbursed vaccine case and 398 million EUR and 5,849 QALYs gained with PCV as a NIP vaccine.
Figure 2. Probabilistic sensitivity analysis results of Danish adults aged ≥18 years at high and moderate risk and all Danes aged ≥65 years in the two potential vaccination situations. (a) cost-effectiveness plane for PCV20 as a reimbursed vaccine and (b) cost-effectiveness plane for PCV20 as a NIP vaccine.
Discussion
This study investigated the cost-effectiveness of PCV20 as a reimbursed vaccine given in addition to PPV23 as well as PCV20 given instead of PPV23 as a NIP vaccine in multiple adult Danish populations. The results showed that PCV20 vaccination strategies were the dominant strategy compared to PPV23 vaccination in both cases, i.e. it resulted in more QALYs gained at a lower cost. The results of the PSA showed that PCV20 strategies were dominant in the scenario with vaccination of adults aged ≥18 years at moderate or high risk and all aged ≥65 years in all iterations. In addition, the DSA indicated that the results were robust when changing one parameter at a time. Only in the case of PCV20 as a reimbursed vaccine shortening the time horizon to 5 years limited the cost-effectiveness in the target group of ≥65 years at moderate or high risk of pneumococcal disease. We content that a 5-year time horizon for preventive initiatives like vaccines could be considered rather shortsighted, especially in the context of conjugate vaccines assumed to have longer duration of protection. Danish guidelines on cost-effectiveness analyses do recommend use of a time horizon as long as important future differences in health gains and costs are capturedCitation66. Increasing the time horizon to just 10 years did make PCV20 cost-effective.
Head-to-head comparisons of PPV23 and PCV20 or PCV13 have never been conducted. However, when PCV13 and PPV23 were evaluated in retrospective studies for their effectiveness in the same age groups using similar methodology and outcomes measures, PCV13 was reported to be effective against CAP, whereas PPV23 showed little or no effectiveness against CAP. Retrospective studies in Germany and the United States have shown efficacy of PPV23 against VT CAP and all cause CAP as low as 2-3% opposed to efficacy of PCV13 against VT CAP as high as 72.8%Citation67–69. A recent systematic literature review concluded that PPV23 has some but limited protection against CAP, with the highest effect in the younger age groups and with waning over timeCitation40. The studies on vaccine efficacy of PPV23 against CAP in this review and in the literature in general is, however, either based on observational studies or a few randomized controlled trials investigating CAP vaccine efficacy of PPV23 on small and selected samples with limited generalizability. Cost-effectiveness analyses based on these studies may result in different conclusionsCitation70.
A recent publication by Lawrence et al.Citation64 found that PPV23 had limited effectiveness against CAP among elderly ≥65 years of age. However, the study could not reject the null hypothesis of no efficacy of PPV23 against CAP due to lack of power. Though we assumed no vaccine efficacy of PPV23 against CAP in the base case, a DSA using the point estimate of PPV23 efficacy against CAP reported by Lawrence et al. confirmed that PCV20 were the dominant strategies in all scenarios. A similar result was found using pooled estimates on PPV23 efficacy against CAP reported in Falkenhorst et al.Citation65
Notable differences in waning between PPV23 and PCV20 have been observed and are part of the reason why PCV20 results in better outcomes than PPV23 in this study. Between 2003 and 2005, PPV23 was recommended for all persons older than 65 years in the UK, and the vaccination coverage today is around 75%. However, it was found that the vaccine efficacy declined after just a few years and was not significant beyond year 5Citation40. In comparison, in the CAPiTA trial, it was found that the PCV13 vaccine, did not have any waning during the first 5 years after administrationCitation33,Citation35.
Strengths and limitations
The present study has both strengths and limitations. As a strength the data for the three risk groups, including mortality, relied on high quality real-life data from the national registries covering the entire Danish population. Comparing these real-life cases with those of the model in year 1 (sepsis, meningitis, and IP pneumonia) showed that the cases estimated by the model were comparable to the cases found in the Danish registries. This indicates that the model was well-calibrated and reliable.
In the analyses it was assumed that serotype distribution for IPD could be used for CAP as well. When comparing with data from an older Danish study by Benfield et al.Citation71 focusing on serotype distribution in CAP in Denmark or recent Swedish CAP serotype data by Theilacker et al.Citation72, it can be concluded that the assumption of transferring the IPD serotype distribution to CAP seems to be fair.
Some estimates for both costs and utility inputs used in the model were conservative and may underestimate the benefit of a PCV20 program. The included disutility for IPD was a conservative estimate, the quality of life in patients with IP pneumonia, which would be expected to be less severe than IPDCitation48. With PCV20 reducing the number of IPD cases compared to PPV23 the introduction of more disutility associated with IPD would have improved the cost-effectiveness of PCV20. Another assumption was that OP pneumonia was not associated with any disutility or costs for GP visits or treatment. As the number of OP pneumonia cases were lower for patients receiving PCV20 in either situation, inclusion of OP pneumonia costs or disutility would have improved the cost-effectiveness of PCV20 further.
The cost-effectiveness analyses made in the NIP vaccine case were based on public-available vaccine list prices for PCV20 and PPV23. However, NIP vaccines in Denmark have lower vaccine costs due to payer negotiated confidential net-prices. This might have improved the cost-effectiveness of the PCV20 alternative further.
The cost-effectiveness analyses did not have a full societal perspective and did not include indirect cost in the form of production lost. The implication of this is, however, regarded as low, because majority of those in the scenarios are 65 years of age and above, and thereby close to the official retirement age in Denmark. If indirect costs were added, this would primarily have been a benefit for PCV20 given the more disease episodes avoided.
Policy implications
Comparing with the current vaccine strategy in Denmark – PPV23 NIP for adults aged 18 years and older at moderate or high risk and all aged ≥65 years – the analyses in this study have shown that PCV20 will be a more cost-effective option for a future NIP in the coming years. Decision-makers could also consider to lower the age-based NIP inclusion regardless of risk profile, because the PCV20 NIP strategy dominates the PPV23 NIP strategy down to 50 years of age. Of the two vaccine financing strategies the NIP strategy is the most preferable from a cost-effectiveness point of view, as it leads to the largest gains in terms of disease avoided, QALYs gained and total costs saved compared to the reimbursement strategy. Vaccination using PCV20 has though the potential to reduce the financial burden related to managing diseases while also increasing public health.
Conclusion
The findings of the current study suggest that vaccinating different age and pneumococcal disease risk groups of the Danish population with PCV20 as either a reimbursed vaccine given in addition to PPV23 or as a NIP vaccine given instead of PPV23 would reduce the incidence and mortality of IPD and CAP compared to PPV23 vaccination alone. This reduction is based on the higher efficacy and decreased waning of PCV20 compared to PPV23. When the incidences of IPD and CAP are reduced, the health is improved while the resource use and financial burden related to managing the diseases decrease. Thus, vaccination with PCV20 resulted in both greater health gain and a net cost reduction at a lifetime horizon compared to PPV23. These health and QALY gains, and total costs saved will be highest in the more preferred case of PCV20 as a NIP vaccine instead of PPV23.
Transparency
Author contributions
All authors have made a significant contribution to the work and meet the criteria for authorship. MS, JV and PBP have been included in the conception and design of the study and have revised the manuscript critically for intellectual content. JO, MBM and HS were responsible for the model development and have analyzed and interpreted the data and drafted the manuscript. All authors have approved the final version and are accountable for all aspects of this work.
Reviewer disclosures
Peer reviewers on this manuscript have received an honorarium from JME for their review work but have no other relevant financial relationships to disclose.
Supplemental Material
Download MS Word (56.4 KB)Supplemental Material
Download MS Word (48.9 KB)Acknowledgements
No assistance in the preparation of this article is to be declared.
Declaration of funding
This research was funded by Pfizer Denmark ApS.
Declaration of interest of financial/other interests
MS and PBP are employees at Pfizer Denmark. JV is an employee at Pfizer Inc. MS, JV and PBP own shares in Pfizer Inc. outside submitted work. JO, MBM and HS are employees at Incentive Denmark Aps, which was a paid vendor to Pfizer Denmark on the project. Pfizer is a manufacturer of vaccines.
Data availability statement
All input data used in the model are publicly available or can be retrieved from the Danish National Patient Register, the Danish Cause of Death Register, and other publicly available sources. The model will not be shared.
References
- Blasi F, Mantero M, Santus P, et al. Understanding the burden of pneumococcal disease in adults. Clin Microbiol Infect. 2012;18:7–14.
- Welte T, Torres A, Nathwani D. Clinical and economic burden of community-acquired pneumonia among adults in Europe. Thorax. 2012;67(1):71–79.
- Ganaie F, Saad JS, McGee L, et al. A new pneumococcal capsule type, 10D, is the 100th serotype and has a large cps fragment from an oral Streptococcus. McDaniel LS, editor. mBio. 2020;11(3):e00937.
- Grant LR, Slack MPE, Yan Q, et al. The epidemiologic and biologic basis for classifying older age as a high-risk, immunocompromising condition for pneumococcal vaccine policy. Expert Rev Vaccines. 2021;20(6):691–705.
- Shea KM, Edelsberg J, Weycker D, et al. Rates of pneumococcal disease in adults with chronic medical conditions. Open Forum Infect Dis. 2014;1(1):ofu024.
- van Hoek AJ, Andrews N, Waight PA, et al. The effect of underlying clinical conditions on the risk of developing invasive pneumococcal disease in England. J Infect. 2012;65(1):17–24.
- Andersen MA, Niemann CU, Rostgaard K, et al. Differences and temporal changes in risk of invasive pneumococcal disease in adults with hematological malignancies: results from a nationwide 16-Year cohort study. Clin Infect Dis. 2021;72(3):463–471.
- Navarro-Torné A, Dias JG, Hruba F, et al. Risk factors for death from invasive pneumococcal disease, Europe, 2010. Emerg Infect Dis. 2015;21(3):417–425.
- Statens Serum Institut. Pneumokokvaccination uden for børnevaccinationsprogrammet i Danmark [Pneumococcal Vaccination Outside the Danish Childhood Vaccination Program] [Internet]. 2020 [cited 2022 Apr 23]. Available from: https://en.ssi.dk/-/media/arkiv/dk/vaccination/risikogrupper/rapport-vedr-pneumokokvacc-udenfor-boernevaccprogram-14042020.pdf?la=en.
- Pelton SI, Shea KM, Weycker D, et al. Rethinking risk for pneumococcal disease in adults: the role of risk stacking. Open Forum Infect Dis. 2015;2(1):ofv020.
- Harboe ZB, Dalby T, Weinberger DM, et al. Impact of 13-valent pneumococcal conjugate vaccination in invasive pneumococcal disease incidence and mortality. Clin Infect Dis. 2014;59(8):1066–1073.
- Hanquet G, Krizova P, Dalby T, et al. Serotype replacement after introduction of 10-Valent and 13-Valent pneumococcal conjugate vaccines in 10 countries, Europe. Emerg Infect Dis. 2022;28(1):137–138.
- Statens Serum Institut. Invasive pneumococcal disease and coverage of pneumococcal vaccination in the childhood vaccination programme, 2018 and 2019. Report No. 10. Copenhagen March 2020 Available from: https://en.ssi.dk/surveillance-and-preparedness/surveillance-in-denmark/annual-reports-on-disease-incidence/invasive-pneumococcal-disease-2018-2019
- Statens Serum Institut. Annual report: Invasive pneumococcal disease and coverage of pneumococcal vaccination in the childhood vaccination programme 2018 and 2019 [Internet]. 2020 [cited 2022 Feb 18]. Available from: https://en.ssi.dk/surveillance-and-preparedness/surveillance-in-denmark/annual-reports-on-disease-incidence/invasive-pneumococcal-disease-2018-2019.
- Statens Serum Institut. EPI-NYT No 7/8 - 2010 -PCV 7 coverage & Invasive Pneumococcal Disease (IPD) 2008/2009 [Internet]. 2010 [cited 2022 Feb 24]. Available from: https://en.ssi.dk/news/epi-news/2010/no-7-8–-2010
- Statens Serum Institut. EIP-NEWS (No 51b - 2012) - Pneumococcal vaccination of persons at increased risk of invasive pneumococcal disease [Internet]. 2012. Available from: https://en.ssi.dk/news/epi-news/2012/no-51b.
- Statens Serum Institut. EPI-NYT Uge 14/16 - Vaccinationsprogram mod pneumokoksygdom til personer der er fyldt 65 år og til risikogrupper [Pneumococcal vaccination program for persons 65 years or older and risk groups] [Internet]. 2020 [cited 2022 Feb 22]. Available from: https://www.ssi.dk/aktuelt/nyhedsbreve/epi-nyt/2020/uge-14-16. –2020.
- Statens Serum Institut. EPI-NYT No 49/50 - Extension of the invasive pneumococcal disease vaccination programme [Internet]. 2022. [cited 2022 Feb 22]. Available from: https://en.ssi.dk/news/epi-news/2021/no-49-50-2021.
- Sundhedsministeriet [The Danish Ministry of Health]. Bekendtgørelse om gratis vaccination mod influenza, pneumokokker og COVID-19 til visse persongrupper (BEK nr 1260 af 09/09/2022) [Notice about free influenza, pneumococcal and COVID-19 vaccination] [Internet]. 2022. [cited 2022 Oct 5]. Available from: https://www.retsinformation.dk/eli/lta/2022/1260.
- Pollard AJ, Perrett KP, Beverley PC. Maintaining protection against invasive bacteria with protein–polysaccharide conjugate vaccines. Nat Rev Immunol. 2009;9(3):213–220.
- Clutterbuck EA, Lazarus R, Yu L-M, et al. Pneumococcal conjugate and plain polysaccharide vaccines have divergent effects on antigen-specific B cells. J Infect Dis. 2012;205(9):1408–1416.
- Pletz MW, Maus U, Krug N, et al. Pneumococcal vaccines: mechanism of action, impact on epidemiology and adaption of the species. Int J Antimicrob Agents. 2008;32(3):199–206.
- Jochems SP, Weiser JN, Malley R, et al. The immunological mechanisms that control pneumococcal carriage. Dehio C, editor. PLoS Pathog. 2017;13(12):e1006665.
- Collins AM, Wright AD, Mitsi E, et al. First human challenge testing of a pneumococcal vaccine. Double-blind randomized controlled trial. Am J Respir Crit Care Med. 2015;192(7):853–858.
- German EL, Solórzano C, Sunny S, et al. Protective effect of PCV vaccine against experimental pneumococcal challenge in adults is primarily mediated by controlling colonisation density. Vaccine. 2019;37(30):3953–3956.
- Adler H, German EL, Mitsi E, et al. Experimental human pneumococcal colonization in older adults Is feasible and safe, not immunogenic. Am J Respir Crit Care Med. 2021;203(5):604–613.
- European Commission. Union Register of medicinal products for human use - Apexxnar [Internet]. 2022 [cited 2022 Feb 28]. Available from: https://ec.europa.eu/health/documents/community-register/html/h1612.htm.
- European Medicines Agenc. Summary of Product Characteristics (Apexxnar) [Internet]. 2022 [cited 2022 Sep 27]. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/apexxnar.
- Palmborg A, Skovdal M, Molden T, et al. Invasive pneumococcal disease (IPD) among elderly in Nordic countries: different needs for broader protection. ECCMID poster (abstract #03702) 2021.
- Cannon K, Elder C, Young M, et al. A trial to evaluate the safety and immunogenicity of a 20-valent pneumococcal conjugate vaccine in populations of adults ≥65 years of age with different prior pneumococcal vaccination. Vaccine. 2021;39(51):7494–7502.
- Essink B, Sabharwal C, Cannon K, et al. Pivotal phase 3 randomized clinical trial of the safety, tolerability, and immunogenicity of 20-valent pneumococcal conjugate vaccine in adults aged ≥18 years. Clin Infect Dis. 2022;75(3):390–398.
- Klein NP, Peyrani P, Yacisin K, et al. A phase 3, randomized, double-blind study to evaluate the immunogenicity and safety of 3 lots of 20-valent pneumococcal conjugate vaccine in pneumococcal vaccine-naive adults 18 through 49 years of age. Vaccine. 2021;39(38):5428–5435.
- Bonten MJM, Huijts SM, Bolkenbaas M, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med. 2015;372(12):1114–1125.
- Hak E, Grobbee DE, Sanders EaM, et al. Rationale and design of CAPITA: a RCT of 13-valent conjugated pneumococcal vaccine efficacy among older adults. Neth J Med. 2008;66(9):378–383.
- Patterson S, Webber C, Patton M, et al. A post hoc assessment of duration of protection in CAPiTA (community acquired pneumonia immunization trial in adults). Trials Vaccinol. 2016;5:92–96.
- Moberley S, Holden J, Tatham DP, et al. Vaccines for preventing pneumococcal infection in adults. Chichester (UK): John Wiley & Sons, Ltd; 2008. [cited 2022 Feb 23]. p. CD000422.pub2. Available from: https://doi.wiley.com/10.1002/14651858.CD000422.pub2.
- Tin Tin Htar M, Stuurman AL, Ferreira G, et al. Effectiveness of pneumococcal vaccines in preventing pneumonia in adults, a systematic review and meta-analyses of observational studies. Arez AP, editor. PLoS One. 2017;12(5):e0177985.
- Latifi-Navid H, Latifi-Navid S, Mostafaiy B, et al. Pneumococcal disease and the effectiveness of the PPV23 vaccine in adults: a two-stage Bayesian meta-analysis of observational and RCT reports. Sci Rep. 2018;8(1):11051.
- Andrews NJ, Waight PA, George RC, et al. Impact and effectiveness of 23-valent pneumococcal polysaccharide vaccine against invasive pneumococcal disease in the elderly in England and Wales. Vaccine. 2012;30(48):6802–6808.
- Djennad A, Ramsay ME, Pebody R, et al. Effectiveness of 23-valent polysaccharide pneumococcal vaccine and changes in invasive pneumococcal disease incidence from 2000 to 2017 in those aged 65 and over in England and Wales. EClinicalMedicine. 2018;6:42–50.
- Berild JD, Winje BA, Vestrheim DF, et al. A systematic review of studies published between 2016 and 2019 on the effectiveness and efficacy of pneumococcal vaccination on pneumonia and invasive pneumococcal disease in an elderly population. Pathogens. 2020;9(4):259.
- Statens Serum Institut. Vacciner der ikke betales af det offentlige [Vaccines not payed by the state] [Internet]. 2022 [cited 2022 Oct 10]. Available from: https://www.ssi.dk/vaccinationer/vacciner-der-ikke-betales-af-det-offentlige
- Data extraction from the Danish National Patient Register. Landspatientregisteret [National Patient Register]; 2017–2019.
- Danmarks Statistik [Statistics Denmark]. Population figures in Denmark (1st quarter 2018) - table FOLK1A [Internet]. [cited 2022 Jan 15]. Available from: https://www.dst.dk/da/Statistik/emner/borgere/befolkning/befolkningstal.
- Woodhead MA, Macfarlane JT, Mccracken JS, et al. Prospective study of the aetiology and outcome of pneumonia in the community. The Lancet. 1987;329(8534):671–674.
- Danmarks Statistik [Statistics Denmark]. Deaths by sex, age and cause of death (Table DOD1) [Internet]. [cited 2022 Jan 15]. Available from: https://www.statistikbanken.dk/DOD1.
- Ara R, Brazier JE. Using health state utility values from the general population to approximate baselines in decision analytic models when condition-specific data are not available. Value Health. 2011;14(4):539–545.
- Mangen M-JJ, Huijts SM, Bonten MJM, et al. The impact of community-acquired pneumonia on the health-related quality-of-life in elderly. BMC Infect Dis. 2017;17(1):208.
- Disease data for pneumococcal disease from ECDC Surveillance Atlas [Internet]. ECDC; 2019. Available from: https://www.ecdc.europa.eu/en/pneumococcal-disease/surveillance-and-disease-data/atlas.
- Danish Ministry of Finance. Dokumentationsnotat – den samfundsøkonomiske diskonteringsrente [Note - the socio-economic discount rate] [Internet]. 2021 [cited 2022 Feb 11]. Available from: https://fm.dk/media/18371/dokumentationsnotat-for-den-samfundsoekonomiske-diskonteringsrente_7-januar-2021.pdf
- Mangen M-JJ, Rozenbaum MH, Huijts SM, et al. Cost-effectiveness of adult pneumococcal conjugate vaccination in The Netherlands. Eur Respir J. 2015;46(5):1407–1416.
- Heo JY, Seo YB, Choi WS, et al. Effectiveness of pneumococcal vaccination against pneumococcal pneumonia hospitalization in older adults: a prospective, test-negative study. J Infect Dis. 2022;225(5):836–845.
- Kim JH, Chun BC, Song JY, et al. Direct effectiveness of pneumococcal polysaccharide vaccine against invasive pneumococcal disease and non-bacteremic pneumococcal pneumonia in elderly population in the era of pneumococcal conjugate vaccine: a case-control study. Vaccine. 2019;37(21):2797–2804.
- Essink B, Sabharwal C, Xu X, et al. 3. Phase 3 pivotal evaluation of 20-valent pneumococcal conjugate vaccine (PCV20) safety, tolerability, and immunologic noninferiority in participants 18 years and older. Open Forum Infect Dis. 2020;7(Supplement_1):S2–S2.
- Praktiserende Laegers Organisation. PLO takster (2022) [Internet]. 2022. Available from: https://www.laeger.dk/sites/default/files/honorartabel_2022_oktober.pdf.
- Brogaard SL, Nielsen MBD, Nielsen LU, et al. Health care and social care costs of pneumonia in Denmark: a register-based study of all citizens and patients with COPD in three municipalities. COPD. 2015;10:2303–2309.
- Sundhedsdatastyrelsen [The Danish Health Data Authority]. DRG-takster [DRG tariffs] [Internet]. 2022. Available from: https://sundhedsdatastyrelsen.dk/da/afregning-og-finansiering/takster-drg/takster-2022.
- Gustafsson N, Stallknecht SE, Skovdal M, et al. Societal costs due to meningococcal disease: a national registry-based study. Clinicoecon Outcomes Res. 2018;volume10:563–572.
- Medicinpriser.dk. Medicinpriser.dk [Internet] 2022 [cited 2022 Sep 5]. Available from: https://medicinpriser.dk/Default.aspx?id=15&vnr=373611
- Medicinrådet [The Danish Medicines Council]. Vaerdisaetning af enhedsomkostninger, version 1.6 [Valuation of unit costs, version 1.6] [Internet]. 2022 [cited 2022 Apr 24]. Available from: https://medicinraadet.dk/media/aunbprvq/v%C3%A6rdis%C3%A6tning-af-enhedsomkostninger-vers-1-6_adlegacy.pdf.
- Forbrugerprisindeks [Consumer price index] [Internet]. Danmarks Statistik [Statistics Denmark] 2022. Available from: https://www.statistikbanken.dk/PRIS111.
- Danmarks Nationalbank [Danish national bank]. Valutakurser DKK til EUR (16. September 2022) [Exchange rate DKK to EUR (16 September 2022)] [Internet]. 2022 [cited 2022 Feb 16]. Available from: https://www.nationalbanken.dk/valutakurser.
- Medicinrådet [Danish Medicines Council]. Medicinrådets metodevejledning for vurdering af nye laegemidler (Version 1.2) [The Danish Medicines Council’s process guide for assessing new pharmaceuticals] [Internet]. 2021 [cited 2022 Apr 24]. Available from: https://medicinraadet.dk/media/hciai0yz/medicinr%C3%A5dets_metodevejledning_for_vurdering_af_nye_l%C3%A6gemidler-vers-_1-2_adlegacy.pdf
- Lawrence H, Pick H, Baskaran V, et al. Effectiveness of the 23-valent pneumococcal polysaccharide vaccine against vaccine serotype pneumococcal pneumonia in adults: a case-control test-negative design study. Kretzschmar MEE, editor. PLoS Med. 2020;17(10):e1003326.
- Falkenhorst G, Remschmidt C, Harder T, et al. Effectiveness of the 23-Valent pneumococcal polysaccharide vaccine (PPV23) against pneumococcal disease in the elderly: systematic review and meta-analysis. PLoS One. 2017;12(1):e0169368.
- Sundheds- og AEldreministeriet. Vejledning om udarbejdelse af sundhedsøkonomiske analyser af laegemidler (VEJ nr 9153) [Internet]. 2018. Available from: https://www.retsinformation.dk/eli/retsinfo/2018/9153.
- Chandler T, Furmanek S, Carrico R, et al. 23-Valent pneumococcal polysaccharide vaccination does not prevent community-acquired pneumonia hospitalizations due to vaccine-type Streptococcus pneumoniae. Microorganisms. 2022;10(3):560.
- Kolditz M, Schmitt J, Pletz MW, et al. Impact of the 13-valent pneumococcal conjugate vaccine on the incidence of all-cause pneumonia in adults aged ≥60 years: a population-based, retrospective cohort study. Clin Infect Dis. 2019;68(12):2117–2119.
- McLaughlin JM, Jiang Q, Isturiz RE, et al. Effectiveness of 13-valent pneumococcal conjugate vaccine Against hospitalization for community-acquired pneumonia in older US adults: a test-negative design. Clin Infect Dis. 2018;67(10):1498–1506.
- Birck AM, Nordin Christensen L, Pedersen MH, et al. Health economic evaluation of introducing a PPSV23-based vaccination programme to adults aged 65 and above, and an extension to the 60-64 age group in Denmark. Expert Rev Vaccines. 2021;20(10):1327–1337.
- Benfield T, Skovgaard M, Schønheyder HC, et al. Serotype distribution in non-bacteremic pneumococcal pneumonia: association with disease severity and implications for pneumococcal conjugate vaccines. Beall B, editor. PLoS One. 2013;8(8):e72743.
- Theilacker C. Pneumococcal serotype distribution in adults hospitalized with radiologically-confirmed community-aquired pneumonia in Malmö, Sweden [Internet] 2020. Available from: https://cslide.ctimeetingtech.com/isppd20/attendee/eposter/poster/904?q=Theilacker.
