Abstract
Objective
This real-world retrospective database study quantified the costs of biomarker testing in a US population of patients with lung or thyroid cancers.
Materials and methods
The commercial claims IBM Marketscan database, a de-identified real-world dataset, was used to identify patients diagnosed with lung or thyroid cancer between 1/2015 and 12/2019. Eligible patients were 18 years or older with two or more lung or thyroid diagnosis codes. Patients were excluded who had evidence of prior cancer diagnoses. Subgroup analyses evaluated eligible patients with metastatic disease. Descriptive statistics were used to evaluate commercial insurance plan payer and patient out-of-pocket costs for diagnostic testing overall as well as by test procedure code and payer type. Costs were adjusted to 2020 US dollars.
Results
A total of 23,633 patients with lung cancer were eligible, 13,320 of whom had metastatic disease. There were 36,867 patients with thyroid cancer, 2,241 of whom had metastatic disease. Biomarker codes were observed among 68.4/75.8% (lung/metastatic lung) and 18.2/42.3% (thyroid/metastatic thyroid). Few patients had codes for comprehensive biomarker tests (5.2/6.7% lung/metastatic lung, 0.3/2.2% thyroid/metastatic thyroid) Among those with biomarker tests, the median per-patient total payer lifetime costs of all biomarker testing were $394/$462 (lung/metastatic lung) and $148/$232 (thyroid/metastatic thyroid). Total lifetime biomarker costs for payers ranged from a median of $128 (consumer-driven health plans) to $477 (preferred provider organizations). Median lifetime patient out-of-pocket costs were $0.00 for both tumor types and all payer types except for consumer-driven health plans ($12 for thyroid and $10 for metastatic lung).
Conclusions
While comprehensive testing adds to the cost of biomarker testing, these data suggest the relatively low lifetime cost of biomarker testing for both payers and patients. Costs for biomarker testing should not be a limitation to access among these populations with commercial insurance plans in the US.
PLAIN LANGUAGE SUMMARY
This real-world retrospective database study found that there is a relatively low lifetime total cost of biomarker testing for the care of patients with lung or thyroid cancers. While comprehensive testing adds to the cost of biomarker testing, these data suggest the relatively low lifetime cost of biomarker testing for both payers and patients. Payer costs for biomarker testing do not appear to be limitation to access among populations with commercial insurance plans in this study.
Introduction
Lung and thyroid cancers are very different diseases in their diagnosis, treatment, and prognosis, and each represents a unique setting for evaluating the costs of biomarker testing. Lung cancer is diagnosed among an estimated 236,740 people in the United States (U.S.) each year and is associated with the highest number of deaths of any cancer, with a total of 130,180 deaths estimated in 2022Citation1. Lung cancer is characterized by a wide range of targeted therapies, particularly in non-small cell lung cancer (NSCLC), that can be utilized following the identification of an actionable biomarker (e.g. FDA-approved therapies exist for alterations in EGFR, BRAF, KRAS, ROS1, ALK, RET, NTRK, or MET)Citation2. Current guidelines for the care of patients with NSCLC recommend broad-based biomarker testing for all patientsCitation3. Thyroid cancer, on the other hand, is far less common (43,800 diagnoses and 2,230 deaths in 2022)Citation1. While there are fewer actionable biomarkers recommended in this tumor type, there are approved targeted therapies with high efficacy for these patientsCitation4. Treatment guidelines in the U.S. recommend germline testing for RET among patients with medullary thyroid cancer (representing <2% of all thyroid cancers), testing for actionable alterations in RET, ALK, or NTRK is limited to patients with advanced or metastatic disease not amenable to radioactive iodine therapy for other thyroid cancers (e.g. follicular, Hürthle cell, and papillary carcinomas)Citation4. These two diseases were selected due to their very different approaches to treatment (one very biomarker-driven, and the second much less so), and for their commonalities in the development of novel agents requiring biomarker evidence for targeted treatment.
Clinical biomarker testing practices vary widely in patients with lung and thyroid cancers despite guideline-recommended approachesCitation5–8. Data from the early 2020s across multiple databases show that only half to three-quarters of patients with NSCLC in the US receive guideline-recommended broad/next-generation sequencing (NGS)-based biomarker testingCitation5,Citation7,Citation8. Evidence for testing for RET was observed among 60% of patients with medullary thyroid cancerCitation6, while data for testing patterns are limited for other histologies. The cost of biomarker testing has been suggested as a barrier and issue to address for coverage and reimbursement of diagnostic testingCitation9,Citation10.
This retrospective observational database study was designed to quantify the costs of biomarker testing in a real-world setting across commercial insurance payer types for lung and thyroid cancers, which were selected to represent cancers where biomarker testing for actionable alterations was well established and emerging, respectively. It was hypothesized that a quantitative evaluation of the details of biomarker costs and coding, as well as per-patient total lifetime costs to the payer (commercial insurance plan) and the patient could help identify potential barriers to overcome to ensure patient access to genomic testing and may be an access barrier to life-extending targeted therapies.
Materials and methods
Data source
This retrospective observational study was conducted using the IBM Marketscan Research DatabasesCitation11. The commercial claims data used for this study contain longitudinal patient-level de-identified medical claims data from employers and health plans for more than 90 million covered employees, spouses, and their dependents. This administrative claims database includes a variety of fee-for-service, preferred provider organizations, and capitated health plans. These data are considered representative of these commercially-insured populations in the U.S. MarketScan data are fully complaint with US privacy laws and regulations, and as de-identified data do not qualify as human subjects research in accordance with 45 CFR 46.102(f) and are therefore exempt from Institutional Review Board requirementsCitation12. Data from January 2015 through December 2020 were accessed for this study.
Study design and patient population
Two cohorts were eligible for this study. The lung cancer cohort required the presence of at least two International Classification of Disease (ICD) diagnosis codes for lung cancer on different dates at least 30 days apart (ICD-9 162.2–162.9 or ICD-10 C34–C34.92), as is standard practice in administrative claims data to ensure accurate diagnosisCitation13,Citation14. The first eligible code was required to be observed between 1 January 2015 and 30 December 2019. Patients were excluded with cancer codes observed before this date. Similarly, for inclusion in the thyroid cancer cohort, two or more diagnosis codes (ICD-9 193 or ICD-10 C73) were required on at least two separate occasions at least 30 days apart between 1 January 2015 and 30 December 2019, with no cancer codes observed before this date. Study cohorts were mutually exclusive as patients with codes for both lung and thyroid cancer were excluded from this analysis. Data were available through 30 December 2020 to allow for up to a year of follow-up data after the last potential eligible diagnosis date. Eligible patients in each cohort were considered to have the metastatic disease if there was evidence of at least one metastatic disease code on or after the first eligible diagnosis code for lung or thyroid cancer.
Statistical analysis of costs of biomarker testing
Current Procedural Terminology (CPT) billing codes were used to identify claims paid for biomarker testing within each eligible study cohort. Biomarker testing included both single-gene (CPT: 81235, 81288, 81311, 81301, 81210, 81275, 81276, 88342, 88341, 88344, 88364, 88365, 88360, 88377, 88374, 88367, 88368, 88373, 88369, 88366, 88365, 81402, 81403, 81404, 81519, 81228, 81229, or 0018 U) and comprehensive/NGS-based testing (CPT: 81445, 81450, 81455, 0022 U, 0037 U, 0013 U, 0012 U, 0026 U, 0179 U, 0204 U, 0208 U, 0048 U, or 0047 U). Payer and patient-out-of-pocket costs specific to these diagnostic codes were obtained and summarized descriptively by biomarker code and payer type (e.g. health maintenance organization, preferred provider organization, point-of-service). Costs were limited to the line-item cost of biomarker codes (the claim record including the individual cost of each CPT code) included in this study and excluded all other costs of care. Total lifetime biomarker costs included the cumulative costs of all claims for biomarker tests on or after the eligible diagnosis code through the end of the study period. Descriptive statistics (medians, quartiles 1, 3 of the interquartile range; means and standard deviations) were used to examine costs, which were adjusted to US 2020 dollars using the consumer price index (CPI), medical care componentCitation15. The extent of potential missing codes was evaluated by reporting the number of patients receiving targeted therapies without evidence of any biomarker codes after diagnosis. Post-hoc sensitivity analyses were conducted excluding patients with any biomarker codes observed before the index date to evaluate the impact of exclusion of pre-diagnosis biomarker costs that may have been associated with other diseases or conditions or with disease identification rather than treatment. All analyses were conducted using SAS Enterprise version 7.1.
Results
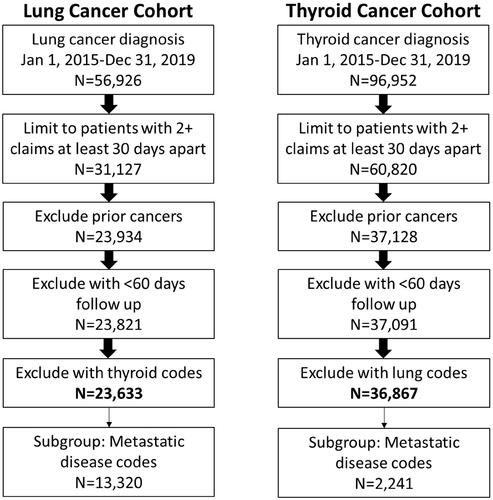
As shown in , a total of 23,633 patients with lung cancer met eligibility criteria and were included in this study (13,320 in the metastatic lung subgroup), and 36,867 were included with thyroid cancer (2,241 in the metastatic thyroid subgroup). The mean duration of follow-up was 14.2 months (standard deviation [SD] = 12.0) and 22.8 months (SD = 14.3) for the lung and thyroid cohorts respectively. The characteristics and payer types associated with each cohort and subgroup are summarized in . Patients were an average of 57 (SD = 6.6, lung) and 46 (SD = 11.5, thyroid) years of age at diagnosis. The most common commercial plan type was a preferred provider organization (PPO) across all cohorts.
Table 1. Patient characteristics at diagnosis.
The majority of patients in the lung cancer cohort (68.4%) and among those with metastatic lung cancer (75.8%) had at least one code paid for biomarker testing (). Among those with evidence of biomarker testing, 7.6 and 8.9% of patients with lung and metastatic lung cancer respectively, had at least one code paid for comprehensive/NGS-based biomarker testing. Few patients with thyroid cancer (18.2%) and less than half of those with metastatic thyroid cancer (42.3%) had evidence of biomarker testing (). Of those tested, 1.8 and 5.2% of patients with thyroid and metastatic thyroid cancer, respectively, had at least one code for comprehensive/NGS-based testing. Characteristics of patients undergoing biomarker testing appeared similar (generally within five percentage points or less) to those who did not have any claims for any biomarker testing in the database for all cohorts.
Table 2. Characteristics of patients with lung cancer, with and without biomarker testing codes.
Table 3. Characteristics of patients with thyroid cancer, with and without biomarker testing codes.
Cost data were available for 95.2, 95.0, 91.1, and 93.2% of patients with codes observed among the lung cancer, metastatic lung cancer, thyroid cancer, and metastatic thyroid cancer groups, respectively. Median and mean costs are summarized in . Median [mean] lifetime payer costs per patient ranged from $147.60 [335.60] to $461.50 [1,019.17] across tumor types. For patients with metastatic disease, the highest payer costs were consumer-driven health plans (CDHP) and exclusive provider organizations (EPO), with median per-patient costs of $499.70 (quartiles 1 and 3 of the interquartile range [IQR]: $213, $1,076; CDHP, metastatic lung) and $399.00 (IQR: $233, $834); EPO, metastatic thyroid). Median patient out-of-pocket costs were $0.00 across all payer types and tumor types except for CDHPs for thyroid cancer (median out-of-pocket lifetime costs $11.80; IQR: $0, $55) and CDHPs for metastatic lung cancer (median out-of-pocket lifetime costs $10.40; IQR: $0, $133). Median [mean] lifetime per-patient payer costs for patients who received at least one comprehensive/NGS-based code was $2,303 (IQR: $402, $4,191) [$$2,302.90, SD: $402, $4,191] and $2,334 (IQR: $421, $4,283) [$2,636.44; SD: $2,336.20] for lung cancer overall and for metastatic lung cancer, respectively. Median [mean] payer per-patient lifetime costs for those who received at least one comprehensive/NGS-based code were $1,469 (IQR: $486, $2,928) [$1,761.65; SD: $1,385.60)] for patients with thyroid cancer and $1,648 (IQR: $479, $3,018) [$1,923.37; SD: $1,651.70] for metastatic thyroid cancer. Median patient out-of-pocket costs were $0.00 for all cohorts. Sample sizes were small for thyroid cancer, in particular, to evaluate lifetime biomarker costs, such as comprehensive/NGS test codes by payer type.
Table 4. Median (interquartile range, IQR) and mean (standard deviation, SD) lifetime per-patient payer costs of biomarker testing, by payer and tumor type.
Table 5. Median and interquartile range (IQR) costs of single-gene and comprehensive/NGS-based testing codes.
There was a little observed risk of missing codes for biomarker testing, with only 2.9% (n = 65, metastatic thyroid) and 3.3% (n = 445, metastatic lung) of patients observed receiving targeted therapy without any observed codes for diagnostic testing. Sensitivity analyses were conducted limiting each cohort to patients without any history of biomarker testing before the index diagnosis (N = 19,531, 82.6% of the primary cohort patients with lung cancer; N = 33,977, 92.2% of the primary cohort of patients with thyroid cancer). Median lifetime payer costs per patient within this sensitivity analysis cohort ranged from $144.90 to $463.00 across tumor types. Median patient out-of-pocket costs were $0.00 across all payer types and tumor types except for PPO and CDHPs for thyroid cancer (median out-of-pocket lifetime costs were $4.20, IQR: $0, $35, and $12.80, IQR: $0, $58, respectively) and CDHPs for lung cancer, metastatic lung cancer, and metastatic thyroid cancer (median out-of-pocket lifetime costs $12.10, IQR: $0, $109; $14.50, IQR: $0, 131, and $4.30, IQR: $0, $86, respectively). Median lifetime per-patient payer costs for patients in the sensitivity analysis cohort who received at least one comprehensive/NGS-based code was $2,213.70 (IQR: $401, $4,130) and $2,302.90 (IQR: $401, $4,220) for lung cancer overall and for metastatic lung cancer, respectively. Median payer per-patient lifetime costs for those who received at least one comprehensive/NGS-based code were $1,451.20 (IQR: $486, $2,928) for patients with thyroid cancer and $808.60 (IQR: $337, $3,802) for metastatic thyroid cancer in the sensitivity analysis cohort. Median out-of-pocket patient costs for patients in the sensitivity analysis with at least one comprehensive/NGS-based code were $0 for all study cohorts. Costs per individual biomarker code across tumor types is presented in .
Discussion
This study quantified the costs paid per code and as lifetime per-patient costs for both the payer and patients with a variety of commercial health care plans for patients diagnosed with lung or thyroid cancers. The median lifetime costs were low for the patient ($0.00) and for the payer (<$500 for all payer types). These costs should be considered in the context of usual care. The costs of care for a single patient treated for advanced or metastatic lung cancer (where diagnostic testing is recommended) have been estimated between $130,000 to more than $300,000, and are rising as novel targeted therapies are increasingly being used for optimal patient careCitation16–21. While systemic treatment is not always needed for all patients with thyroid cancer, among those who do require treatment, the first two years of care have been estimated at $100,000 to more than $250,000Citation19,Citation22,Citation23. Not only are healthcare costs for diagnostic testing low, but represent an estimated <1% of the total cost of patient care for these two diseases from the healthcare system perspective. Given that biomarker testing can identify potential life-extending therapies, these costs are numerically low and also proportionally low in the total cost of care. There were several outliers with high costs. This type of distribution is not unique to costs for biomarker testing. Highly right-skewed data are known to be an issue with real-world costs across all sectors of health care, and are expected to be non-normally distributed; median data may therefore better reflect the distribution of costs but are limited in terms of use as input data for future economic analysis. Health care cost data always have a long tail representing a few individuals with extremely high costsCitation24,Citation25. To reduce the issue of excessive skewness, all patients with zero costs due to lack of utilization of the resource were excluded in the cost analysis; costs were only calculated among users of diagnostic tests for this study to best represent the actual costs of receiving biomarker testing.
This study observed a very low rate of reimbursed comprehensive/NGS-based testing codes, despite the comprehensive inclusion of these billing codes. This is not unexpected, as coding practices are not yet fully refined for these tests. For example, a health care provider or system may use several individual CPT test codes (co-billed or “stacked”), to code the various biomarkers included in comprehensive testingCitation26. The submission of a set of singular codes may represent a different biomarker included in a broad-based test, vs. the use of a solitary comprehensive/NGS test codeCitation27. This should be further explored in a dataset linked to clinical records, to evaluate the codes used for actual tests conducted, and could not be fully investigated in this study, and the clinical implications of what is actually being done in patient care cannot be fully elucidated. Additionally, denied or unpaid claims are not represented in this database, and these coverage issues cannot be fully evaluated, but are expected to be low given the very low number of patients receiving targeted therapy (only about 3% in each cohort) without evidence of biomarker testing observed in this database. Among patients with lung cancer, it is estimated that up to one-half of all patients with non-squamous non-small cell lung cancer, which represents 80–85% of all lung cancers, may have a biomarker-positive disease that is associated with improved outcomes with these novel therapiesCitation28. Since biomarker-directed therapy is only recommended for those with a biomarker-positive disease, it would not be expected that all patients receive these therapies, but given the known rates of positivity, the use of targeted therapies observed in this study is exceedingly low. Additionally, a sensitivity analysis excluding patients with any biomarker codes outside of the study time window found nearly identical findings as the primary analysis, suggesting that excluding any potential additional biomarker testing in the workup to identify the disease did not substantially influence the overall lifetime costs of biomarker testing for these patients.
While the utilization of comprehensive/NGS-based biomarker codes was low (<10% for all lung cancer cohorts and <5% for all thyroid cancer cohorts), the costs paid for biomarker testing that included these tests were higher for the payer than for biomarker testing without comprehensive biomarker codes. Median lifetime per-patient payer costs ranged from $1,469 to $2,303 which still represents only about 1% of the total healthcare system cost of patient care when put in the context of published estimates. Median patient out-of-pocket costs remained $0 regardless of the use of comprehensive/NGS codes. These findings, even for these broad-based tests, do not support the hypothesis that the payer cost of biomarker testing should be a barrier to access to recommended care for these patients. The results may be limited by the inclusion of insurance-based claims data, which do not include other forms of potential payment for testing, such as patient self-pay. Affordability cannot be examined in this database due to the lack of data on patient socioeconomic status; therefore, even at 1% of total healthcare system costs for these cancers, the dollar amount for a given patient for out-of-pocket costs may still be a barrier to care. However, based on this study, the cost of biomarker testing as a percentage of total care should not be a barrier to coverage for a payer responsible for patients with lung or thyroid cancers. There is a need to identify coverage gaps to provide opportunities for comprehensive testing to allow patients to access FDA-approved targeted therapies. Biomarker testing has been shown to be cost-effective for clinical lung cancer care and its value should be reevaluated where coverage may be limitedCitation28.
It is estimated that more than half of all Americans are covered by employer-sponsored health care plansCitation29. The findings from this study of individuals with lung or thyroid cancers within the MarketScan databases are likely representative of this larger population of patients with a similar diagnosis. Generalizability is limited, however, and these findings do not apply to patients insured by government-sponsored plans (e.g. Medicare/Medicaid) or by commercial plans that may have been purchased directly or through local or community programs, patients who are uninsured, or who may be covered by plans purchased through the Health Insurance Marketplace or a state-based exchange. Additionally, these findings are not generalizable to other diseases not included in this study. Additional work is needed to evaluate the costs of biomarker testing among other insured populations and tumor types.
Transparency
Declaration of funding
This was an unfunded research study conducted with employee time and material support from Eli Lilly and Company.
Declaration of financial/other relationships
LMH and DM are employees of Eli Lilly and Company. PMK, TM, and ANS are employees of LOXO@Lilly.
Author contributions
All authors meet the International Committee of Medical Journal Editors (ICMJE) requirements for authorship. LMH, PMK, and TM conceptualized the study. DM conducted statistical analyses. All authors interpreted the data. LMH drafted the full manuscript. All authors have reviewed and approved the final version for submission.
Acknowledgements
The study team would like to thank Nancy Hedlund for providing an outline for the initial draft of the manuscript.
Data availability statement
Data used in this study were supplied by International Business Machines Corporation under license for scientific research at Eli Lilly and Company. Data are available for research purposes by licensing directly from IBM, Inc. (www.ibm.com/products/marketscan-research-databases).
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Previous presentations
A poster containing an earlier version of data from this study was presented at ISPOR International Conference (Washington, DC) on 15–18 May 2022.
References
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA A Cancer J Clin. 2022;72(1):7–33.
- Imyanitov EN, Iyevleva AG, Levchenko EV. Molecular testing and targeted therapy for non-small cell lung cancer: current status and perspectives. Crit Rev Oncol Hematol. 2021;157:103194.
- NCCN clinical practice guidelines in oncology (NCCN guidelines) non-small cell lung cancer, version 3.2022 [cited 2022 Jun 16]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
- NCCN clinical practice guidelines in oncology (NCCN guidelines) thyroid carcinoma version 2.2022 [cited 2022 Jun 16]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/thyroid.pdf
- Hess LM, Krein PM, Haldane D, et al. Biomarker testing for patients with advanced/metastatic nonsquamous NSCLC in the United States of America, 2015 to 2021. JTO Clin Res Rep. 2022;3(6):9.
- Parikh R, Hess LM, Esterberg E, et al. Diagnostic characteristics, treatment patterns, and clinical outcomes for patients with advanced/metastatic medullary thyroid cancer. Thyroid Res. 2022;15(1):2.
- Sireci N, Krein P, Hess L, et al. Biomarker testing patterns in patients with stage IV non-small cell lung cancer (NSCLC) in the US community-based oncology practice setting. Boston (MA): ASCO Quality Care; 2021.
- Robert NJ, Nwokeji ED, Espirito JL, et al. Biomarker tissue journey among patients (pts) with untreated metastatic non-small cell lung cancer (mNSCLC) in the US oncology network community practices. Wolters Kluwer Health; 2021.
- Desai K, Hooker G, Gilbert K, et al. Real-world trends in costs of next generation sequencing (NGS) testing in US setting. Wolters Kluwer Health; 2021.
- Normanno N, Apostolidis K, de Lorenzo F, et al. Cancer biomarkers in the era of precision oncology: addressing the needs of patients and health systems. Semin Cancer Biol. 2022;84:293–301.
- Butler AM, Nickel KB, Overman RA, et al. IBM MarketScan research databases. In: Sturkenboom M, Schink T, editors. Databases for pharmacoepidemiological research. Cham: Springer International Publishing; 2021. p. 243–251.
- HHS. Human subject regulations decision charts: 2018 requirements; 2020 [cited 2021 Feb 3]. Available from: https://www.hhs.gov/ohrp/regulations-and-policy/decision-charts-2018/index.html#c1
- Nordstrom BL, Simeone JC, Malley KG, et al. Validation of claims algorithms for progression to metastatic cancer in patients with breast, non-small cell lung, and colorectal cancer. Front Oncol. 2016;6:18.
- Whyte JL, Engel-Nitz NM, Teitelbaum A, et al. An evaluation of algorithms for identifying metastatic breast, lung, or colorectal cancer in administrative claims data. Med Care. 2015;53(7):e49–e57.
- Statistics UBoL. Consumer price index; 2020. Available from: https://www.bls.gov/cpi/
- Korytowsky B, Radtchenko J, Nwokeji ED, et al. Understanding total cost of care in advanced non-small cell lung cancer pre-and postapproval of immuno-oncology therapies. Am J Manag Care. 2018;24(20 Suppl):S439–S447.
- Zhang X, Beachler DC, Masters E, et al. Health care resource utilization and costs associated with advanced or metastatic nonsmall cell lung cancer in the United States. J Manag Care Spec Pharm. 2022;28(2):255–265.
- Lin S, Luo S, Gu D, et al. First-line durvalumab in addition to etoposide and platinum for extensive-stage small cell lung cancer: a US-based cost-effectiveness analysis. Oncologist. 2021;26(11):e2013–e2020.
- NCI. Financial burden of cancer care; 2022.
- Aguilar-Serra J, Gimeno-Ballester V, Pastor-Clerigues A, et al. Cost-effectiveness analysis of the first‐line EGFR‐TKIs in patients with advanced EGFR-mutated non-small-cell lung cancer. Expert Rev Pharmacoecon Outcomes Res. 2022;22(4):637–646.
- Peng Y, Zeng X, Peng L, et al. First-line atezolizumab for metastatic NSCLC with high PD-L1 expression: a United States-based cost-effectiveness analysis. Adv Ther. 2021;38(5):2447–2457.
- Berger A, Edelsberg J, Chung K, et al. Healthcare (HC) utilization and costs in patients (pts) with newly diagnosed metastatic thyroid cancer (mTC). J Clin Oncol. 2007;25(18_suppl):17082.
- Rivas AM, Nassar A, Zhang J, et al. ThyroSeq® V2. 0 molecular testing: a cost-effective approach for the evaluation of indeterminate thyroid nodules. Endocr Pract. 2018;24(9):780–788.
- Mihaylova B, Briggs A, O'Hagan A, et al. Review of statistical methods for analysing healthcare resources and costs. Health Econ. 2011;20(8):897–916.
- Cantoni E, Ronchetti E. A robust approach for skewed and heavy-tailed outcomes in the analysis of health care expenditures. J Health Econ. 2006;25(2):198–213.
- Trogan G. What do payers want in oncology diagnostics? Insights from a national survey of top commercial and medicare health plans. Am Health Drug Benefits. 2011;4. Available from: https://www.ahdbonline.com/issues/2011/august-2011-vol-2014-no-2014-special-issue/2790-article-2790?page=2010,2012
- Hsiao SJ, Mansukhani MM, Carter MC, et al. The history and impact of molecular coding changes on coverage and reimbursement of molecular diagnostic tests: transition from stacking codes to the current molecular code set including genomic sequencing procedures. J Mol Diagn. 2018;20(2):177–183.
- Zou D, Ye W, Hess LM, et al. Diagnostic value and cost-effectiveness of next generation sequencing-based testing for treatment of patients with advanced/metastatic non-squamous non-small cell lung cancer in the US. J Mol Diagn. 2022;24(8):901–914.
- NCHS. High-deductible health plan enrollment among adults aged 18–64 with employment-based insurance coverage; 2018.