Abstract
Introduction
People with recurrent epileptic seizures are typically treated with anti-seizure medications (ASMs). Around a third of epilepsy patients fail to achieve an adequate response to ASMs and may be eligible to receive vagus nerve stimulation (VNS) therapy for their drug-resistant epilepsy (DRE) if they are unsuited to surgery. VNS received approval from the United States (US) Food and Drug Administration agency. However, there has to date been no comprehensive cost effectiveness evaluation of VNS within the US setting. This study was designed, using a US Medicare perspective, to estimate costs and quality-adjusted life years (QALYs) associated with VNS as an adjunct to ongoing ASM therapy, compared to ASMs alone.
Methods
We developed a cohort state transition model in Microsoft Excel, with four health states defined by different percentage reductions in seizure frequency, with a 3-month cycle and transition probabilities derived from published clinical trials and registry data. Sensitivity analyses were conducted to understand the impact of parameter uncertainty. Costs included the VNS device, placement, programming, battery changes, and removal; ASM therapy; adverse events associated with VNS (dyspnea, hoarseness, and cough); and costs associated with seizure burden (i.e. hospitalizations, emergency department visits, neurologist visits).
Results
Under base case assumptions, treatment with VNS was associated with a 0.385 QALY gain and a $109,678 saving per patient, when compared with ASM therapy alone. The incremental net monetary benefit (iNMB) was $128,903 at a threshold of $50,000 per QALY, with the positive iNMB indicating that VNS is a highly cost effective treatment. This result is explained by the modeled reduction in relative seizure frequency and associated reduction in healthcare resource use that the VNS group experienced. Sensitivity analyses supported this conclusion.
Conclusions
VNS was evaluated as a cost effective addition to the current standard of care in the treatment of DRE in the US Medicare context.
PLAIN LANGUAGE SUMMARY
Anti-seizure medications (ASMs) are drugs commonly prescribed to people with epilepsy to help prevent seizures from reoccurring. But these drugs do not work for all people: around a third keep having seizures despite taking the medication—a condition called drug-resistant epilepsy (DRE). For such people, their main options involve trying different combinations of ASMs, having brain surgery, or having a medical device implanted. In the United States (US), vagus nerve stimulation (VNS) therapy is approved by the Food and Drug Administration agency for DRE patients who are still having focal onset seizures despite trying ASM therapy. Using methods defined by the US evaluation body, the Institute for Clinical and Economic Review, we made an economic model to assess how cost effective VNS would be as an add-on to ASM therapy. The evaluation utilizes a previously published model, which was updated to use costs, health-related quality of life, and mortality estimates relevant to the US Medicare setting. The analysis demonstrated that VNS could generate cost savings when used as an add-on ASM treatment in the US Medicare setting. VNS may reduce the number of seizures, and subsequently improve patient quality of life and result in substantially lower costs for Medicare (e.g. in emergency and hospital care for a person having a seizure). We tested uncertainties in our model using standard methods—these additional analyses allow us to conclude that VNS is highly likely to be a cost effective addition to the current standard of care for DRE treatment from a US perspective.
Introduction
Around a third of people with epilepsy fail to achieve an adequate response to treatment with anti-seizure medications (ASMs)Citation1. Drug-resistant epilepsy (DRE) is a serious and potentially life-limiting conditionCitation2 with significant health system burdenCitation3. The prevalence of DRE varies geographically and is dependent on the use of the definition of drug resistanceCitation4,Citation5 but is universally found to be associated with more frequent hospitalization and emergency department visits when compared to patients responding to ASMsCitation6,Citation7. In the United States (US), the estimated financial burden of DRE is $4 billion per annum in direct and indirect costsCitation8. Patients with DRE have significantly poorer quality of lifeCitation7, with depressionCitation9 and cognitive impairmentCitation10 most commonly reported.
Vagus nerve stimulation (VNS) has been an important treatment option in DRE for three decades, with over 125,000 patients having received it since 1989Citation11. In the US, VNS is approved for use by the Food and Drug Administration (FDA) agency as an adjunctive treatment in reducing the frequency of seizures in patients aged four or older with focal onset seizures, who are not responsive to ASMsCitation12. As an already established treatment for DRE, there is abundant evidence demonstrating the clinical efficacy of VNSCitation13–15. A recent systematic review and meta-analysis of 30 studies in people with DRECitation16 observed similar efficacy for three modalities of neurostimulation during initial blinded phases of randomized controlled trials (RCTs), albeit with different eligibility criteria.
Whilst several international studies have assessed the cost effectiveness of VNS and have found VNS a cost effective strategy to manage DRE in adults in countries, such as the Netherlands, China, and EnglandCitation17–19, cost effectiveness studies of VNS in the US remain scarceCitation16. Recent literature reviews searching for cost effectiveness analyses found no US cost effectiveness analyses of VNS in adult DRE, with only studies analyzing budget impact or cost consequence, pediatric populations, or responsive neurostimulation (RNS) devices foundCitation15,Citation16. A recent study found that RNS was cost effective vs. ASM alone, with an incremental cost effectiveness ratio (ICER) of $28,825 per quality-adjusted life year (QALY)Citation20. The objective of this study was to develop the first comprehensive cost-utility analysis (CUA) of VNS as an adjunct therapy to ongoing ASM in adult DRE patients. As per a previously published cost effectiveness study of neurostimulation in DRE patients, the perspective adopted was of US MedicareCitation20. This is in line with the Institute for Clinical and Economic Review reference case, which recommends the inclusion of costs paid by third-party payerCitation21. Moreover, DRE patients are eligible for Medicare reimbursementCitation22, with ∼50% of VNS patients in the US covered by MedicareCitation23.
Methods
The main model design elements and methods, including the health state structure and effectiveness parameters, are based on a published English National Health Service (NHS) cost effectiveness model for VNS in DRECitation19. Since the publication for the English NHS analysis contains a full account of the model development, the Methods section for our US analysis provides only a brief recapitulation of the main model setup, followed by full details regarding the steps taken to adapt this model for a de novo analysis applicable to the US setting.
Design and population
Our CUA complies with the US Institute for Clinical and Economic Review reference caseCitation21 to estimate the costs and benefits of two DRE management strategies:
ASM regimen as typically administered in DRE management, with no VNS.
VNS administered as an adjunct to the ASM regimen.
Other management strategies, such as RNS and deep brain stimulation (DBS), were considered as comparators; however, they were not included in the analysis due to a scarcity of comparative evidence with VNS.
Health benefits were estimated as QALYs, and costs took a US Medicare perspective. To account for time preference, costs and benefits were discounted at an annual rate of 3%, as specified in the US reference caseCitation21. A time horizon of 10 years was used to capture the most relevant costs and benefits relating to treatment with DRE, and to reflect the extent of available evidence. The ICER, calculated as incremental costs over incremental QALYs, is evaluated against a threshold; if it falls below the threshold, the intervention of interest can be considered cost effective. In this analysis, the US reference case range of $50,000 per QALY to $150,000 per QALY was usedCitation21.
Two pivotal RCTs (named E03 and E05) evaluating the efficacy of high vs. low stimulation VNS therapy given adjunctively to ASMs were used to inform the model cohort population characteristics and are summarized in the Technical SupplementCitation24,Citation25.
Model structure
We used the same cohort state transition model as the English NHS analysisCitation19. Patients could be in one of four health states defined by percentage reductions in seizure frequency achieved from baseline, with the lowest category (<50% reduction) corresponding to “no response” and the highest (100% reduction) representing “seizure-free”. Death was also modeled as an absorbing state. A graphical summary is provided in and .
Figure 1. Model structure for VNS + ASM group. ASM: anti-seizure medication; VNS: vagus nerve stimulation.
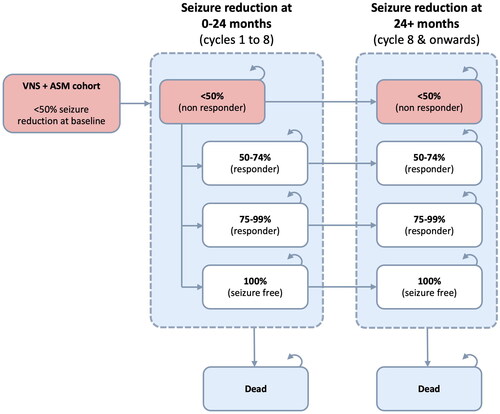
Figure 2. Model structure for ASM only group. ASM: anti-seizure medication; VNS: vagus nerve stimulation.
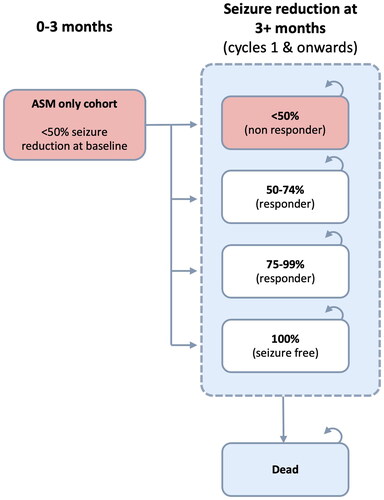
Although not directly quantifying epilepsy severity, these health-state categories are meaningful with respect to therapeutic goals and have been used in other published health economic analyses in this fieldCitation26–28.
Transitions occurred on a 3-month cycle basis. All patients started in the “no response” health state, and patients in the VNS arm could transition between health states any time up to two years after implantation, consistent with published long-term observational dataCitation29. After this, their health state remained fixed (subject to death or discontinuation). By contrast, ASM only patients moved between health states in the first 3-month cycle, whereafter their response remained static.
Clinical evidence—usage and assumptions
Efficacy of VNS and health state transitions
The sourcing and application of efficacy parameters were identical to the English NHS modelCitation19. Individual patient-level data from the international trials of VNS E03 and E05 informed efficacy estimates, both for the VNS and “ASM only” groups, for the first three months of the modelCitation24,Citation25. Longer-term effectiveness of VNS was based on estimates from a systematic review of VNS studiesCitation29, validated and cross-checked against real-world registry data and open-label trial extension dataCitation30.
For “ASM only” patients, we found limited evidence with which to model longer-term changes in response. Therefore, we took the assumption that modeled “ASM only” patients would maintain in perpetuity the distribution of response categories achieved by “low stimulation” arm patients in the E03 and E05 studies. The simplicity of the assumption matches the data scarcity and allows for transparent sensitivity analysis.
Adverse events
Three adverse events commonly observed in the VNS clinical trials were included in the analysis as annual risks: hoarseness, cough, and dyspneaCitation30. Adverse effects associated with ASMs were not modeled since the base case assumed that both modeled cohorts received the same stable ASM regimens throughout the time horizon.
The most common complication of VNS implantation is surgical site infection, which has been estimated to occur in 1.3% of patientsCitation31. As per the previously published modelCitation19, any health-related quality of life (HRQoL) impact of surgical site infection was assumed to be negligible in the context of the time horizon, given the infrequent and one-off nature of the eventCitation31. Further, the Hospital-Acquired Condition Reduction program financially disincentivizes infections, with some costs of infection falling outside the Medicare perspectiveCitation32. A sensitivity analysis, whereby the cost of implementing VNS was increased substantially, was conducted to explore uncertainty of this parameter (i.e. to understand if conclusions would change even if infection rates were high and included in addition to the Medicare reimbursement cost).
Device explantation or replacement due to battery depletion
Patients receiving VNS could discontinue treatment due to non-response (leading to the deactivation of the device) or if explantation (for any cause) was needed, with the latter involving an additional procedure cost. Replacement of VNS devices due to battery depletion was associated with a respective cost, but patients retained the health benefits of VNS. Time to device explantation and battery replacement were modeled using survival models fit to Kaplan-Meier estimates from real-world registered implant data from the US, detailing the use of VNS (AspireSR 106) in 26,261 patients for up to 7.14 yearsCitation33.
Health-related quality of life
A quality-of-life utility score for each health state was obtained using data from a prospective study of 81 patients by Messori et al.Citation34, which estimated utilities using the time trade-off methodCitation35 for a set of seizure frequency categories. These categories, which reflected absolute numbers of seizures, could be mapped with reasonable similarity onto the percentage seizure reduction categories. The utilities used for each health state are summarized in .
Table 1. Health-related quality of life values used in model.
Health state utilities were adjusted over time to reflect the expected decline in utility as patients age. It was assumed that seizure-free patients would have a utility equal to the US general population over time. To adjust the utilities for each health state over time, we first calculated the relative difference between the health state utilities and the utility of the US general population adjusted for the baseline mean age and gender mix of the modeled DRE population, i.e. 0.96Citation36. We then applied these relative differences to the US baseline population utility value corresponding to the mean age of patients at each cycle in the model to calculate the adjusted health state utilities.
Consistent with the English NHS model, the three types of VNS adverse events were modeled to have an HRQoL decrementCitation19.
Costs and resource use
Health state costs
Healthcare resource unit costs, summarized in the Technical Supplement, were obtained from publicly available sources, in line with the US reference caseCitation21. Primary care costs were not included as care for DRE patients was assumed to take place in a secondary care setting. Secondary care (hospital) episodes were costed using two statistical briefs of Healthcare Cost and Utilization Project data published by the Agency for Healthcare Research and Quality, which reflects Medicare costsCitation37,Citation38. Costs were inflated to 2021 using medical care inflation rates from the Bureau of Labor StatisticsCitation39.
The cost of outpatient visits associated with each health state in the model is based on the national payment amount of $103.82 for service in a non-facility setting (current procedural terminology: 99,214), assuming a Medicare reimbursement of 80% of the visitCitation40.
Healthcare resource assumptions
The method for estimating healthcare resource use (HCRU) in each health state was identical to the published English NHS modelCitation19, except that estimates for the 50–74% seizure reduction health state were obtained from a published US studyCitation41. The annual health state resource use frequencies were multiplied by unit costs to give annual costs as shown in , with frequencies varied by the ranges shown for sensitivity analysis.
Table 2. Summary of annual healthcare resource costs and utilization.
Anti-seizure medication costs
No direct, publicly available evidence on the schedules of ASMs administered to DRE patients was identified for the US context. The model used an annual ASM cost estimate of $21,772 (inflated cost: $24,021.91) reported in a US cost effectiveness study of surgery for drug-resistant temporal lobe epilepsyCitation43. This analysis assumes that the costs and effects of ASM are the same for the modeled VNS and ASM only groups.
VNS costs—implantation, explantation, and battery replacement
An overview of the values used to estimate the cost of VNS implantation, as well as explantation and replacement where applicable, is given in . Implantation procedure costs include the costs of full system implant in hospital settings and full system placement in facility settings; the cost reflects that ∼93% of VNS procedures are expected to take place in an outpatient or ambulatory surgical care settingCitation46.
Table 3. Resource use and costs for VNS.
Adverse event costs
The expected resource use and associated cost for a patient experiencing hoarseness, cough, or dyspnea over the course of 1 year were estimated at $156. This corresponds to 1.5 neurologist visits to reflect the general treatment and management of the adverse event, including potential changes in device programmingCitation40. The impact of the changing of the programming of the device on seizure reduction following an adverse event was not specifically modeled due to a lack of available data, in line with the previously published modelCitation19. The annual cost was converted to a mean cost per cycle ($39) and applied proportionally to the percentage of the cohort experiencing an event in any given cycle.
Sensitivity and scenario analyses
Deterministic sensitivity analysis
To identify the key drivers of model outcomes, single parameters or groups thereof were varied as appropriate - by a plausible minimum and maximum range reported from the source, or by ±15% where ranges were not available, keeping all other inputs constant.
Probabilistic sensitivity analysis
Parameter uncertainty was assessed by probabilistic sensitivity analysis (PSA) using 5,000 simulations and the likelihood of being cost effective at various thresholds evaluated. The parameter distributions used in PSA are provided in the Technical Supplement.
Other sensitivity analyses
A set of threshold and scenario analyses were designed to test the model’s sensitivity to the following concepts:
mapping HCRU to health states
assumptions regarding longer-term treatment effect
expected inpatient and elective cost burden for a DRE patient, designed to reflect and explore the impact of potential discrepancies between Medicare reimbursement and the total cost of provision of VNS, or regional differences in cost within the US of healthcare provision more generally (this latter scenario was run via a two-way sensitivity analysis that varied hospitalization and additional device costs).
All scenario analyses and their results are reported in detail in the Technical Supplement.
Results
Base case
The cost effectiveness analysis estimates that the total cost over a 10-year time horizon is $689,959 for the VNS group and $799,637 for the ASM only group. The total QALYs are estimated at 6.051 for the VNS group and 5.666 for the ASM only group. The incremental total cost difference is $109,678 favoring VNS, with an estimated incremental QALY gain of 0.385. Therefore, VNS is expected to be cost effective and dominate ASM only (i.e. cost saving and conferring more QALYs than the comparator). A breakdown of the results is provided in .
Table 4. Base-case cost effectiveness results.
Deterministic sensitivity analysis
As shows, no input tested had the potential to change conclusions regarding cost effectiveness when considering the ICER threshold range of $50,000–$150,000 per QALY (noting that a positive incremental net monetary benefit [iNMB] indicates cost effectiveness at the threshold tested). A tornado plot is provided in .
Figure 3. Deterministic sensitivity analysis—tornado diagram. ASM: anti-seizure medication; ED: Emergency Department; NMB: net monetary benefit; QALY: quality-adjusted life year; SMR: standardized mortality ratio; TRAE: treatment-related adverse event; VNS: vagus nerve stimulation. Notes. Where a parameter sensitivity analysis involved change to a single value, lower and upper bound values are shown on the diagram; ¥lower or upper bound applied to the group of inputs informing the parameter simultaneously.
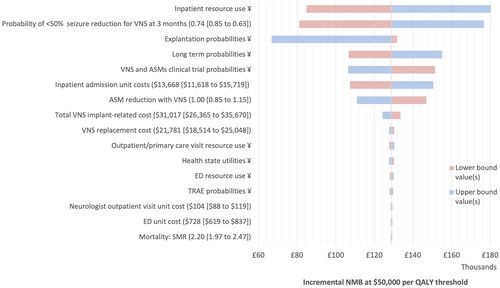
Table 5. Top 15 most sensitive parameters.
The model is most sensitive to the resource estimates and unit cost applied for hospitalization. The model is also sensitive to the probability of <50% seizure reduction for VNS in the first cycle, mostly because HCRU costs are directly linked in our model to seizure reduction. This echoes findings in the Raspin et al. study, which gives further explanation to the relationships between the parameters and the results displayed hereCitation19.
An increase in the aggregate cost of the VNS device and implantation of just over $75,000 was the threshold at which VNS no longer dominated ASM only (full tabulation is provided in the Technical Supplement). This provides reassurance that should additional costs (i.e. for infection) need reimbursement, VNS would still be considered a cost effective strategy.
Scenario analyses
Mapping healthcare resource use to health states
Healthcare resource consumption is a key driver of cost effectiveness in this therapy area, yet the methods and assumptions used in this model are subject to uncertainty. Therefore, a range of alternative scenarios was tested and written up in the Technical Supplement. In all scenarios tested, VNS remained a dominant strategy compared to ASM alone.
Long-term clinical effectiveness
Exploratory scenarios were tested in which the long-term effectiveness of VNS, or its modeled rate of explantation, were varied. In all scenarios, VSM remained cost saving or cost effective compared to ASM alone.
Expected inpatient and elective cost burden for a DRE patient
Hospitalization is the most costly form of HCRU in the model, so a set of scenarios around the cost and usage of inpatient care was explored and is reported in the Technical Supplement. VNS continued to dominate vs. ASMs alone for hospitalization costs over $4,000 and remained cost effective at a $50,000 threshold with a hospitalization cost of over $2,000.
The analysis was also rerun using the procedure associated with the CPT code 99215 for outpatient visits ($183.07 in facility settings), which is reimbursed up to $146.50 by Medicare. This change did not impact on the conclusion of dominance of VNS when compared to ASM therapy alone.
Probabilistic sensitivity analysis
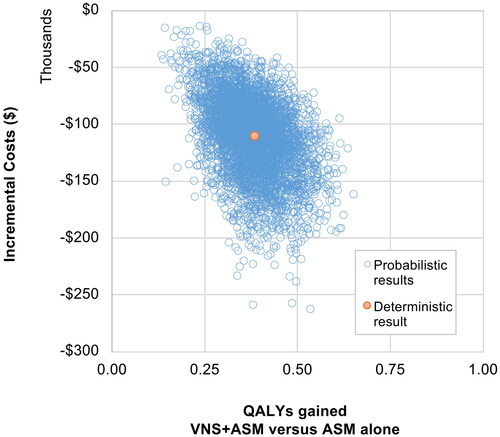
In PSA, VNS was dominant in 100% of the 5,000 iterations, with a probabilistic iNMB of $129,337 (95% CI: $64,823–$203,833) using a threshold of $50,000. A PSA scatter plot is provided in .
Discussion
Overview
This novel analysis evaluates the cost effectiveness of VNS in the US Medicare setting, using contemporary cost estimates and granular patient-level efficacy data in a detailed long-term model. The base case results suggest that VNS is a cost saving intervention in the US setting, with a dominant ICER value ($109,678 cost savings and 0.385 QALYs gained over a ten-year horizon). The majority of savings seen in the VNS group are realized through hospitalizations avoided compared to the amount expected in the ASM only group. The higher the expected cost of hospitalization, the greater the saving that can be realized through reduced seizure burden.
In the US, DRE patients have access to several non-pharmacological interventions, including VNS, RNS, DBS, and surgery (if suitable). Although there have been no prior US-based cost effectiveness analyses of VNS in an adult population, published analyses for RNS and surgery provide informative comparisons of methodology.
A recent study by Youngerman et al. estimated the cost effectiveness of RNS vs. ASM aloneCitation20, using comparable methods to link HCRU and utility values to defined health states. The study found RNS was associated with an incremental cost vs. ASM, which was considered cost effective given the incremental health benefits. The relative difference in HRQoL between health states for the RNS model was greater than for this study; with Youngerman et al. using utilities elicited from the general population in South Korea for hypothetical focal onset seizure health states, and our analysis using utilities elicited from patients with medically refractory epilepsy in Italy. Had the Youngerman et al. source been used in our study, a higher incremental QALY gain would be achieved in favor of VNS. Another key difference between study methodology is the modeling of adverse events, with our study opting to model the risk of battery replacement and explantation of the device—as well as the potential for adverse events, such as cough, dyspnea, and hoarseness.
US-based studies evaluating the cost effectiveness of surgery have also been carried out for drug-resistant temporal lobe epilepsyCitation43 and Lennox-Gastaut Syndrome (LGS)Citation47. These studies used alternative model structures to our analysis to capture clinical risks and outcomes associated with surgery. In the temporal lobe epilepsy study, surgery was found to be cost saving and more effective—that is, dominant—compared to medical management in surgically eligible patientsCitation43. In the LGS analysis, corpus callosotomy was to be less cost effective than VNS with an incremental cost of $451,952 per positive seizure outcome gained over a 1-year time horizonCitation47.
Despite limited evidence of cost effectiveness of VNS in DRE in the US, our US-based study supports the finding that VNS is considered a cost effective option managing DRE in adults, as found in other international cost effectiveness studies (such as a Dutch study comparing VNS to DBS and care usualCitation17 and a Chinese evaluation of VNS vs. ASMCitation18.
The most relevant international cost effectiveness study of VNS to consider is the 2021 English NHS model by Raspin et al.Citation19, which used the same health state structure and clinical effectiveness data sources. Similar to the current study, the main driver of cost effectiveness in the English NHS model was inpatient costs—these were avoided in the VNS group in amounts that offset device and service costs, making VNS a cost effective intervention compared to ASM alone.
When modeled for the US Medicare perspective, threshold analysis results suggest that even if payers have to pay up to $75,000 in additional device costs (i.e. those potentially not covered by Medicare reimbursement), VNS as an adjunct to ASM therapy remains cost saving when compared to ASM therapy alone. Additional threshold analyses of our model found that VNS remained dominant as long as the hospitalization unit cost was over $4,000 and cost effective (at a $50,000/QALY threshold) as long as it was over $2,000.
With HCRU differences generating such large per-patient savings, alternative data sources and assumptions were tested in scenario analyses to inform the modeled HCRU estimates. Our healthcare resource utilization estimates came from a 2007 US-based observational studyCitation41 which is likely to be the most relevant for a US setting. The model continued to support the conclusion that VNS is cost effective across numerous other scenario analyses (see Technical Supplement).
Key assumptions considered in detail
Since the model structure in large part mirrors that of the English NHS analysis, the main considerations around model structure and assumptions are described in that publicationCitation19. Briefly, there are four main considerations. First, ASM seizure reduction rates were sourced from the control arms of the E03 and E05 trials. These control arms were not true placebo but rather “low stimulation” VNS, so the model may underestimate the true relative incremental benefit of VNS. Second, the “ASM only” group was assumed to permanently retain the level of response achieved by control arm patients in the E03 and E05 trials after three months (around 15.7% reached “responder” health states). This assumption is simplistic and captures neither long-term therapeutic decisions over time about ASM in DRE patients nor potential changes to seizure frequency. However, the assumption reflects the lack of long-term data availableCitation16 and potentially favors the “ASM only” group, in whom absolute response numbers have been observed to declineCitation48–50. Third, a constant rate of mortality was applied across all health states. However, sudden death in epilepsy risk is higher for people with ongoing seizures than for those who are seizure freeCitation51. Finally, the E03 and E05 trials used older devices, yet our model device costings were based on the currently available SenTiva model of VNS generator. The SenTiva device has an “AutoStim” feature that triggers stimulation when there is a change in heart rate, which may provide better seizure control than previous modelsCitation52,Citation53, and have a different lifespan and likelihood of explantation.
There were also specific assumptions to our US-based model worth noting. Medicare reimbursement costs—rather than unit financial costs—were used. This means that, in general, the modeled costs of healthcare, in particular for hospitalization and inpatient care associated with seizures, may underestimate the true cost borne by the hospitals. In turn, this means that seizures avoided through VNS may in fact be associated with greater healthcare resource savings. Additionally, implantation procedures were also costed using appropriate Medicare reimbursement amounts. However, since this reflects a potential situation whereby hospital providers are not fully covered by Medicare for the costs of implanting and maintaining VNS devices, we also performed sensitivity analyses in which the implantation and maintenance costs were added to the direct up-front device cost as a single bundle. Even in this most pessimistic scenario, VNS remained dominant compared to using ASMs alone. Further analyses using cost estimates from alternative perspectives, such as Medicaid or private insurance could be conducted to further understand the impact of VNS on the US healthcare system.
Strengths and limitations
Strengths
This study used individual data from 310 patients in E03 and E05 to inform efficacy. Long-term follow-up data came from registries collected in 3,182 patients over 24 months and 1,194 patients over 48 months across multiple countries. A systematic review combining data from 2,689 patients was also used, demonstrating that treatment response on VNS is sustained, if not enhanced, over time in real clinical practiceCitation29. Furthermore, survival analysis of long-term data in a sample of 26,286 patients was used to estimate time to battery replacement and explantation for VNS over time.
Limitations
Using percentage reductions in seizure rates to define health states is considered reasonable, but it necessitates mapping estimates of HRQoL and HCRU from published studies, which typically report their values against absolute seizure rates. Where possible, we tied percentage reductions to commonly reported clinical outcomes in epilepsy, including “seizure freedom” (100% reduction), “non-responder” (<50% reduction), and “responder” (>50% reduction). However, this approach does not address potential differences in absolute baseline seizure rates between the referenced studies. Where possible, we matched and adjusted values to counter this type of variety, but some misalignment remains. It was also not possible to determine how well the severity of seizures aligns between publications. For example, we assumed that “refractory seizures” captured in the HRQoL publication by Messori et al. were not severe due to the relatively high quality of life reported by patients who were experiencing them. By contrast, 55.0–70.4% of patients in the E03 trial experienced focal to bilateral tonic clonic (referenced in the trial as secondary generalized) seizures, which can have a considerable impact on a patient’s HRQoL.
It was necessary to adapt and use HCRU data reported from multiple US and non-US healthcare settingsCitation27,Citation41,Citation42 to complete the matrix of health state costs. This posed further challenges since each publication differed in its definition and categorization of response. There is also uncertainty in whether patients in the “<50% response” category may have more costly inpatient stays compared to patients who have achieved some level of seizure control due not only to possible differences in seizure severity, but also their pattern of long-term recurrence. The model uses the same unit cost per healthcare item, irrespective of which seizure reduction health state it is used in. Cost savings of VNS could be underestimated if the “<50% response” group ends up consuming more healthcare resources when hospitalized.
Finally, the model does not include the benefits of a relative reduction in seizure frequency for carers of an epilepsy patient or the societal costs of lost productivity, such as those reported by Gupta et al.Citation54. It is conceivable that incorporation of such benefits would increase the cost effectiveness of VNS.
Conclusion
Under base case assumptions and when taking a US Medicare perspective, VNS is considered a cost saving intervention as an adjunct to ASMs in patients with DRE who are not suitable for surgery. There is uncertainty in how VNS impacts on the reduction of disabling seizure and therefore its relationship with HRQoL, HCRU, and costs. The analyses presented should be considered exploratory until further data are forthcoming. If future research indicates a different ratio of HCRU between health states, then the conclusions of the analysis could change. This said, a wide range of potential scenarios, including a scenario whereby the cost of providing VNS was increased substantially, were modeled and results consistently indicated that VNS was more cost effective than ASM alone.
Transparency
Declaration of funding
This study was funded by LivaNova PLC.
Declaration of financial/other relationships
FB, JM, and VD have received support from LivaNova PLC as paid employees. CR, JA, and VP have served as paid consultants from Symmetron Ltd., they were contracted by LivaNova PLC to undertake the study. EF has been a member of scientific advisory boards for Biogen, Eisai, Neurelis, SK Life Science, Sage Pharmaceuticals, and the Centers for Disease Control and Prevention, and has received research support from UCB Pharma.
Author contributions
All authors contributed to the critical revision and final review of the manuscript. CR, JA, and VP developed the economic model and undertook the initial analysis and interpretation of results. FB, JM, and VD provided advice on the conceptual model and, FB, JM, VD, and EF assisted in the provision of data that informed the model. CR and JA drafted the manuscript, with kind medical writing assistance from Jana Tillotson. VP, EF, FB, JM, and VD provided a review of model structure, inputs and contributed to the interpretation of results. All authors contributed to the critical revision and final review of the manuscript.
Acknowledgements
The authors are grateful to Jana Tillotson, Medical Writer, for her kind assistance in preparing the draft manuscript. The authors are thankful to Šárka Sirůčková (Project Coordinator, Symmetron) for her kind assistance in the manuscript submission process.
Reviewer disclosures
Peer reviewers on this manuscript have received an honorarium from JME for their review work but have no other relevant financial relationships to disclose.
Supplemental Material
Download MS Word (71.1 KB)References
- Kwan P, Arzimanoglou A, Berg AT, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc task force of the ILAE commission on therapeutic strategies. Epilepsia. 2010;51(6):1069–1077.
- Neligan A, Bell GS, Johnson AL, et al. The long-term risk of premature mortality in people with epilepsy. Brain. 2011;134(Pt 2):388–395.
- Platt M, Sperling MR. A comparison of surgical and medical costs for refractory epilepsy. Epilepsia. 2002;43(s4):25–31.
- Neligan AJ. The incidence and prevalence of epilepsy; 2015 [cited 2020]. Available from: https://www.epilepsysociety.org.uk/sites/default/files/attachments/Chapter01Neligan-2015.pdf
- Hauser WA, Beghi E. First seizure definitions and worldwide incidence and mortality. Epilepsia. 2008;49 (Suppl 1):8–12.
- Strzelczyk A, Griebel C, Lux W, et al. The burden of severely drug-refractory epilepsy: a comparative longitudinal evaluation of mortality, morbidity, resource use, and cost using German health insurance data. Front Neurol. 2017;8:712–712.
- Villanueva V, Girón JM, Martín J, et al. Quality of life and economic impact of refractory epilepsy in Spain: the ESPERA study. Neurología. 2013;28(4):195–204.
- Murray MI, Halpern MT, Leppik IE. Cost of refractory epilepsy in adults in the USA. Epilepsy Res. 1996;23(2):139–148.
- Garcia ME, Garcia-Morales I, Gil-Nagel A. Prevalence of depressive symptoms and their impact on quality of life in patients with drug-resistant focal epilepsy (IMDYVA study). Epilepsy Res. 2015;110:157–165.
- Dalic L, Cook MJ. Managing drug-resistant epilepsy: challenges and solutions. Neuropsychiatr Dis Treat. 2016;12:2605–2616.
- LivaNova. Data on file, private communications; 2022.
- FDA. Summary of safety and effectiveness day (SSED), VNS Therapy, notice of approval; 2017 [cited 2022 Nov 3]. Available from: https://www.accessdata.fda.gov/cdrh_docs/pdf/p970003s207b.pdf
- Morris GL, Gloss D, Buchhalter J III, et al. Evidence-based guideline update: vagus nerve stimulation for the treatment of epilepsy: report of the guideline development subcommittee of the American Academy of Neurology. Epilepsy Curr. 2013;13(6):297–303.,
- NICE. The epilepsies: the diagnosis and management of the epilepsies in adults and children in primary and secondary care. National Institute for Health and Care Excellence; 2012 [updated 2020 Nov 28; cited 2022 Nov 3]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK247130/pdf/Bookshelf_NBK247130.pdf
- Shaw B, King V, Robalino S, et al. Vagal nerve stimulation for epilepsy and depression. Draft evidence report; 2020.
- Touma L, Dansereau B, Chan AY, et al. Neurostimulation in people with drug‐resistant epilepsy: systematic review and meta‐analysis from the ILAE surgical therapies commission. Epilepsia. 2022;63(6):1314–1329.
- Chan HY, Wijnen BFM, Majoie MHJM, et al. Economic evaluation of deep brain stimulation compared with vagus nerve stimulation and usual care for patients with refractory epilepsy: a lifetime decision analytic model. Epilepsia. 2022;63(3):641–651.
- Zhang J, Li W, Zhang Z. PND12 vagus nerve stimulation versus anti-epileptic drug in Chinese patients with drug-resistant epilepsy: a cost-effectiveness analysis. Value Health Reg Issues. 2020;22:s76.
- Raspin C, Shankar R, Barion F, et al. An economic evaluation of vagus nerve stimulation as an adjunctive treatment to anti-seizure medications for the treatment of drug-resistant epilepsy in England. J Med Econ. 2021;24(1):1037–1051.
- Youngerman BE, Mahajan UV, Dyster TG, et al. Cost-effectiveness analysis of responsive neurostimulation for drug-resistant focal onset epilepsy. Epilepsia. 2021;62(11):2804–2813.
- ICER. ICER’s reference case for economic evaluations: principles and rationale current as of January 31, 2020. Boston; ICER; 2020.
- CMS.gov. Vagus nerve stimulation (VNS) for treatment resistant depression (TRD) [CAG-00313R2]; 2019 [updated 2023 Jan 11; cited 2022 Nov 3]. Available from: https://www.cms.gov/medicare-coverage-database/view/ncacal-decision-memo.aspx?proposed=N&NCAId=292#:∼:text=The%20Centers%20for%20Medicare%20%26%20Medicaid,follow%2Dup%20duration%20of%20at]
- LivaNova. Private emails. Data on file; 2023.
- The VNS Study Group. A randomized controlled trial of chronic vagus nerve stimulation for treatment of medically intractable seizures. Neurology. 1995;45(2):224–230.
- Handforth A, DeGiorgio CM, Schachter SC, et al. Vagus nerve stimulation therapy for partial-onset seizures. A randomized active-control trial. Neurology. 1998;51(1):48–55.
- Bolin K, Berggren F, Forsgren L. Lacosamide as treatment of epileptic seizures–cost utility results for Sweden. Acta Neurol Scand. 2010;121(6):406–412.
- Kristian B, Wachtmeister K, Stefan F, et al. Retigabine as add-on treatment of refractory epilepsy–a cost-utility study in a Swedish setting. Acta Neurol Scand. 2013;127(6):419–426.
- Purser MF, Mladsi DM, Beckman A, et al. Expected budget impact and health outcomes of expanded use of vagus nerve stimulation therapy for drug-resistant epilepsy. Adv Ther. 2018;35(10):1686–1696.
- Englot DJ, Rolston JD, Wright CW, et al. Rates and predictors of seizure freedom with vagus nerve stimulation for intractable epilepsy. Neurosurgery. 2016;79(3):345–353.
- Morris GL III, Mueller WM. Long-term treatment with vagus nerve stimulation in patients with refractory epilepsy. The vagus nerve stimulation study group E01–E05. Neurology. 1999;53(8):1731–1735.
- Selner AN, Rosinski CL, Chiu RG, et al. Vagal nerve stimulation for epilepsy in adults: a database risk analysis and review of the literature. World Neurosurg. 2019;121:e947–e953.
- CMS.gov. Hospital-acquired condition reduction program; 2022 [cited 2023 Jan 11]. Available from: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/HAC-Reduction-Program
- LivaNova. Data on file. Extracted from internal database on 06/09/2020; 2020.
- Messori A, Trippoli S, Becagli P, et al. Adjunctive lamotrigine therapy in patients with refractory seizures: a lifetime cost-utility analysis. Eur J Clin Pharmacol. 1998;53(6):421–427.
- Devlin NJ, Tsuchiya A, Buckingham K, et al. A uniform time trade off method for states better and worse than dead: feasibility study of the ‘lead time’ approach. Health Econ. 2011;20(3):348–361.
- Jiang R, Janssen MFB, Pickard AS. US population norms for the EQ-5D-5L and comparison of norms from face-to-face and online samples. Qual Life Res. 2021;30(3):803–816.
- Kimberly W. McDermott LL. Overview of operating room procedures during inpatient stays in U.S. hospitals, 2018 – Statistical brief #281; 2021 [cited 2022 Nov 3]. Available from: https://www.hcup-us.ahrq.gov/reports/statbriefs/sb281-Operating-Room-Procedures-During-Hospitalization-2018.jsp#:∼:text=In%202018%2C%209.6%20million%20inpatient,females%20aged%2018%2D44%20years
- Brian J. Moore PD, Liang L. Ph.D. costs of emergency department visits in the United States 2017; 2020.
- BLS. Medical Care Services: Consumer Price Index; 2016, in United States. Washington (DC): Department of Labor; 2016.
- CMS. Physician fee schedule pricing_information-99213, d.e. Baltimore: CMS; 2022.
- Bernstein AL, Hess T. Vagus nerve stimulation therapy for pharmacoresistant epilepsy: effect on health care utilization. Epilepsy Behav. 2007;10(1):134–137.
- Manjunath R, Paradis PE, Parisé H, et al. Burden of uncontrolled epilepsy in patients requiring an emergency room visit or hospitalization. Neurology. 2012;79(18):1908–1916.
- Sheikh SR, Kattan MW, Steinmetz M, et al. Cost-effectiveness of surgery for drug-resistant temporal lobe epilepsy in the US. Neurology. 2020;95(10):e1404–e1416.
- LivaNova. VNS therapy codes. Data on file; 2022.
- LivaNova. Price list. Data on file; 2022.
- AMA. Resource-Based Relative Value Scale (RBRVS): frequency data-64568; 2018.
- Abel TJ, Remick M, Welch WC, et al. One-year cost-effectiveness of callosotomy vs vagus nerve stimulation for drug-resistant seizures in Lennox-Gastaut syndrome: a decision analytic model. Epilepsia Open. 2022;7(1):124–130.
- Cho Y-J, Heo K, Kim W-J, et al. Long-term efficacy and tolerability of topiramate as add-on therapy in refractory partial epilepsy: an observational study. Epilepsia. 2009;50(8):1910–1919.
- Husain A, Chung S, Faught E, et al. Long-term safety and efficacy in patients with uncontrolled partial-onset seizures treated with adjunctive lacosamide: results from a phase III open-label extension trial. Epilepsia. 2012;53(3):521–528.
- Kuba R, Novotná I, Brázdil M, et al. Long-term levetiracetam treatment in patients with epilepsy: 3-year follow up. Acta Neurol Scand. 2010;121(2):83–88.
- Tomson T. Mortality in epilepsy. J Neurol. 2000;247(1):15–21.
- Boon P, Vonck K, van Rijckevorsel K, et al. A prospective, multicenter study of cardiac-based seizure detection to activate vagus nerve stimulation. Seizure. 2015;32:52–61.
- Fisher RS, Eggleston KS, Wright CW. Vagus nerve stimulation magnet activation for seizures: a critical review. Acta Neurol Scand. 2015;131(1):1–8.
- Gupta S, Ryvlin P, Faught E, et al. Understanding the burden of focal epilepsy as a function of seizure frequency in the United States, Europe, and Brazil. Epilepsia Open. 2017;2(2):199–213.