Abstract
Background
The chimeric antigen receptor (CAR) T-cell therapy idecabtagene vicleucel (ide-cel) is approved for the treatment of adult patients with relapsed/refractory multiple myeloma (RRMM) who have already received an immunomodulatory agent, a proteasome inhibitor, and an anti-CD38 antibody and have progressed on their last therapy. The objective of this study was to assess the cost-effectiveness of ide-cel versus conventional care in Canada and France.
Methods
A partitioned survival model was used to estimate the cost-effectiveness of ide-cel (target dose 450 × 106 CAR T cells) in its approved indication in terms of life-years (LYs), quality-adjusted LYs (QALYs), and costs. Patient-level data from the KarMMa Phase II clinical trial (clinicaltrials.gov NCT03361748) and KarMMa-RW study were used to inform the model; overall and progression-free survival were extrapolated using standard parametric functions after the observed periods. The model adopted Canadian and French societal perspectives over a lifetime horizon. Costs, utilities, discounting (Canada: 1.5%, France: 2.5%), and general population mortality were country-specific.
Results
The base case demonstrated that ide-cel was associated with more additional LYs (+2.64 and +2.51) and QALYs (+2.31 and +2.54) than conventional care at incremental costs of CAN$588,490 and €392,251 in Canada and France, respectively. The resulting incremental cost-effectiveness ratio (ICER) for ide-cel was $255,245 per QALY in Canada, and €154,593 per QALY in France.
Conclusion
Ide-cel was associated with significant survival improvements in terms of both LYs and QALYs in patients with progressive triple-class-exposed RRMM. The ICER for ide-cel was similar to that of other approved and reimbursed RRMM therapies.
1. Introduction
Multiple myeloma (MM) has a worldwide incidence of 160,000 cases annually and is most common in late adulthood, with two-thirds of newly diagnosed MM patients being over 65 years of ageCitation1. Treatment advances over the past two decades, particularly the introduction of immunomodulatory agents, proteasome inhibitors, and anti-CD38 monoclonal antibodies, have improved MM outcomes in early treatment lines. By contrast, the prognosis for patients who develop relapsed or refractory MM (RRMM) remains poor and treatment options remain limited, particularly in later lines. Historically, conventional care for later line RRMM has been with doublet or triplet salvage chemotherapy regimens such as carfilzomib-dexamethasone (Kd), dexamethasone-pomalidomide (Pd), carfilzomib-dexamethasone-pomalidomide (KPd), daratumumab-dexamethasone-pomalidomide (DPd), and carfilzomib-cyclophosphamide-dexamethasone (KCd). Overall, the median overall survival (OS) for RRMM patients treated with conventional care is only 9.3 months and the median progression-free survival (PFS) is only 3.4 monthsCitation2.
Idecabtagene vicleucel (ide-cel) is a novel B-cell maturation antigen (BCMA)-directed chimeric antigen receptor (CAR)-modified T cell therapy approved in 2021 by the United States (US) Food and Drug Administration for the treatment of triple-class-exposed (TCE) patients (i.e. patients who have already received an immunomodulatory agent, a proteasome inhibitor, and an anti-CD38 monoclonal antibody) who have received four or more prior lines of therapyCitation3. In ex-US markets such as Europe, Canada, and Japan, ide-cel is approved for the treatment of TCE patients who have demonstrated disease progression on their last therapyCitation4–6. In those markets, patients would tend to receive ide-cel after three prior lines of therapy. Approvals were based on Phase II, single-arm trial (KarMMa; clinicaltrials.gov NCT03361748); in Europe, the approved treatment consists of a single infusion with a target dose of 420 × 106 CAR-positive viable T cells (equivalent to 450 × 106 CAR-positive T cells) and in Canada, ide-cel is approved with a target dose of 450 × 106 CAR-positive T cellsCitation4,Citation5.
There are numerous cost-effectiveness analyses of conventional care in patients with MM at different lines of treatmentCitation7. The objective of this study was to develop a cost-effectiveness analysis for ide-cel vs. conventional care for the treatment of RRMM in TCE patients who have demonstrated disease progression on their last therapy. Another objective was to assess the comparative economic and clinical value of ide-cel in a broader RRMM context.
2. Methods
2.1. Overview
In line with previous economic evaluations and health technology assessments (HTAs) of treatments for RRMM, a partitioned survival model was developed to evaluate the cost-effectiveness of ide-cel (target dose 450 × 106 CAR T cells, equivalent to the dose approved by the European Medicines Agency) vs. conventional care for the treatment of TCE RRMM patients who have received at least three prior lines of treatment and have demonstrated disease progression on the last therapy, over a lifetime horizon (15 years)Citation8–12. The core model was subsequently adapted for different countries and healthcare systems. In this manuscript, we describe the model adaptations for the Canadian and French societal perspectives.
For ide-cel, OS and PFS data were derived from the KarMMa Phase II trial (clinicaltrials.gov NCT03361748)Citation13. For conventional care, survival data were derived from KarMMa-RW, a global patient-level retrospective study that included real-world TCE RRMM patients with characteristics like those of the KarMMa population but treated with conventional careCitation14. The two study populations were balanced using propensity scoring and inverse probability of treatment weighting. Survival beyond the study observation periods was extrapolated using standard parametric functions and country-specific general population mortality. The model tracked progression, post-progression therapy, and direct medical expenditures over time. Subsequent costs were accounted for in both treatment strategies.
The model estimated the cost-effectiveness of ide-cel for RRMM in terms of life-years (LYs), quality-adjusted LYs (QALYs), and lifetime costs, with the primary outcome being the incremental cost-effectiveness ratio (ICER). Costs were reported in 2021 Canadian dollars (CAN$) for Canada and Euros (€) for France. Costs, utilities, discount rates, and general population mortality were country-specific. Base case assumptions are summarized in Table S1.
2.2. Model structure
The model consisted of three health states: pre-progression, post-progression, and death (Figure S1). Health state occupancy, i.e. the proportion of patients in each health state, was estimated using the area under the PFS and OS curves in the two studies. All patients entered the model in the pre-progression state. The proportion of patients moving to the post-progression state was calculated as the difference between the probability of surviving with/without progression over time (i.e. OS in the population) and that of remaining free from progression over time (i.e. PFS in the population). The occurrence of adverse events (AEs) was accounted for in both treatment strategies in terms of influence on cost and disutility estimates for the pre-progression health state but did not affect health state transition probabilities. A 1-month cycle length was applied to accommodate a variety of treatment administration schedules.
2.3. Clinical data
Clinical data for ide-cel were derived from the single-arm KarMMa trial at a median follow-up of 24 monthsCitation13,Citation15. For patients receiving ide-cel at a target dose of 450 × 106 CAR-positive T cells, the ORR was 82%, the median PFS was 12.2 months, and the median OS was 24.8 months. The most common any-grade AEs were cytopenias (96.9%) and cytokine release syndrome (CRS; 83.6%).
For conventional care, clinical data were derived from KarMMa-RW, a global non-interventional, retrospective, multi-center study to generate real-world evidence on TCE patients with RRMMCitation14. The data for KarMMa-RW were retrospectively collected from patients in North America and Europe from several data sources including clinical sites, the Connect® MM Registry, the COTA database, the Flatiron Health electronic health record database, and the Guardian Research Network. RRMM patients (n = 190) for KarMMa-RW were selected based on the eligibility criteria of the KarMMa trial. A patient-level analysis was conducted utilizing propensity scores to balance the KarMMa and KarMMa-RW populations for covariates that can influence treatment selection. The propensity scores were then evaluated to generate the relative treatment effects between ide-cel and conventional care using a trimmed inverse probability of treatment weighting approachCitation14.
The efficacy data for conventional care were generated based on >90 different regimens in KarMMa-RW. Thus, an assumption was made that the clinical efficacy across different regimens was equal. For patients receiving conventional care, the ORR was 31.4%, the median PFS was 3.5 months, and the median OS was 14.2 months. AEs were not evaluated in the KarMMa-RW study. Thus, in the cost-effectiveness model, the same set of grade 3 and 4 AEs that were selected for the ide-cel arm was selected for the conventional care arm. The AE occurrence rates with conventional care for Canada were informed by the literatureCitation16–20. The AE occurrence rates with conventional care for France were assumed to be represented by those of the three anti-myeloma combination chemotherapy regimens most frequently used in the study (i.e. KPd, KCd, DPd); inputs were gathered from the key clinical trials of these therapiesCitation21–23.
The analysis uses economic modelling to develop a CEA analysis for ide-cel vs. conventional care and therefore patient consent or ethics approval was not required for this study. The KarMMa clinical trialCitation8 (p. 705–716) was conducted in accordance with the International Council for Harmonization guidelines for Good Clinical Practice and the principles of the Declaration of Helsinki. All patients provided informed consent. For the KarMMa-RW studyCitation14 (p. 116), data collection was retrospective and each data source was in compliance with applicable national and local ethical, legal, and privacy regulations.
2.4. Survival analyses
Due to the violation of the proportional hazard assumption within the study period (Figure S2), parametric curves were fitted to the PFS and OS from the KarMMa trial and KarMMa-RW study independently and were extrapolated over the lifetime of patients. The Kaplan-Meier PFS and OS extrapolations for the ide-cel and conventional care populations are presented in Figure S3.
Seven standard parametric models were tested for OS and PFS: exponential, Weibull, log-normal, log-logistic, generalized gamma, gamma, and Gompertz distributions. The within-trial fit and the clinical plausibility of the extrapolations over the lifetime horizon were both assessed to identify the most appropriate distribution. The base case model was based on goodness-of-fit statistics (Akaike Information Criterion and Bayesian Information Criterion), visual inspection of fit to the observed Kaplan-Meier data, and clinical plausibility. Long-term PFS and OS outcomes were also presented to clinical experts to assess whether the extrapolation of PFS and OS beyond the study observation periods was reasonable and aligned with clinical expectations. In the base-case, the log-normal and exponential distribution was set as the default distribution for PFS and OS curves for ide-cel, respectively. For conventional care, the gamma distribution was set as the default distribution for both PFS and OS curves. Country-specific general mortality was applied when the age-projected mortality from the parametric distributions was lower than the age-specific mortality of the general populations of Canada and France.
2.5. Cost inputs
The analysis included costs associated with drug acquisition and administration, treatment-related monitoring, routine care, AE management, subsequent treatment, terminal care, and productivity losses. All relevant costs in the model are reported in 2021 CAN$ and Euros; therefore, where relevant, unit costs were inflated to 2021 prices using the consumer price indexes (CPIs) from Canada and France (). Costs were discounted at 1.5% for Canada and 2.5% for France.
Table 1. Cost inputs included in the model.
Ide-cel pre-treatment costs included leukapheresis, bridging therapy, and lymphodepleting chemotherapy (LDC) costs. These were applied before the first cycle of the model. The model included a one-time administration cost for ide-cel. In addition, post-infusion, a 14-day hospitalization was assumed to monitor for symptoms of CRS or neurotoxicity. Treatment-related monitoring costs (i.e. radiological, and biological exams) for ide-cel were also incorporated into the model.
Conventional care costs were based on the list of relevant comparators for each country. For Canada, conventional care costs were represented by a weighted average cost of a mix of treatment regimens based on the recommended distribution by the Canadian Agency for Drugs and Technologies in Health (CADTH)Citation24. For France, the drug acquisition cost of conventional care was also the average cost of a mix of treatment regimens based on their distribution in KarMMa-RWCitation14 and French market shares.
Routine care costs, including haematologist visits, hospitalization, concomitant drug use, and concomitant procedures/surgeries were incorporated into the model for both ide-cel and conventional care. Costs of AEs were included for both treatments for all grade 3/4 AEs that occurred in ≥5% of patients included in the KarMMa trial and assuming that grade 3 and above AEs require hospitalization in all cases. AE-related costs are provided in Table S2 of the supplementary materials.
The model included a one-off cost of subsequent treatment at progression, which was the same for both ide-cel and conventional care. For Canada, the cost of subsequent treatment was assumed to be 100% based on the cost of cyclophosphamide + dexamethasone. For France, the cost of subsequent treatment was based on a mix of treatment options used in the fifth-line and beyond in the French market. Terminal care was applied as a one-off cost for patients entering the “death” health state. Finally, the model included costs associated with productivity loss among RRMM patients obtained from published literature using the human capital approachCitation25,Citation26. The productivity loss was applied for 5 years based on a starting age of 60.5 years and assuming a general retirement age of 65 yearsCitation27.
2.6. Health state utilities
Health state utilities represent the strength of individuals’ preferences for different health states on a scale from 0 (death) to 1 (perfect health). The utility estimates for ide-cel were derived from patient-level EQ-5D-5L questionnaire data collected in KarMMa, and summarized separately for the pre-progression (for month 1 0.783 in Canada and 0.864 in France; for month 2 and beyond 0.825 in Canada and 0.901 in France) and post-progression health states (0.796 in Canada and 0.881 in France) (). Country-specific tariffs for Canada and France were applied to derive the utilities used in the model. The utility value of ide-cel in the pre-progression state was split into ‘month 1′ and ‘month 2 and beyond’ to reflect that the single infusion of ide-cel in the first month can be associated with a disutility.
Table 2. Utility estimates for idecabtagene vicleucel and conventional care for Canada and France.
The utility values for conventional care pre-progression (0.795 in both Canada and France) and post-progression (0.676 in both Canada and France) were derived from the KarMMa trial, KarMMa-RW study, and published literature. The utility estimate for the pre-progression state was calculated as the utility values of both patients with a very good partial tumour response and patients who did not achieve a very good partial tumour response in the KarMMa trial, weighted by the distribution of these patient cohorts in the KarMMa-RW study. The utility value of conventional care in the post-progression state was obtained from the Weisel et al. studyCitation28. As with the clinical data, the utility values were assumed to be the same for all conventional care regimens.
Disutilities associated with AEs were applied for conventional care based primarily on previous HTA submissions to the United Kingdom National Institute for Health and Care Excellence (NICE)Citation9. No separate AE-related disutility were applied for the ide-cel arm in the model; these were accounted for in the utility estimates derived from the EQ-5D-5L in the KarMMa trial.
2.7. Outcomes
We estimated expected LYs, QALYs, and total costs for the ide-cel and conventional care strategies. The ICER was also calculated as the ratio of the difference in costs and the difference in effects (e.g. QALYs) between strategies.
2.8. Sensitivity analyses
One-way deterministic sensitivity analyses (DSA) and probabilistic sensitivity analyses (PSA) were conducted to assess the impact of uncertainties in the model parameters on the outcomes of the base case.
In the one-way DSA, key parameters were varied using their lower and upper bounds based on 95% confidence intervals (CIs) either as reported or where unavailable based on a 10% variation around the parameter point estimate (Table S3). The results were summarized as a tornado diagram to highlight the variables that had the most impact on the ICER.
With the PSA, new parameter values were sampled from the (posterior) distributions for efficacy, safety, utilities, and costs for each iteration of the model. The model was evaluated by averaging output values from a second-order Monte Carlo simulation run for 1000 iterations, which accounted for uncertainty in model parameters. Next, the incremental cost, incremental QALYs, and ICER were calculated. Uncertainty in the outcomes of interest was expressed with 95% CIs (Table S3). The joint uncertainty distribution of incremental costs and QALYs was plotted on a cost-effectiveness plane. The key parameters tested in the PSA included OS and PFS parameters (drawn from corresponding parametric survival functions with covariant matrix between shape and scale parameters), utilities (drawn from beta distributions), and AE disutilities and costs (drawn from gamma distributions).
3. Results
3.1. Base case
In the base case analysis, the ide-cel cohort accrued discounted LYs of 4.17 and 4.03 and discounted QALYs of 3.37 and 3.57 at a discounted cost of CAN$739,818 and €562,626 in Canada and France, respectively. The conventional care cohort accrued corresponding discounted LYs of 1.53 and 1.51 and discounted QALYs of 1.06 and 1.03 at discounted costs of CAN$ 151,3128 and €170,376. This equated to incremental gains in LYs of 2.64 and 2.51 and QALYs of 2.31 and 2.54 with ide-cel versus conventional care at discounted incremental costs of CAN $588,490 and €392,251 in Canada and France, respectively. The resulting ICER for ide-cel was CAN $255,245 per QALY gained in Canada, and €154,593 per QALY gained in France ( and ).
Table 3. Base case analysis – incremental costs and outcomes for Canada.
Table 4. Base case analysis – incremental costs and outcomes for France.
3.2. Deterministic sensitivity analysis results
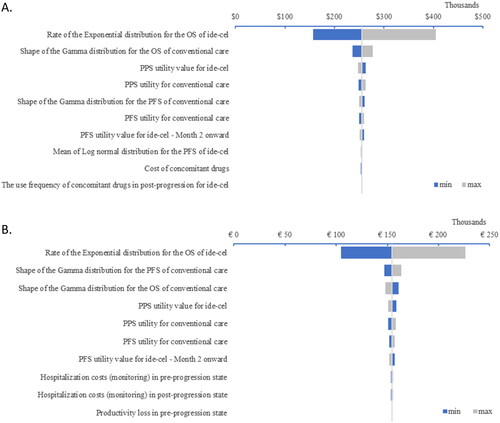
The greatest drivers of uncertainty in the cost-effectiveness analysis are shown in . ICERs were most influenced by parameters related to the relative efficacy of interventions in terms of PFS and OS, and by the utility values of interventions.
Figure 1. Deterministic sensitivity analysis results in Canada (A) and France (B). These analyses were conducted to assess the impact of uncertainties in the model parameters on the outcomes of the base case. The tornado diagram highlights the variables that had the most impact on the incremental cost-effectiveness ratio. Abbreviations. OS, overall survival; PFS, progression-free survival; PPS, post-progression survival.
3.3. Probabilistic sensitivity analysis results
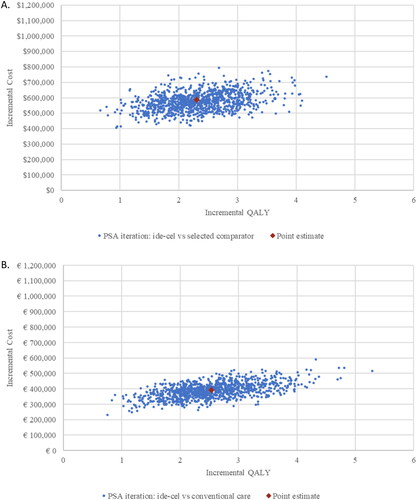
The results of 1000 Monte Carlo simulations for Canada and France are displayed in the incremental cost-effectiveness plane in . All simulations indicated that ide-cel is associated with higher costs but also more QALYs gained.
Figure 2. Probabilistic sensitivity analysis results, cost-effectiveness plane in Canada (A) and France (B). These analyses were conducted to assess the impact of uncertainties in the model parameters on the outcomes of the base case. In the figures, the joint uncertainty distribution of incremental costs and QALYs is plotted on a cost-effectiveness plane. Abbreviations. PSA, probabilistic sensitivity analysis; QALY, quality-adjusted life-year.
4. Discussion
Patients with RRMM who have already received an immunomodulatory agent, a proteasome inhibitor, and an anti-CD38 monoclonal antibody and who have demonstrated disease progression on their last therapy have few treatment options and a poor prognosis. The higher efficacy of ide-cel versus conventional care has been established through an indirect treatment comparison with patient-level data from KarMMa-RWCitation14, but the economic implications of this treatment have not been previously evaluated. In this study, ide-cel was compared with conventional care in terms of both costs and efficacy using a three-state partitioned survival model. The results from the model demonstrate that ide-cel was significantly more effective than conventional care over a lifetime horizon. A single infusion with ide-cel yielded more incremental LYs (+2.64 and +2.51) and QALYs (+2.31 and +2.54) gained in Canada and France compared with continuous therapy with conventional care.
The higher total costs associated with ide-cel were predominantly driven by the drug acquisition costs as well as improved OS, resulting in additional costs incurred over a longer period of time than with conventional care. In addition to the immediate cost of therapy, other costs are potential concerns for patients receiving CAR T therapies. CRS and neurotoxicity have been identified as major AEs associated with CAR T therapy and are associated with increased healthcare costs. Thus, in the model, the total cost of AEs was higher for ide-cel than for conventional care (total cost of AEs with ide-cel in Canada $21,736 and in France €10,607; total cost of AEs with conventional care in Canada $4,667 and in France €2,762). However, the AE profile of ide-cel is manageable, similar to that of other CD-19 CAR TsCitation29,Citation30.
No significant reduction in health-related quality of life (HRQoL) or LYs gained related to AEs has been observed for ide-cel. In fact, the results of KarMMa indicate a significant and clinically meaningful improvement in most HRQoL measuresCitation13,Citation31. Clinically meaningful improvements were achieved by month 1 post-ide-cel infusion for pain and disease symptoms and by month 2 for fatigue, cognitive functioning, and global health scale; these effects persisted for 15–18 months after a single infusion of ide-cel.
The model results associated with the total costs and QALYs for conventional care were validated against previous HTA submissions and ICER report on chemotherapies in late-line MM treatment, which showed costs for conventional care ranging from CAN $100,876 to CAN $165,113 and total QALYs rangingCitation24,Citation32 from 1.00 to 1.15. Previous therapies for MM have generated an incremental QALY gainCitation11,Citation33,Citation34 of 0.39 to 0.97. In comparison, a single infusion of ide-cel was associated with an incremental QALY gain two to three times greater than that of conventional care, suggesting that ide-cel treatment has the ability to generate unprecedented clinical benefit for patients.
Furthermore, the ICER of ide-cel vs. conventional care (CAN$255,245 per QALY gained for Canada and €154,593 per QALY gained for France) falls into the same range as other novel treatments for heavily pretreated RRMM, such as daratumumab-bortezomib-dexamethasone (DVd; ICER CAN $394,401/€333,391, adjusted for inflation and currency converted) vs. bortezomib-dexamethasone and Pd (ICER CAN$312,794/€264,407 adjusted for inflation and currency converted) vs. carfilzomibCitation35,Citation36. Notably, these are therapies that have been widely approved for reimbursement in heavily pretreated RRMM patients. For example, although traditionally Canada favours a willingness-to-pay threshold of CAN $50,000, both Pd and DVd were recommended for reimbursement with conditions by CADTH. Similarly, the French Haute Autorité de Santé has recommended therapies such as daratumumab-lenalidomide-dexamethasone and isatuximab-Pd for RRMM, despite both regimens having ICERs of >€500,000.
Importantly, in this study, the cost-effectiveness of ide-cel was evaluated based on adopting a conservative approach to estimating treatment effects and relevant cost-effectiveness outcomes. Treatment with ide-cel was associated with a much higher response rate than conventional care, with an ORR of 82% versus only 31.4%. This was not taken into account in the partitioned survival model structure, which included no differentiation of OS and PFS for patients with long-term response to therapy and prolonged survival. Different methodologies, such as mixture-cure or response-based model structures, would better capture these benefits and could be explored in the future for ide-celCitation37,Citation38.
5. Study advantages and limitations
This is one of the first studies to estimate the cost-effectiveness of CAR T therapy for TCE RRMM patients who have progressed on their last therapy. The main strength of the analysis is the use of patient-level data for both ide-cel and conventional care to estimate the most robust relative treatment effect in the absence of head-to-head data. The main limitation is the immaturity of the OS, and possibly PFS, data for ide-cel. To account for uncertainty in the OS and PFS extrapolations used in the analysis, extensive sensitivity analyses were conducted to determine the impact of this limitation on the final cost-effectiveness outcomes. In addition, the long-term estimates of OS and PFS with standard parametric models were validated by clinical experts. Although additional survival models, such as piecewise, mixture-cure, or spline-based models, could be used to further determine the impact of the survival extrapolations on outcomes, for the current model a conservative approach was used to determine the cost-effectiveness of ide-cel vs. conventional care, in line with previously published health-economic research on RRMM patients.
It is important to note that although the model accounted for indirect costs such as productivity loss as well as direct healthcare costs, for some patients there may be additional costs that it was not possible to include – for example, costs related to caregivers or the need to travel to a CAR T treatment centre. Other assumptions made in the model, such as the utility data used for conventional care, were also tested in sensitivity and scenario analyses and no significant impact on the ICER was observed.
6. Conclusions
Progressive RRMM in patients who are TCE is associated with severe morbidity and premature mortality and represents an area of unmet need for effective treatment. Ide-cel, a novel therapy recently approved for this patient population, has been shown to increase both OS and PFS in these patients. In this cost-effectiveness modelling study, ide-cel was associated with significant improvements in LYs and QALYs. Though associated with higher costs, the ICER for ide-cel was similar to that of other approved and reimbursed RRMM therapies. These findings can inform both healthcare providers and payers regarding treatment decision-making from the clinical and economic value perspective.
Transparency
Acknowledgements
Medical writing and editorial support were provided by LATITUDE (Powered by AXON) and were funded by Bristol Myers Squibb.
Reviewer disclosures
Peer reviewers on this manuscript have received an honorarium from JME for their review work but have no other relevant financial relationships to disclose.
Supplemental Material
Download MS Word (447.8 KB)Declaration of financial/other relationships
KK, F-EC, and DD are employees of Bristol Myers Squibb. WZ and MV are employees of Precision Health, who were commissioned by Bristol Myers Squibb to conduct/be involved in this study.
Additional information
Funding
References
- Ludwig H, Novis Durie S, Meckl A, et al. Multiple myeloma incidence and mortality around the globe; interrelations between health access and quality, economic resources, and patient empowerment. Oncologist. 2020;25(9):e1406-13–e1413.
- Gandhi UH, Cornell RF, Lakshman A, et al. Outcomes of patients with multiple myeloma refractory to CD38-targeted monoclonal antibody therapy. Leukemia. 2019;33(9):2266–2275.
- Celgene. ABECMA (idecabtagene vicleucel) [prescribing information]. Summit, NJ: Celgene Corporation, a Bristol-Myers Squibb Company; 2021.
- Celgene. Abecma (idecabtagene vicleucel) [summary of product characteristics]. Utrecht, Netherlands: Celgene Europe B.V; 2021.
- Bristol Myers Squibb. Health Canada approves ABECMATM (idecabtagene vicleucel), the first and only anti-BCMA CAR T cell therapy for relapsed and refractory multiple myeloma. 2021. Available from: https://www.bms.com/ca/en/media/press-release-listing/2021-05-31-press-release.html#:∼:text=Health%20Canada%20Approves%20ABECMATM,Relapsed%20and%20Refractory%20Multiple%20Myeloma&text=%E2%80%9CToday’s%20approval%20is%20an%20important,i. n%20Canada%2C%E2%80%9D%20says%20Dr.Pu.
- Bristol Myers Squibb. Bristol Myers Squibb receives approval for Abecma (idecabtagene vicleucel), the first CAR T therapy approved for the treatment of multiple myeloma in Japan; 2022. Available from: https://news.bms.com/news/details/2022/Bristol-Myers-Squibb-Receives-Approval-for-Abecma-idecabtagene-vicleucel-the-First-CAR-T-Therapy-Approved-for-the-Treatment-of-Multiple-Myeloma-in-Japan/default.aspx.
- Seefat MR, Cucchi DGJ, Dirven S, et al. A systematic review of cost-effectiveness analyses of novel agents in the treatment of multiple myeloma. Cancers. 2021;13(22):5606.
- National Institute for Health Care and Excellence [INTERNET]. Panobinostat for treating multiple myeloma after at least 2 previous treatments [TA 380]; 2016. Available from: https://www.nice.org.uk/guidance/ta380.
- National Institute for Health Care and Excellence [INTERNET]. Daratumumab monotherapy for treating relapsed and refractory multiple myeloma: technology appraisal guidance [TA510]; 2018. Available from: https://www.nice.org.uk/guidance/ta510.
- National Institute for Health Care and Excellence [INTERNET]. Pomalidomide for multiple myeloma previously treated with lenalidomide and bortezomib [TA 427]; 2016. Available from: https://www.nice.org.uk/guidance/ta427.
- National Institute for Health Care and Excellence [INTERNET]. Ixazomib with lenalidomide and dexamethasone for treating relapsed or refractory multiple myeloma [TA505]; 2018. Availalble from: https://www.nice.org.uk/guidance/ta505/.
- Beinfeld M, Lee S, McQueen B, et al. Anti B-cell maturation antigen CAR T-cell and antibody drug conjugate therapy for heavily pretreated relapsed and refractory multiple myeloma. J Manag Care Spec Pharm. 2021;27(9):1315–1320.
- Anderson LD, Shah N, Jagannath S, et al. OAB-027: Idecabtagene vicleucel (ide-cel, bb2121), a BCMA-directed CAR T-cell therapy, for the treatment of patients with relapsed and refractory multiple myeloma (RRMM): updated results from KarMMa. Clin Lymphoma Myeloma Leuk. 2021;21(Suppl 2):S17–S18.
- Jagannath S, Lin Y, Goldschmidt H, et al. KarMMa-RW: comparison of idecabtagene vicleucel with real-world outcomes in relapsed and refractory multiple myeloma. Blood Cancer J. 2021;11(6):116.
- Munshi NC, Anderson LD, Jr, Shah N, et al. Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N Engl J Med. 2021;384(8):705–716.
- Belcher SM, Watkins Bruner D, Hofmeister CC, et al. Characterizing pain experiences: african American patients with multiple myeloma taking around-the-clock opioids. Clin J Oncol Nurs. 2020;24(5):538–546.
- Benyounes A, Ma N, Kocoglu M, et al. Racial disparities in multiple myeloma patients with durable stringent complete response. Clin Lymphoma Myeloma Leuk. 2019;19(10):e287.
- Blue BJ, Luo S, Sanfilippo KM, et al. Race-based differences in routine cytogenetic profiles of patients with multiple myeloma. Br J Haematol. 2017;176(2):322–324.
- Boyd KD, Ross FM, Chiecchio L, et al. Gender disparities in the tumor genetics and clinical outcome of multiple myeloma. Cancer Epidemiol Biomarkers Prev. 2011;20(8):1703–1707.
- Bunce CM, Drayson MT. Dissecting racial disparities in multiple myeloma-clues from differential immunoglobulin levels. Blood Cancer J. 2020;10(4):44.
- Chari A, Vogl DT, Gavriatopoulou M, et al. Oral selinexor-dexamethasone for triple-class refractory multiple myeloma. N Engl J Med. 2019;381(8):727–738.
- Shah JJ, Stadtmauer EA, Abonour R, et al. Carfilzomib, pomalidomide, and dexamethasone for relapsed or refractory myeloma. Blood. 2015;126(20):2284–2290.
- Bringhen S, Petrucci MT, Larocca A, et al. Carfilzomib, cyclophosphamide, and dexamethasone in patients with newly diagnosed multiple myeloma: a multicenter, phase 2 study. Blood. 2014;124(1):63–69.
- Canadian Agency for Drugs and Technologies in Health [INTERNET]. CADTH reimbursement review: idecabtagene vicleucel (Abecma): Canadian Journal of Health Technologies; 2022. Available from: https://doi.org/https://www.cadth.ca/sites/default/files/DRR/2022/PG0240-Abecma.pdf
- Prica A, Hay AE, Crump M, et al. Evaluating the indirect costs of care associated with salvage chemotherapy for relapsed and refractory aggressive-histology lymphoma: a subset analysis of the Canadian cancer trials group (CCTG) LY.12 clinical trial. Curr Oncol. 2021;28(2):1256–1261.
- Jackson G, Galinsky J, Alderson DC, et al. Productivity losses in patients with newly diagnosed multiple myeloma following stem cell transplantation and the impact of maintenance therapy. Eur J Haematol. 2019;103(4):393–401.
- Statistics Canada. Table 14-10-0060-01 Retirement age by class of worker, annual. [last updated 2023 Jan 6]. DOI:10.25318/1410006001-eng
- Weisel K, Paner A, Engelhardt M, et al. Quality-of-life outcomes in patients with relapsed/refractory multiple myeloma treated with elotuzumab plus pomalidomide and dexamethasone: results from the phase 2 randomized ELOQUENT-3 study. Blood. 2018;132(Supplement 1):2288–2288.
- Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large b-cell lymphoma. N Engl J Med. 2019;380(1):45–56.
- European Medicines Agency [INTERNET]. CARVYKTI (Ciltacabtagene Autoleucel) Summary of product characteristics; 2022. Avaialble from: https://www.ema.europa.eu/en/documents/product-information/carvykti-epar-product-information_en.pdf.
- Delforge M, Shah N, Miguel JSF, et al. Health-related quality of life with idecabtagene vicleucel in relapsed and refractory multiple myeloma. Blood Adv. 2022;6(4):1309–1318.
- Institute for Clinical and Economic Review [INTERNET]. Anti b-cell maturation antigen CAR T-cell and antibody drug conjugate therapy for heavily pre-treated relapsed and refractory multiple myeloma. 2021. Available from: https://icer.org/wp-content/uploads/2020/10/ICER_Multiple-Myeloma_Final-Report_Update_070921.pdf.
- Canadian Agency for Drugs and Technologies in Health [INTERNET]. Pan-canadian oncology drug review registered clinician feedback on a pCODR expert review committee initial recommendation pomalidomide (Pomalyst) for multiple myeloma; 2014. Available from: https://www.cadth.ca/sites/default/files/pcodr/pcodr-pomalyst-mm-fn-egr.pdf.
- Scottish Medicines Consortium [INTERNET]. Daratumumab (Darzalex). Advice. SMC1205/17; 2017. Available from: https://www.scottishmedicines.org.uk/medicines-advice/daratumumab-darzalex-resubmission-120517/.
- Pelligra CG, Parikh K, Guo S, et al. Cost-effectiveness of pomalidomide, carfilzomib, and daratumumab for the treatment of patients with heavily pretreated relapsed-refractory multiple myeloma in the United States. Clin Ther. 2017;39(10):1986–2005.e5.
- Zhang TT, Wang S, Wan N, et al. Cost-effectiveness of daratumumab-based triplet therapies in patients with relapsed or refractory multiple myeloma. Clin Ther. 2018;40(7):1122–1139.
- Roth JA, Sullivan SD, Lin VW, et al. Cost-effectiveness of axicabtagene ciloleucel for adult patients with relapsed or refractory large B-cell lymphoma in the United States. J Med Econ. 2018;21(12):1238–1245.
- Qi CZ, Bollu V, Yang H, et al. Cost-effectiveness analysis of tisagenlecleucel for the treatment of patients with relapsed or refractory diffuse large B-cell lymphoma in the United States. Clin Ther. 2021;43(8):1300–1319.e8.
- Ontario Case Costing Initiative. 2019. https://data.ontario.ca/dataset/ontario-case-costing-initiative-occi.
- Canadian Agency for Drugs and Technologies in Health. Pan-Canadian Oncology Drug Review Registered Clinician Feedback on a pCODR Expert Review Committee Initial Recommendation Daratumumab (Darzalex) for Multiple Myeloma. 2017. https://www.cadth.ca/sites/default/files/pcodr/pcodr_daratumumab_darzalex_mm_2ndln_fn_egr.pdf.
- Canadian Agency for Drugs and Technologies in Health. Pan-Canadian Oncology Drug Review Final Economic Guidance Report Bortezomib (Velcade) for Multiple Myeloma. 2013. https://www.cadth.ca/sites/default/files/pcodr/pcodr-velcademm-fn-egr.pdf.
- Canadian Agency for Drugs and Technologies in Health. Pan-Canadian Oncology Drug Review Final Economic Guidance Report Carfilzomib (Kyprolis) for Multiple Myeloma. 2017. https://www.cadth.ca/sites/default/files/pcodr/pcodr_carfilzomib_kyprolis_mm_rel_fn_egr.pdf.
- Ontario Drug Benefit Formulary. https://www.formulary.health.gov.on.ca/formulary/.
- Aide au Codage. https://www.aideaucodage.fr/ccam.
- Canadian Institute for Health Information. Cost of a Standard Hospital Stay. https://yourhealthsystem.cihi.ca/hsp/inbrief.#!/indicators/015/cost-of-a-standard-hospital-stay/;mapC1;mapLevel2;/.
- Ontario Health Insurance Plan. Schedule of Benefits for Laboratory Services, 2020. https://health.gov.on.ca/en/pro/programs/ohip/sob/lab/lab_mn2020.pdf.
- Autorité de Santé H. Rapport d‘activité. 2015. [Internet]. Disponible sur: https://www.has-sante.fr/upload/docs/application/pdf/2016-07/rapport_activite_2015.pdf.
- Gonzalez-McQuire S, Yong K, Leleu H, et al. Healthcare resource utilization among patients with relapsed multiple myeloma in the UK, France, and Italy. J Med Econ. 2018;21(5):450–467.
- de Oliveira C, Pataky R, Bremner KE, et al. Phase-specific and lifetime costs of cancer care in Ontario, Canada. BMC Cancer. 2016;16(1):809.
