Abstract
Aim
To assess the cost-effectiveness of adjuvant pembrolizumab versus observation for patients with resected stage IIB/IIC melanoma from a third-party payers’ perspective in Switzerland over a lifetime horizon.
Materials and Methods
A Markov state transition model with four health states (recurrence-free [RF], locoregional recurrence, distant metastases [DM], and death) was developed to determine the cost-effectiveness of pembrolizumab versus observation as an adjuvant treatment in patients with stage IIB/IIC melanoma who have undergone complete resection. The model utilized data from the KEYNOTE-716 randomized controlled trial (ClinicalTrials.gov, NCT03553836). The incremental cost-effectiveness ratio (ICER) (Swiss Franc [CHF] per life year or quality-adjusted life years [QALYs] gained) was calculated. A probabilistic sensitivity analysis and deterministic sensitivity analysis were conducted to assess the robustness of the base case results.
Results
Model results demonstrated that pembrolizumab is highly cost-effective as an adjuvant treatment for resected stage IIB/IIC melanoma versus observation in Switzerland. Base case results showed an ICER of CHF 27,424/QALY (EUR 27,342/QALY; exchange rate: 1 CHF = 0.997 EUR) for pembrolizumab versus observation. Results were most sensitive to changes to transition probabilities from the RF state. Most sensitivity and scenario analyses resulted in ICERs below the willingness-to-pay threshold (WTP) of CHF 100,000. At this WTP, pembrolizumab had a 78.9% probability of being cost-effective versus observation.
Limitations
Due to a limited follow-up period in the KEYNOTE-716 trial, data from other clinical trials in the advanced melanoma setting were synthesized in a network meta-analysis and used to inform transition probabilities from DM to death in the cost-effectiveness model, to overcome the absence of these data from the trial.
Conclusion
The model demonstrated that pembrolizumab is highly cost-effective versus observation in patients with resected stage IIB/IIC melanoma in Switzerland. The ICER was below the WTP threshold of CHF 100,000, commonly used for cost-effectiveness models in Switzerland.
Introduction
Melanoma poses a substantial burden to society, with more than 20,000 deaths reported annually in Europe, with higher mortality rates in Nordic and Western Europe compared to Eastern EuropeCitation1. In Switzerland, melanoma is one of the five most common types of cancerCitation2. Between 2014 and 2018, the average number of new malignant melanoma cases per year in Switzerland was 1,600 in men and 1,400 in womenCitation3. In the same time period, the average number of melanoma deaths annually was 180 and 130 in men and women, respectivelyCitation3.
Melanoma staging (0 to IV) is defined using the TNM system based on tumor thickness and ulceration, nearby lymph node involvement, and distant lymph node or organ involvementCitation4. Stage II (A–C) melanoma is localized; however, patients are at high-risk of the tumor spreading to other parts of the body (metastatic melanoma)Citation4. Patients with stage IIB and IIC melanoma have deeper or thicker tumors (>4.0 mm) that often spread to nearby lymph nodesCitation5. For some patients with stage II cutaneous melanoma, surgical resection can be curative. However, surgery may leave some melanoma cells, resulting in an increased risk for recurrenceCitation5. The overall risk of recurrence to locoregional or metastatic disease in patients with stage II melanoma is approximately 27.2%, with a 19–37% probability of cases resulting in mortality within 5 yearsCitation6,Citation7. Of note, high mortality rates are associated with metastatic melanoma, which accounts for 90% of deaths attributable to skin cancerCitation8. For patients with stage IIB or IIC, the melanoma-specific survival probability at 10 years is 94% and 75%, respectivelyCitation9.
For patients with melanoma at high-risk of recurrence, doctors may suggest the use of adjuvant therapy following surgery, to prevent or delay the recurrence of melanomaCitation5. Until recently, adjuvant therapy with interferon (IFN) alpha was the only potential alternative treatment option to observation for patients with melanomaCitation10. However, IFN alpha is associated with several drawbacks including life-threatening toxicities and major impacts on patient health-related quality-of-life (HRQoL)Citation11. Additionally, a meta-analysis of 15 clinical trials reported modest treatment efficacy of IFN alpha for patients with melanoma, with an approximately 3.0% overall survival advantage and 3.5% event-free survival advantage from baseline to 5 yearsCitation12. Due to an unclear indication of specific dose or treatment duration and evidence of significant toxicity, the European Society for Medical Oncology (ESMO) no longer recommends IFN alpha as an adjuvant therapy for melanomaCitation10. Further, the National Comprehensive Cancer Network (NCCN) recommends locoregional radiation therapy as an adjuvant treatment for patients with stage IIB/IIC melanomaCitation13. However, this recommendation is based on category 2B evidence (i.e. lower-level evidence)Citation13. Therefore, observation is considered the standard of care for resected stage II melanoma in SwitzerlandCitation10. In order to decrease the risk of disease recurrence and progression, a clinically effective and cost-effective adjuvant therapy is needed for patients with stage II melanoma.
In Switzerland, metastatic melanoma is associated with a substantial economic burden that increases as the disease progressesCitation8,Citation14. Estimated full treatment pharmacotherapy costs in Switzerland have been reported to range between CHF 1,329.89 and CHF 104,311.59 (CHF of 2015)Citation8,Citation14. On average, monthly outpatient visit costs increase with disease progression, from CHF 27.40 during pre-progression, to CHF 92.40 during progression and to CHF 170.23 during post-progressionCitation8. Therefore, delaying or preventing the recurrence to metastatic melanoma from early-stage melanoma is important to minimize the substantial clinical and economic burden associated with the diseaseCitation2,Citation8.
Pembrolizumab blocks the interaction between programmed cell death protein 1 receptor (PD-1 receptor) and programmed cell-death ligands (PD-L1 and PD-L2), which ultimately re-establishes anti-tumor immunity in affected patientsCitation15. Further, pembrolizumab minimizes immune-related adverse events (AEs) by restoring tumor-induced immune deficiencies in the tumors’ microenvironmentCitation15.
The KEYNOTE-054 double-blinded phase III trial 5-year analysis has shown that adjuvant treatment with pembrolizumab has resulted in a sustained improvement in long-term recurrence-free survival (RFS) (55%) and distant metastasis-free survival (DMFS) (61%) compared with placebo in patients with resected stage III melanomaCitation16. Following these improvements in patients with stage III melanoma, there is an ongoing randomized controlled phase III trial (KEYNOTE-716) assessing the safety and efficacy of adjuvant pembrolizumab in patients with surgically resected stage IIB/IIC melanomaCitation17,Citation18. In the interim analysis of the KEYNOTE-716 trial (N = 976), adjuvant pembrolizumab has been shown to significantly reduce the risk of disease recurrence or death compared to placebo (11% versus 17%, respectively, p = 0.0066)Citation19. Further, the number of patients with a distant recurrence was approximately 50% lower with pembrolizumab compared to placebo (6% versus 12%, respectively, p-value not reported)Citation19.
Based on the evidence from these trials, pembrolizumab has been approved as an adjuvant treatment by the United States’ (US) Food and Drug Administration (FDA) and the European Medicine Agency (EMA) for resected stage IIB/IIC and III melanomaCitation20,Citation21. Furthermore, the NCCN recommends pembrolizumab for the adjuvant treatment of stage IIB/IIC melanoma based on category 1 evidence (i.e. high-level evidence)Citation13. In Switzerland, pembrolizumab was approved by Swissmedic as an adjuvant treatment for resected stage III melanomaCitation22,Citation23. However, it is not yet approved for resected stage II melanomaCitation24.
The aim of this study was to assess the cost-effectiveness of adjuvant pembrolizumab versus observation for patients with resected stage IIB/IIC melanoma in Switzerland from a third-party payers’ perspective.
Materials and methods
Population
The model population consisted of patients aged 12 years or older who underwent complete surgical resection of stage IIB/IIC melanoma. Population eligibility criteria and baseline characteristics were consistent with the KEYNOTE-716 trial (ClinicalTrials.gov, NCT03553836) and aligned with the EMA product label. Overall, 39.7% of the population were female and the starting age of the patient cohort at model entry was 59.3 yearsCitation17,Citation18,Citation21.
Model structure
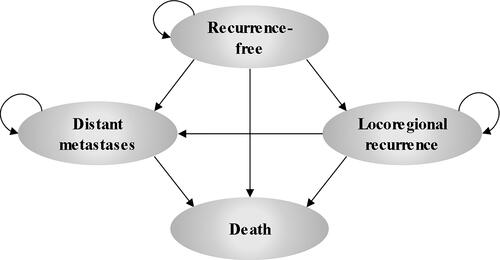
A Markov state transition model with four health states (recurrence-free [RF], locoregional recurrence [LR], distant metastases [DM], and death) was developed () to determine the cost-effectiveness of pembrolizumab versus observation as adjuvant treatment in patients with stage IIB/IIC melanoma who have undergone complete resection. RF is defined as the absence of a local recurrence or occurrence of DM, while LR is defined as the cancer returning in the primary site or nearby and DM refers to a tumor that has spread to areas of the body distant from the primary tumor. RFS, DMFS, and overall survival (OS) curves were generated using the Markov trace. The model had a cycle length of 1 week with half-cycle corrections. In the base case, it was assumed that patients who achieved long-term RFS (≥ 10 years) were cured. The percentage reduction in recurrence risk was assumed to linearly increase from 0% at 7 years to 95% at 10 years and beyond, and applied to efficacy outcomes in both treatment arms. In the base case, no treatment waning was applied as adjuvant pembrolizumab is expected to have an ongoing immunotherapeutic effect in reducing the risk of the first recurrence event. The analyses were conducted from a third-party payers’ perspective in Switzerland. A lifetime horizon of 40.7 years (maximum 100 years of age) was used to comprehensively capture differences in costs and outcomes between treatment arms. A 3% annual discount rate was applied to all cost and health benefits, to align with standard practiceCitation25–28. The model was developed in Microsoft Excel® 2016.
Intervention and comparator
The intervention in this model was adjuvant pembrolizumab and the comparator was observation. In the model base case, patients received pembrolizumab at a flat intravenous dose of 200 mg (2 mg/kg up to 200 mg for pediatric patients) on day 1 of each 21-day cycle, for up to 17 cycles. This aligns with the approved pembrolizumab dosing regimen for the treatment of stage III melanoma in Switzerland and the dosing regimen tested in the KEYNOTE-716 trial for the treatment of resected stage IIB/IIC melanomaCitation19,Citation22. The KEYNOTE-716 trial protocol and all amendments were approved by the appropriate institutional review board or ethics committee at each participating institution and all patients provided written informed consent before study entryCitation19.
Transition probabilities
Key transition probabilities for both the intervention and comparators arms were estimated based on survival analyses of individual patient-level data from the KEYNOTE-716 trial and supported by robust published evidence including a systematic literature review (SLR) and network meta-analysis (NMA) in advanced melanoma, as well as a real-world retrospective database analysisCitation17,Citation18,Citation29–31. Of note, the NMA compared the efficacy of pembrolizumab in the KEYNOTE-006 trial versus comparators for the treatment of advanced or metastatic melanoma, and these data were used to inform the transition probabilities from the DM state to death. Transition probabilities from RFS to LR, DM, or death, were calculated for each weekly cycle, as a function of all three cause-specific hazardsCitation32. The modeled percentage of patients who were RF and DM free declined from year 1 to year 10 (90.4% to 42.1% and 95.0% to 43.6%, respectively). Data from observational studies were used to validate the model and mirrored this decrease in the percentage of patients who were RF and DM free (85.5% to 29.8% and 91.5% to 33.3% from year 1 to year 10, respectively).
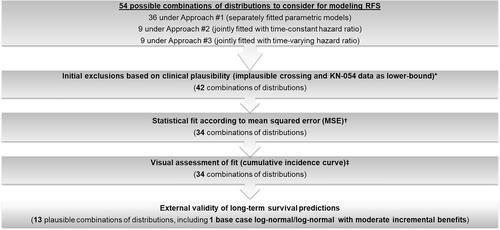
The cause-specific hazard within each cycle was estimated based on parametric models fitted to individual patient level data from the KEYNOTE-716 trialCitation33. Base case parametric functions were chosen based on clinical plausibility, mean squared errors versus observed RFS and DMFS, visual assessment of fit, and plausibility of long-term extrapolations. Of the 54 tested combinations of parametric distributions, 13 final combinations were chosen based on these criteria, of which one (log-normal function from RD to LR and RF to DM) was used for the base case and the remaining 12 were considered in the scenario analyses ( [overview of selection of parametric model]; Supplementary Table S5 [validation of parametric models] and Figure S1 [base case model fit versus observed data]). The base case parametric distribution was modeled as separately fitted models for each treatment arm, with a log-normal function for the transition from RF to LR and a log-normal function for RF to DM. These models provided the best balance between goodness-of-fit with observed data and plausible long-term extrapolations in each arm. Due to the small number of direct transitions from RF to death observed in KEYNOTE-716, exponential distributions were fitted for this transition in each arm.
Figure 2. Selection of base case parametric distribution functions for RFS. * Twelve out of 54 combinations of parametric distributions resulted in implausible crossing of the survival curves for pembrolizumab and observation, and were therefore excluded from base case consideration. Six of these 12 combinations were also excluded based on the use of 4-year RFS and DMFS data from KEYNOTE-054 (adjuvant stage III melanoma setting) as lower bounds. † Because MSEs were generally lower in the pembrolizumab arm than the observation arm, statistical fit in the observation arm was prioritized. Therefore, combinations ranked among the 10 worst-fitting for both RFS and DMFS in the observation arm were excluded (eight combinations excluded). ‡ Predicted RFS and DMFS in the observation arm up to 7 years was required to fall within ±5 percentage points of external RFS and DMFS data. External validation was performed using data from Bajaj et al. 2020, the US Oncology Network study and the RFS Kaplan-Meier curve from the KEYNOTE-716 trialCitation34,Citation35. Abbreviations. DMFS, distant metastasis-free survival; MSE, mean squared error; KN, KEYNOTE; RF, recurrence-free; RFS, recurrence-free survival; US, United States.
Utility inputs
Health state utility values for the base case and scenario analyses were derived through EQ-5D-5L data collected from the KEYNOTE-716 trial and analyzed in a linear mixed-effects model with patient-level random effects (Supplementary Appendix 2). Utility values for RF (without toxicity), LR recurrence, DM (pre-progression), and DM (post-progression) were 0.9414, 0.9261, 0.8868, and 0.8868, respectively. Disutilities used were associated with AEs and general aging. Both utility and disutility for health states were derived through repeated measures regression analyses of patient-level EQ-5D-5L data, using the German utility algorithm for the base case and the European utility algorithm for the scenario analysesCitation36,Citation37. The German value set for the EQ-5D-5L used in the base case was developed through interviews with a representative sample of the general population in Germany (N = 1,158) as part of a time trade-off and discrete choice experimentCitation38. Further details on utility inputs can be found in the Supplementary Appendix 2: Model input information Table S6.
Cost and resource use inputs
Cost data presented in this model were reported in CHF (2022) and estimated from a Swiss third-party payers’ perspective. The consumer price index was sourced from the Federal Office for Statistics and used to inflate costs, as neededCitation39. The following main costs components were considered in the model: treatment costs, salvage surgery, and subsequent treatment costs for recurrent disease (including drug acquisition and administration costs), BRAF mutation testing costs, disease management by health state, terminal care costs, and AE costs. The model considered AEs that were grade ≥3 and that occurred at a frequency of ≥5% in either treatment arm. Grade 2 diarrhea was also included as the costs of managing this AE were expected to be high. Details of each cost and resource input and the corresponding source of each parameter are provided in Supplementary Appendix 2: Model input information Table S6. Only direct healthcare costs to the payer were included.
Healthcare resource use assumptions in this indication were based on the recommended schedule of surveillance for resected stage IIB/IIC disease according to United Kingdom clinical guidelines in cutaneous melanoma and National Institute for Health and Care Excellence (NICE) guidelines for melanoma assessment and management, and validated with Swiss clinical expertsCitation40,Citation41. Healthcare resource use inputs included in the model were salvage surgery, outpatient visits, inpatient stays, home care, laboratory tests, and radiological exams.
Analyses
To test the robustness of the base case results, a deterministic sensitivity analyses (DSA), probabilistic sensitivity analysis (PSA), and scenario analyses were performed. The DSA varied each model parameter (testing lower and upper limits [±10% for all inputs except utility, varied by 95% CI]). The PSA used a Monte-Carlo simulation with 1,000 iterations randomly drawn from different model parameter distributions (multivariate/normal, normal, log-normal, gamma, beta). Scenario analyses were conducted to assess the impact of different assumptions for the time horizons and discount rates, efficacy, subsequent therapies for advanced melanoma, drug acquisition and administration costs, assumed rebated drug costs in Switzerland, utilities, time for rechallenge and immune-oncology-eligibility for LR and DM state, and AE costs.
Model validation
Model predictions were compared against observed data from three published external studies that reported long-term RFS and/or OS in real-world cohorts of patients diagnosed with stage IIB or IIC melanomaCitation7,Citation34,Citation42. OS data from the KEYNOTE-716 trial were not mature as the number of events required for analyses per the trial protocol had not been reachedCitation19. Therefore, OS was not included as part of the pre-specified third interim analysis of KEYNOTE-716, and KEYNOTE-716 data were not used to model transition probabilities starting from the DM state. In the absence of such data, the model used evidence from other clinical trials in the advanced melanoma setting to inform transition probabilities from DM to death. RFS, DMFS, and OS projections were validated against long-term data observed in a real-world study using US Oncology Network electronic health recordsCitation35. In addition, clinical experts were consulted to validate efficacy inputs and other key model decisions, such as assumptions about post-recurrence treatments. Health Technology Assessments (HTAs), and published cost-effectiveness studies from other adjuvant oncology indications provided support and precedence for the model assumptions (particularly the selection of base case parametric functions)Citation43–51.
Results
Base case results
Over the 40.7-year time horizon, total costs for pembrolizumab and observation were projected to be CHF 281,481 and CHF 248,637, respectively. Incremental quality-adjusted life years (QALYs) were 1.20 and incremental life years (LY) were 1.36. In the pembrolizumab arm 9.66% of LYs were spent in the RFS state, compared with 7.53% in the observation arm.
The base case incremental cost-effectiveness ratios (ICERs) for pembrolizumab versus observation were CHF 27,424 per QALY gained and CHF 24,105 per LY gained. Pembrolizumab was highly cost-effective versus observation for the treatment of stage IIB/IIC melanoma following complete resection. Model base case results are summarized in . Additional disaggregated results are presented in Supplementary Appendix 1: Additional results Table S1 and Table S2.
Table 1. Base case results.
One-way deterministic sensitivity analyses and scenario analyses
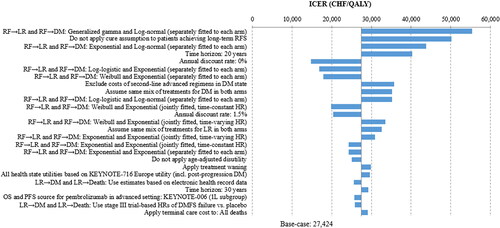
To assess the robustness of the model base case results to parameter uncertainty, a DSA was conducted. Results of the 25 most influential parameters, and other assumptions tested, are presented as a tornado diagram in . The ICER varied from CHF 14,710/QALY to CHF 55,428/QALY, with all scenarios yielding ICERs below the willingness-to-pay (WTP) threshold of CHF 100,000/QALY, commonly used in SwitzerlandCitation25,Citation52. Scenarios that tested alternative parametric distributions to model transitions from the RFS resulted in ICERs ranging from CHF 16,881/QALY to CHF 55,428/QALY. A 30-year time horizon resulted in an ICER comparable to the base case (CHF 29,179/QALY versus CHF 27,424/QALY). Reducing the time horizon to 20 years or 10 years led to increased ICERs (CHF 40,312/QALY and CHF 130,131/QALY, respectively). The ICER decreased to CHF 14,710/QALY when no discount rate was applied to costs and effectiveness measures, and to CHF 20,396/QALY when using an annual discount rate of 1.5% rather than 3.0% (base case). An annual discount rate of 5.0% was also assessed to represent a scenario in which the clinical benefits are delayed more than the base caseCitation53. Over a lifetime horizon, the ICER increased to CHF 39,106/QALY when a 5.0% discount rate was applied. Detailed sensitivity and scenario analyses results are presented in Supplementary Appendix 1: Additional results Table S3 and Table S4.
Figure 3. Tornado diagram showing only the top 25 most influential scenario or sensitivity analyses results. The impacts of the one-way sensitivity analysis on the ICER were too small to be seen on this figure. Abbreviations. CHF, Swiss Franc; DM, distant metastasis; DMFS, distant metastasis free survival; HR, hazard ratio; ICER, incremental cost-effectiveness ratio; LR, locoregional; QALY, quality-adjusted life year; RF, recurrence-free; RFS, RF survival.
Probabilistic sensitivity analysis
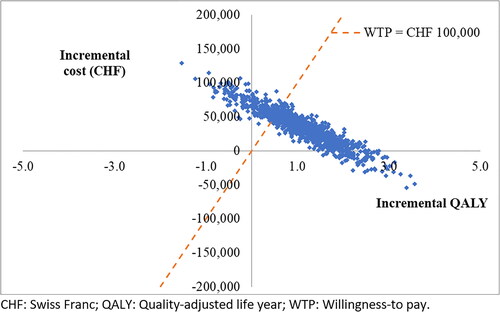
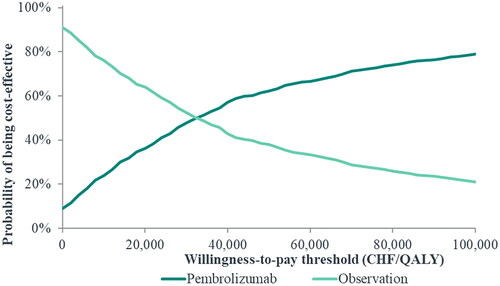
A PSA was conducted to estimate the probability of pembrolizumab being cost-effective relative to observation, based on different WTP thresholds. Across the 1,000 iterations, the average incremental cost was CHF 34,821 and the average incremental QALY gain was 1.10 for pembrolizumab versus observation. The resulting probabilistic ICER per QALY for pembrolizumab versus observation was higher compared to the base case (CHF 31,549/QALY versus CHF 27,424/QALY). Results of the PSA are illustrated in a scatterplot of simulated incremental costs and QALYs for pembrolizumab versus observation (). In addition, cost-effectiveness acceptability curves () show the probability of pembrolizumab being cost-effective versus observation over a range of different WTPs. At a WTP of CHF 100,000, the probability of pembrolizumab being cost-effective versus observation was 78.9%.
Discussion
Results of the KEYNOTE-716 randomized controlled trial have shown that pembrolizumab is a clinically effective adjuvant therapy for resected stage IIB/IIC melanoma. Key results of the trial have demonstrated a significant reduction in the risk of disease recurrence and development of DM, as well as a manageable safety profileCitation19. Moreover, pembrolizumab has been shown to have no detrimental impact on long-term HRQoL versus placeboCitation54,Citation55. The objective of this current study was to evaluate the cost-effectiveness of adjuvant pembrolizumab versus observation from a third-party payers’ perspective in Switzerland.
The results of the model demonstrate that pembrolizumab is highly cost-effective versus observation, with an ICER of CHF 27,424/QALY (EUR 27,342/QALY; exchange rate: 1 CHF = 0.997 EUR), significantly below the WTP threshold of CHF 100,000, commonly used for cost-effectiveness analyses in SwitzerlandCitation25,Citation52. In addition, at a WTP of CHF 100,000, the probability of pembrolizumab being cost-effective was 78.9% versus observation. Of note, the resulting ICER may have been lower if the analysis was conducted from a societal perspective as indirect costs associated with disease progression, such as reduced work productivity and income losses, would be lower in pembrolizumab compared to observation.
In the sensitivity analyses, the ICER decreased when no discount rates were used and the ICER increased when a shorter time horizon was applied; these results suggest that much of the value lies in the longer-term impact of pembrolizumab, i.e. the reduction in disease progression. This reduction in disease progression can decrease future costs as fewer subsequent treatments are required. Although a shorter time-horizon of 10 years may not capture all benefits, as Kaplan-Meier curves for OS amongst patients with stage IIB/IIC melanoma do not plateau during this time frameCitation9, the resulting ICER (CHF 130,131/QALY) remained below the World Health Organization WTP threshold of three-times the gross domestic product per capita for medical interventionsCitation56. This indicates that pembrolizumab may be considered cost-effective at the WHO WTP threshold over a 10-year time horizonCitation56.
This is the first published cost-effectiveness analysis of pembrolizumab for the treatment of stage II melanoma in Switzerland and other countries in Europe. Although pembrolizumab is already approved by the FDA and EMA for the adjuvant treatment of adults and pediatrics with stage IIB or IIC melanoma following complete resection, it is not yet approved in SwitzerlandCitation20,Citation21.
A key strength of this analysis is that efficacy and patient-level data were derived from the KEYNOTE-716 randomized controlled trial, external data, and clinical expert opinions, and validated accordingly. Consistent with methodological guidance from NICE’s Decision Support Unit (a gold standard for HTA agencies across Europe)Citation33,Citation57, the selection of parametric functions to model transitions starting from the RF state were based on goodness-of-fit with the observed data, and clinical plausibility of long-term extrapolations assessed using external data and clinical expert opinion. All transition probabilities starting from the RF and LR states were directly estimated using pivotal trial dataCitation17,Citation18,Citation29–31. Further, given the maturity of time on treatment data from the KEYNOTE-716 trial, no extrapolation was needed.
Extensive scenario analyses were conducted using varying assumptions regarding the combination of subsequent treatments received in each adjuvant treatment arm. In addition, the ability to model survival in the DM state according to the mix of subsequent treatments received under Switzerland-specific clinical practice is an important advantage of this model.
Base case market shares of subsequent treatments used in this analysis were supported by clinical expert opinion and Switzerland-specific market research data. Moreover, utilities used in this analysis were measured using EQ-5D, a widely used and accepted HRQoL measure for clinical trials in oncologyCitation58,Citation59. The disutility associated with AEs was applied as a one-time QALY decrement in the first model cycle. Disutility associated with AEs accounted for the mean duration (in weeks) of each AE episode and the mean number of episodes per patient with each AE, pooled across both treatment arms.
There are several limitations associated with this analysis. Firstly, as OS was not available in the pre-specified third interim analyses of KEYNOTE-716, other clinical trials in the advanced melanoma setting were synthesized from an SLR into an NMA and used to inform transition probabilities from DM to death. This ensured the most robust estimates were applied for the transition probabilities from the DM state when such data could not be sourced from the trial. Nevertheless, there is substantial published evidence that improvements in RFS and DMFS, such as those observed with pembrolizumab relative to placebo in KEYNOTE-716, could translate into an OS benefitCitation29–31,Citation60.
Another limitation was the need to extrapolate long-term RFS data (e.g. 5- or 10-year) available from the follow-up (median 27.4 months) period of the KEYNOTE-716 trialCitation61. Given the inherent uncertainty in the extrapolation of survival outcomes, alternative distributional assumptions were tested in the scenario analyses. This included conservative scenarios assuming a smaller incremental RFS benefit (moderate prediction in base case, relative to other combinations of distributions that met the external validity requirement) of pembrolizumab versus observation than that implied by the base case parametric functions. Across all scenario analyses conducted on RFS, the resulting ICERs of pembrolizumab were below the WTP threshold, supporting the robustness of the base case ICER.
Additional sensitivity analyses to determine the impact of treatment exposure (i.e. number of pembrolizumab cycles) on the resulting ICER could not be conducted due to the diminished sample sizes when stratifying patients by categories of treatment exposure. However, time on treatment was incorporated in the base-case analysis using a Kaplan-Meier curve and, therefore, the analysis did account for varying treatment exposure in the estimation of the ICER and did not assume that all patients completed therapy. Furthermore, sensitivity analyses to determine stratified results for pediatric versus adult patients could not be conducted due to pediatric melanoma being rare and, consequently, only two patients aged ≤ 17 years were recruited in the KEYNOTE-716 trialCitation19.
Due to the memoryless property of Markov models, exponential distributions were assumed for transitions starting from the LR and DM states. This assumption was validated as modeled DMFS for the observation arm closely aligned with the 10-year DMFS Kaplan-Meier curve from the US Oncology Network studyCitation35. However, the ICER showed only moderate variation in DSAs that varied the rates of OS and PFS failure.
A targeted search for HTA submissions in adjuvant oncology settings did not identify any prior submissions for adjuvant treatments for stage II melanoma at the time of the model development. Notably, HTA guidelines for Switzerland do not exist. Consequently, it was not possible to cross-validate the current model results against other, independently developed economic evaluations in the same indication. However, prior HTAs and published cost-effectiveness studies in other adjuvant oncology indications provided support and precedence for the assumptions used in the current modelCitation43–51.
Since the finalization of the model, a health technology appraisal has been published by NICE for pembrolizumab for the adjuvant treatment of resected stage IIB and IIC melanoma in people aged 12 years and over, which aligns with the model structure and comparatorsCitation62. The Evidence Review Group noted the model structure was similar to that used in the technology appraisal for pembrolizumab in stage III melanoma and therefore was suitable for decision-makingCitation62. From the economic model, the ICER was estimated to be around £20,000 per QALY gained when confidential patient access schemes for pembrolizumab and subsequent treatments were appliedCitation62. Overall, the NICE appraisal concluded that pembrolizumab is an acceptable use of NHS resources for the treatment of stage IIB or IIC melanomaCitation62.
Finally, due to the short follow-up data available from the KEYNOTE-716 trial and therefore the small number of patients in the DM state, trial-based estimates of utility in the DM state may not accurately reflect HRQoL during the entire period from DM until death. Consequently, the base case analysis used results from the KEYNOTE-716 German utility algorithm to inform utility in the post-progression DM state. Scenario analyses were also undertaken using the European utility algorithm for health state utilities, and the results supported the base case cost-effectiveness conclusions.
Results of this model support the cost-effectiveness of pembrolizumab for the treatment of patients with resected stage IIB/IIC melanoma in Switzerland. Further research is needed to confirm the cost-effectiveness of pembrolizumab for the treatment of resected stage IIB/IIC melanoma in other European countries. As IFN is no longer recommended as an adjuvant therapy for melanoma due to substantial toxicity and negative impacts on HRQoL, observation is the current standard of care for resected stage IIB/IIC melanoma in SwitzerlandCitation10. Therefore, the burden of disease remains high and there is an unmet need for an effective adjuvant therapy that reduces the risk of disease recurrenceCitation2,Citation8,Citation63,Citation64.
Conclusion
As there are currently limited options for the adjuvant treatment of patients with resected stage IIB or IIC melanoma, there is an urgent need for a therapy that reduces the risk of melanoma recurrence. Results of this analysis show that pembrolizumab is a cost-effective adjuvant treatment option for patients with resected stage IIB or IIC melanoma compared to observation in Switzerland from a third-party payer perspective. Moreover, cost offsets may be achieved through the reduction of subsequent treatment costs. The findings of this study are anticipated to be consistent across other European settings.
Transparency
Declaration of financial/other interests
AFB, GB, and DA are employed by MSD. SZ and RJ are employed by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA (MSD). AB received financial support via the employment institution from MSD.
Author contributions
Conception and design, AFB, GB, SZ; data acquisition, AFB, AB, DA; formal analysis, AFB, AB, DA, RJ; interpretation of results, AFB, AB, DA, GB, RJ, SZ; writing – original draft preparation, AFB, AB, GB, RJ; writing – review and editing, AFB, AB, DA, GB, RJ, SZ. All authors have read and agreed to the published version of the manuscript.
Reviewer disclosures
A reviewer on this manuscript has disclosed that they are an employee of Merck & Co., Inc. but are not in the same team. Another reviewer is a consultant for Merck. The other peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Supplemental Material
Download MS Word (173.2 KB)Acknowledgements
The authors thank Adelphi Values PROVE for the support on the development of this manuscript in cooperation with all authors. The authors thank Dr Pascal Zihlmann and Dr Matthias Rölli for their valuable scientific advice, as well as technical and logistical support.
Data availability statement
All data generated or analyzed during this study are included in this published article and its supplementary information.
References
- Forsea AM. Melanoma Epidemiology and early detection in Europe: diversity and disparities," (in eng. Dermatol Pract Concept. 2020;10(3):e2020033.
- Wanner M, Matthes KL, Karavasiloglou N, et al. 37-year incidence and mortality time trends of common cancer types by sex, age, and stage in the canton of Zurich. Swiss Med Wkly. 2020;150:w20388.
- Schweiz K. Krebs in der Schweiz: wichtige Zahlen. 2022. https://www.krebsliga.ch/ueber-krebs/zahlen-fakten/-dl-/fileadmin/downloads/sheets/zahlen-krebs-in-der-schweiz.pdf
- Skin Cancer Foundation. Melanoma stages, diagnosis and staging. what it means for you. 2022. https://www.skincancer.org/skin-cancer-information/melanoma/the-stages-of-melanoma/
- Melanoma Research Alliance. Stage 2 melanoma. 2022. https://www.curemelanoma.org/about-melanoma/melanoma-staging/stage-2
- Miller R, Walker S, Shui I, et al. Epidemiology and survival outcomes in stages II and III cutaneous melanoma: a systematic review. Melanoma Manag. 2020;7(1):Mmt39.
- Bleicher J, Swords DS, Mali ME, et al. Recurrence patterns in patients with stage II melanoma: the evolving role of routine imaging for surveillance," (in eng. J Surg Oncol. 2020;122(8):1770–1777.
- Christoffersen P, Khan N, Lucas J, et al. Estimation of direct medical costs associated with treatment of metastatic melanoma in Switzerland. Value in Health. 2015;18(7):A451.
- Gershenwald JE, Scolyer RA. Melanoma staging: American joint committee on cancer (AJCC) 8th edition and beyond," (in eng. Ann Surg Oncol. 2018;25(8):2105–2110.
- Michielin O, van Akkooi ACJ, Ascierto PA, ESMO Guidelines Committee. Electronic address: [email protected], et al. Cutaneous melanoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30(12):1884–1901.
- Trinh VA, Zobniw C, Hwu WJ. The efficacy and safety of adjuvant interferon-alfa therapy in the evolving treatment landscape for resected high-risk melanoma. Expert Opin Drug Saf. 2017;16(8):933–940.
- Ives NJ, Suciu S, Eggermont AMM, International Melanoma Meta-Analysis Collaborative Group (IMMCG), et al. Adjuvant interferon-alpha; for the treatment of high-risk melanoma: an individual patient data meta-analysis. Eur J Cancer. 2017;82:171–183.
- National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology - Melanoma: Cutaneous. Version 1. 2022. NCCN https://www.nccn.org/professionals/physician_gls/pdf/cutaneous_melanoma.pdf
- Krensel M, Schäfer I, Augustin M. Cost-of-illness of melanoma in Europe – a systematic review of the published literature. J Eur Acad Dermatol Venereol. 2019;33(3):504–510.
- Sanmamed MF, Chen L. A Paradigm shift in cancer immunotherapy: from enhancement to normalization," (in eng). Cell. 2018;175(2):313–326.
- Eggermont AMM, Kicinski M, Blank CU, et al. Pembrolizumab versus placebo after complete resection of High-Risk stage III melanoma: 5-Year results of the EORTC 1325-MG/KEYNOTE-054 Double-Blinded phase 3 trial," in ESMO congress 2022, paris, France. Ann Oncol. 2022;33(Suppl_7):S912–S913.
- Luke JJ, Ascierto PA, Carlino MS, et al. KEYNOTE-716: phase III study of adjuvant pembrolizumab versus placebo in resected high-risk stage II melanoma," (in eng). Future Oncol. 2020;16(3):4429–4438.
- ClinicalTrials.gov. Safety and efficacy of pembrolizumab compared to placebo in resected high-risk stage II melanoma (MK-3475-716/KEYNOTE-716). 2022. https://clinicaltrials.gov/ct2/show/NCT03553836
- Luke JJ, Rutkowski P, Queirolo P, et al. Pembrolizumab versus placebo as adjuvant therapy in completely resected stage IIB or IIC melanoma (KEYNOTE-716): a randomised, double-blind, phase 3 trial. The Lancet. 2022;399(10336):1718–1729.
- Food and Drug Administration. FDA approves pembrolizumab for adjuvant treatment of Stage IIB or IIC melanoma. 2022. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pembrolizumab-adjuvant-treatment-stage-iib-or-iic-melanoma#:∼:text=FDA%20approves%20pembrolizumab%20for%20adjuvant%20treatment%20of%20Stage%20IIB%20or%20IIC%20melanoma,-Share&text=On%20December%203%2C%202021%2C%20the,IIC%20melanoma%20following%20complete%20resection
- European Medicines Agency. Product information. 2022. https://www.ema.europa.eu/en/documents/product-information/keytruda-epar-product-information_en.pdf
- RefData. Keytruda® product information (Switzerland). 2022. https://www.swissmedicinfo.ch/#section1
- Bundesamt für Gesundheit (BAG). BAG-bulletin. 2019. https://www.bag.admin.ch/bag/de/home/das-bag/publikationen/periodika/bag-bulletin.html
- ScienceIndustries Switzerland. Verhaltenskodex der pharmazeutischen Industrie in der Schweiz (Pharmakodex): Regel 26. 2022. https://www.scienceindustries.ch/_file/27931/Pharmakodex_Version_Mai%202020_D.pdf
- Bhadhuri A, Insinga R, Guggisberg P, et al. Cost effectiveness of pembrolizumab vs chemotherapy as first-line treatment for metastatic NSCLC that expresses high levels of PD-L1 in Switzerland," (in eng. Swiss Med Wkly. 2019;149:w20170.
- Barbier M, Durno N, Bennison C, et al. Cost-effectiveness and budget impact of venetoclax in combination with rituximab in relapsed/refractory chronic lymphocytic leukemia in Switzerland. Eur J Health Econ. 2022;23(5):837–846.
- Schur N, Gudala K, Vudumula U, et al. Cost effectiveness and budget impact of siponimod compared to interferon beta-1a in the treatment of adult patients with secondary progressive multiple sclerosis with active disease in Switzerland. PharmacoEconomics. 2021;39(5):563–577.
- Bommer C, Lupatsch J, Bürki N, et al. Cost–utility analysis of risk-reducing strategies to prevent breast and ovarian cancer in BRCA-mutation carriers in Switzerland. Eur J Health Econ. 2022;23(5):807–821.
- Eggermont AMM, Chiarion-Sileni V, Grob J-J, et al. Adjuvant ipilimumab versus placebo after complete resection of stage III melanoma: long-term follow-up results of the european organisation for research and treatment of cancer 18071 double-blind phase 3 randomised trial," (in eng). Eur J Cancer. Sep 2019;119:1–10.
- Suciu S, Eggermont AMM, Lorigan P, et al. Relapse-free survival as a surrogate for overall survival in the evaluation of stage II-III melanoma adjuvant therapy. J Natl Cancer Inst. 2018;110:87–96.
- Coart E, Suciu S, Squifflet P, et al. Evaluating the potential of relapse-free survival as a surrogate for overall survival in the adjuvant therapy of melanoma with checkpoint inhibitors. Eur J Cancer. 2020;137:171–174.
- Hinchliffe SR, Lambert PC. Flexible parametric modelling of cause-specific hazards to estimate cumulative incidence functions. BMC Med Res Methodol. 2013;13(1):13–14.
- National Institute for Health and Care Excellence. DSU technical support document 19: partitioned survival analysis for decision modelling in health care: a critical review. 2022. https://www.sheffield.ac.uk/nice-dsu
- Bajaj S, Donnelly D, Call M, et al., Melanoma Prognosis: accuracy of the American joint committee on cancer staging manual eighth edition, J Natl Cancer Inst, 2020;112(9):921–928.
- Samlowski W, Silver MA, Hohlbauch A, et al. Real-world clinical outcomes of patients with stage IIB and IIC cutaneous melanoma treated at US community oncology clinics. Future Oncol. 2022;18(33):3755–3767.
- Chaudhuri M. Utility Analysis report for KN716-CSR3 Adjuvant Therapy with Pembrolizumab versus Placebo in Resected High-risk Stage II Melanoma: report adapted for Germany. 2022.
- Ara R, Brazier JE. Populating an economic model with health state utility values: moving toward better practice. Value Health. 2010;13(5):509–518.
- Ludwig K, Graf von der Schulenburg J, Greiner W. German value set for the EQ-5D-5L. Pharmacoeconomics. 2018;36(6):663–674.
- F. S. Office. Consumer prices. 2022. https://www.bfs.admin.ch/bfs/de/home/statistiken/preise/landesindex-konsumentenpreise.assetdetail.21784714.html
- Larkin A, Algurafi A, Barlow B. Position paper: follow-up of high risk cutaneous melanoma in the UK. 2022. https://melanomafocus.org/melanoma-patient-treatment-guide/
- National Institute for Health and Care Excellence. Melanoma: assessment and management: NICE guideline [NG14]. 2022. https://www.nice.org.uk/guidance/ng14
- Kanaki T, Stang A, Gutzmer R, et al. Impact of American joint committee on cancer 8th edition classification on staging and survival of patients with melanoma. Eur J Cancer. 2019;119:18–29.
- Aguiar-Ibáñez R, Wang J, Bensimon A, et al. Challenges and good practices when modeling CostEffectiveness of novel (neo-) adjuvant oncology therapies in health technology appraisals (HTAs). ISPOR Workshop. 2020.
- Crott R, Ali F, Burdette-Radoux S. Cost-utility of adjuvant high-dose interferon alpha therapy in stage III cutaneous melanoma in Quebec. Value Health. 2004;7(4):423–432.
- Cormier JN, Xing Y, Ding M, et al. Cost Effectiveness of adjuvant interferon in Node-Positive melanoma. J Clin Oncol. 2007;25(17):2442–2448.
- Dixon S, Walters SJ, Turner L, et al. Quality of life and cost-effectiveness of interferon-alpha in malignant melanoma: results from randomised trial. Br J Cancer. 2006;94(4):492–498.
- González-Larriba JL, Serrano S, Alvarez-Mon M, et al. Cost-effectiveness analysis of interferon as adjuvant therapy in high-risk melanoma patients in Spain. Eur J Cancer. 2000;36(18):2344–2352.
- Hillner BE, Kirkwood JM, Atkins MB, et al. Economic analysis of adjuvant interferon alfa-2b in high-risk melanoma based on projections from Eastern cooperative oncology group 1684. J Clin Oncol. 1997;15(6):2351–2358.
- Clarke CS, Hunter RM, Shemilt I, et al. Multi-arm Cost-Effectiveness analysis (CEA) comparing different durations of adjuvant trastuzumab in early breast cancer, from the English NHS payer perspective. PLoS One. 2017;12(3):e0172731.
- Erman A, Nugent A, Amir E, et al. Cost-effectiveness analysis of extended adjuvant endocrine therapy in the treatment of post-menopausal women with hormone receptor positive breast cancer. Breast Cancer Res Treat. 2014;145(2):267–279.
- Hedden L, O'Reilly S, Lohrisch C, et al. Assessing the real-world cost-effectiveness of adjuvant trastuzumab in HER-2/neu positive breast cancer," (in eng. Oncologist. 2012;17(2):164–171.
- Salikhanov I, Heinimann K, Chappuis P, et al. Swiss cost-effectiveness analysis of universal screening for lynch syndrome of patients with colorectal cancer followed by Cascade genetic testing of relatives. J Med Genet. 2022;59(9):924–930.
- U.S. Department of Veterans Affairs. Measuring costs for cost-effectiveness analysis. 2022. https://www.herc.research.va.gov/include/page.asp?id=measure-costs-cea
- Khattak MA, Luke JJ, Long GV, et al. Health-related quality of life (HRQoL) with pembrolizumab (pembro) in resected high-risk stage II melanoma in the phase 3 KEYNOTE-716 study. American Society of Clinical Oncology. 2022.
- Bottomley A, Coens C, Mierzynska J, EORTC Melanoma Group, et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma (EORTC 1325-MG/KEYNOTE-054): health-related quality-of-life results from a double-blind, randomised, controlled, phase 3 trial. Lancet Oncol. 2021;22(5):655–664.
- Bertram MY, Lauer JA, Stenberg K, et al. Methods for the economic evaluation of health care interventions for priority setting in the health system: an update from WHO CHOICE. Int J Health Policy Manag. 2021;10(11):673–677.
- National Institute for Health and Care Excellence. DSU technical support document 14: survival analysis for economic evaluations alongside clinical trials - extrapolation with patient-level data. 2022. https://www.sheffield.ac.uk/nice-dsu
- Schwenkglenks M, Matter-Walstra K. Is the EQ-5D suitable for use in oncology? An overview of the literature and recent developments. Expert Rev Pharmacoecon Outcomes Res. 2016;16(2):207–219.
- Bhadhuri A, Kind P, Salari P, et al. Measurement properties of EQ-5D-3L and EQ-5D-5L in recording self-reported health status in older patients with substantial multimorbidity and polypharmacy," (in eng. Health Qual Life Outcomes. 2020;18(1):317.
- Koruth R, Sharma R, Kanters S, et al. Establishing the relationship between relapse-free survival and overall survival in adjuvant high-risk radically resected cutaneous melanoma. In: The Society for Melanoma Research, Fifteenth International Congress, Manchester, England, 2018.
- Long GV, Luke JJ, Khattak MA, et al. Pembrolizumab versus placebo as adjuvant therapy in resected stage IIB or IIC melanoma (KEYNOTE-716): distant metastasis-free survival results of a multicentre, double-blind, randomised, phase 3 trial," (in eng. Lancet Oncol. 2022;23(11):1378–1388.
- National Institute for Health and Care Excellence. Pembrolizumab for adjuvant treatment of resected stage 2B or 2C melanoma: Technology appraisal guidance [TA837]. 2022. https://www.nice.org.uk/guidance/TA837
- Hematology/Oncology. Adjuvant pembrolizumab shows benefit in patients with resected stage IIB, IIC melanoma. 2022. https://www.healio.com/news/hematology-oncology/20220606/adjuvant-pembrolizumab-shows-benefit-in-patients-with-resected-stage-iib-iic-melanoma
- Goodman A. Adjuvant pembrolizumab for high-risk stage II melanoma: Efficacy and safety examined. 2022. https://ascopost.com/news/september-2021/adjuvant-pembrolizumab-for-high-risk-stage-ii-melanoma-efficacy-and-safety-examined/#:∼:text=The%20current%20standard%20of%20care,stage%20IIIA%20and%20IIIB%20melanoma