Abstract
Background and Aims
Combination of a cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitor and an aromatase inhibitor is the standard of care first-line (1L) treatment of hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2−) advanced breast cancer (ABC). Updated clinical data have become available from the MONALEESA-2 and PALOMA-2 trials for ribociclib and palbociclib, respectively. This analysis with updated data assessed the cost-effectiveness of ribociclib versus palbociclib, both in combination with letrozole, in the setting of 1L therapy of postmenopausal women with HR+/HER2− ABC, from a United Kingdom (UK) National Health Service perspective.
Methods
A three state (progression-free, progressed disease, and death) partitioned survival model with a 1-month cycle was developed. Clinical data were derived from MONALEESA-2 (NCT01958021) and PALOMA-2 (NCT01740427). The treatment effect was modeled using hazard ratios (HRs) for progression-free survival and overall survival derived through a matched-adjusted indirect comparison. Trial data and published literature were used to derive utility values. Cost inputs included drug acquisition, disease monitoring, subsequent therapies, and adverse events. Costs and outcomes were discounted by 3.5%, over a 40-year lifetime horizon. One-way and probabilistic sensitivity analyses were performed.
Results
Ribociclib dominated palbociclib, and was both overall cost saving (−£3,273) and more effective (+1.251 quality-adjusted life years [QALYs]). Ribociclib total drug costs were £17,156 lower than palbociclib. At a £30,000 per QALY willingness-to-pay threshold, the probability of ribociclib being cost-effective was almost 100%. Ribociclib remained cost-effective when varying HRs, utilities, drug cost, and health state costs.
Conclusions
Ribociclib is both cost-saving and cost-effective compared with palbociclib for the 1L treatment of postmenopausal women with HR+/HER2− ABC in the UK.
Introduction
Globally, breast cancer is the most commonly occurring cancer in females, with 2.3 million new cases in 2020Citation1. In the United Kingdom (UK), breast cancer accounts for 15% of new cancer casesCitation2. It is estimated that around 30% of patients diagnosed with earlier stages of breast cancer later develop recurrent advanced breast cancer (ABC), whilst approximately 6–10% of new cases are initially diagnosed at the advanced stage (stage IV), where the cancer is inoperable or has spread significantly to other sites in the bodyCitation3,Citation4. The most common form of breast cancer is hormone receptor-positive (HR+) and human epidermal growth factor receptor 2-negative (HER2−); approximately 65% of patients with newly-diagnosed metastatic breast cancer are HR+/HER2−Citation5,Citation6. ABC has a poor prognosis, and distant HR+/HER2− breast cancer has a 5-year survival rate of 32%Citation7.
The mainstay treatment for postmenopausal women with HR+/HER2− ABC was single agent endocrine therapy, for instance with aromatase inhibitors such as letrozole, however resistance to endocrine therapy usually developsCitation8. Therefore, prolongation or restoration of sensitivity to endocrine therapy is needed. In the past few years, several studies have shown improved efficacy with the addition of a CDK4/6 inhibitor to endocrine therapy, which are approved for use by the Food and Drug Administration and European Medicines AgencyCitation9, such that the combination of a CDK4/6 inhibitor and endocrine therapy is now the standard of care first-line (1L) treatment of HR+/HER2− ABC in most parts of the world. Due to the poor prognosis of ABC, effective hormonal therapies are necessary to improve patient outcomes and avoid chemotherapy.
Ribociclib and palbociclib are both CDK4/6 inhibitors that have demonstrated clinical efficacy in separate randomized clinical trialsCitation10–15, and are licensed for use in patients with HR+/HER2− breast cancerCitation16,Citation17. Ribociclib and palbociclib are both administered orally, with licensed doses of 600 mg and 125 mg, respectivelyCitation16,Citation17. Both drugs are administered for 21 consecutive days followed by 7 days off treatment, in combination with an aromatase inhibitorCitation16,Citation17 or fulvestrantCitation18,Citation19. The cost-effectiveness of ribociclib plus letrozole versus palbociclib plus letrozole has been previously evaluated using clinical data from the MONALEESA-2, and PALOMA-1 and PALOMA-2 studies, for ribociclib and palbociclib, respectivelyCitation20. Since this prior analysis, more mature, longer-term progression-free survival (PFS) and overall survival (OS) data have been collected for both CDK4/6 inhibitors. It should be noted that another CDK4/6 inhibitor, abemaciclib, is also licensed for use in patients with HR+/HER2− breast cancerCitation21, and a cost-effectiveness analysis of ribociclib versus abemaciclib has been conducted separatelyCitation22.
The MONALEESA-2 trial (NCT01958021) was a randomized, double-blinded, placebo-controlled phase III study evaluating the efficacy and safety of ribociclib plus letrozole versus placebo plus letrozoleCitation13,Citation14,Citation23–25. The trial demonstrated a statistically significant benefit in PFS in patients receiving ribociclib plus letrozole compared with placebo plus letrozole (hazard ratio [HR] = 0.56; 95% confidence interval [CI] = 0.43–0.72; p = 3.29 × 10−6)Citation14. Since the prior cost-effectiveness analysis, longer-term MONALEESA-2 OS data at a median follow-up of 6.6 years have been published. OS was statistically significantly longer in the ribociclib plus letrozole group than in the placebo plus letrozole group (HR = 0.76; 95% CI = 0.63–0.93; two-sided p = 0.008) Citation24.
Three clinical trials have demonstrated the efficacy and safety of palbociclib plus letrozole: PALOMA-1 (NCT00721409)Citation26, PALOMA-2 (NCT01740427)Citation27, and PALOMA-4 (NCT02297438)Citation28. PALOMA-1 was a phase II, open-label randomized study, while PALOMA-2 and PALOMA-4 were double-blind, randomized phase III trialsCitation10–12,Citation15. As PALOMA-4 was evaluated in patients in Asia onlyCitation15, it was not considered a relevant source for a UK-based cost-effectiveness analysis and therefore was not considered further. PALOMA-1 and PALOMA-2 both reported significant improvements in PFS for palbociclib plus letrozole versus placebo plus letrozole (PALOMA-1: HR = 0.49; 95% CI = 0.32–0.75; p = 0.0004; PALOMA-2: HR = 0.58; 95% CI = 0.46–0.72 p < 0.001)Citation11,Citation12. In the absence of OS data from PALOMA-2 at the time of the prior cost-effectiveness analysis, OS data were previously sourced from the phase II, PALOMA-1 trial. Since then, final OS data have recently been presented from the phase III, PALOMA-2 trial (median follow-up 7.5 years), which demonstrated no statistical difference between palbociclib plus letrozole and placebo plus letrozole (HR = 0.96; 95% CI = 0.78–1.18; one-sided p = 0.3378)Citation12,Citation29.
Following on from the earlier cost-effectiveness analysis, this study aims to provide further confidence to the previous study by incorporating the more mature final OS data from MONALEESA-2 and PALOMA-2 and other recent data sources to evaluate the cost-effectiveness of ribociclib plus letrozole versus palbociclib plus letrozole in a 1L setting among postmenopausal women with HR+/HER2− ABC in the UK.
Methods
The model structure and methodology is aligned with the previously conducted analysisCitation20. A cohort-based partitioned survival model was developed in Microsoft Excel® to estimate the cost-effectiveness of ribociclib versus palbociclib, both in combination with letrozole. The analysis was conducted from the perspective of the health care payer in the UK (National Health Service [NHS] and Personal Social Services [PSS]).
Model overview
A three state (progression-free, progressed disease, and death) partitioned survival model was previously developedCitation20. The proportion of patients alive was estimated using an OS curve, with the proportion of patients alive and progression-free estimated from a PFS curve. The proportion of patients in the progressed disease health state was then estimated as the difference of the alive patients and progression-free patients. Further details on OS and PFS are provided in the next section.
The model used a 1-month cycle, consistent with the clinical review and treatment schedules of ribociclib and palbociclib, which are administered for 21 days followed by 7 days off treatment, and a lifetime horizon of 40 years, based on the model starting age. Both costs and outcomes were discounted by 3.5%. The patient population modeled was based on the patients enrolled in MONALEESA-2 (NCT01958021) with a starting age of 62 yearsCitation14. The patients in MONALEESA-2 were generally similar to those enrolled in PALOMA-2 (NCT01740427). Both MONALEESA-2 and PALOMA-2 included postmenopausal women with HR+/HER2− ABC with no prior treatment for ABC, and required > 12 months from end of (neo)adjuvant treatment to recurrence (treatment-free interval) only if patients received prior non-steroidal aromatase inhibitors for early breast cancerCitation30. It is important to note that the percentage of patients with a treatment-free interval of 12 months or less was similar between the two trials. Of note, while PALOMA-2 used the term ‘disease-free interval’ when reporting the data, the actual interval used was not the time from diagnosis, but from end of (neo)adjuvant treatment to recurrence. Thus, for PALOMA-2 it was the same as the definition of treatment-free interval used in MONALEESA-2. The percentage of patients with a treatment-free interval ≤ 12 months in the ribociclib and palbociclib arms (intention to treat) of MONALEESA-2 and PALOMA-2 was 17.7% and 22.1%, respectivelyCitation12,Citation14.
Clinical inputs
A summary of the model inputs is presented in .
Table 1. Summary of model inputs.
Survival estimates for ribociclib plus letrozole and palbociclib plus letrozole were derived by applying a HR (versus placebo plus letrozole) to the reference arm, which was modeled by fitting parametric functions to PFS and OS data for placebo plus letrozole in the MONALEESA-2 trial. There are no head-to-head data available for ribociclib versus palbociclib, therefore HRs were derived using a matching-adjusted indirect comparison (MAIC)Citation30, using individual patient data from MONALEESA-2 and published summary data from PALOMA-2. Patients enrolled in MONALEESA-2 were weighted to match baseline characteristics in the corresponding arms of PALOMA-2Citation30. After matching, the baseline characteristics for the MONALEESA-2 population were identical to the PALOMA-2 population for each characteristic. It is important to note that all available baseline characteristics reported for PALOMA-2 were used in matching and none of the characteristics were removed. Further details on the MAIC methods and results have been previously published in Jhaveri et al.Citation30. The resulting HRs of ribociclib plus letrozole versus palbociclib plus letrozole were generated via the Bucher methodCitation30. The HRs for PFS and OS generated from the MAIC are presented in .
The parametric distributions were re-evaluated and updated to the most appropriate choice based on the mature MONALEESA-2 data. These were based on statistical goodness of fit indicators (Akaike Information Criterion/Bayesian Information Criterion) as well as clinical plausibility as recommended in the National Institute for Health and Care Excellence Decision Support Unit document on survival analysis modelingCitation31, resulting in a log-logistic distribution being chosen to model OS and a log-normal distribution chosen to model PFS for placebo plus letrozole.
Treatment specific overall response rates were used to model the proportion of patients in the progression-free state in the previous analysisCitation20, however response rates have not been included in the updated model due to having no impact on the cost-effectiveness results.
The respective clinical trial data were used to estimate the probabilities of grade 3 or above adverse events for ribociclib and palbociclibCitation24,Citation32. The costs of managing such adverse events were accounted for in the model ().
Valuation of health benefits
Quality-adjusted life years (QALYs) were estimated using health state utility (HSU) values, and separate HSU values were estimated for patients in the progression-free and progressed disease states, and were considered to be consistent across treatment arms.
In line with the previous analysisCitation20, HSU values in the progression-free state applied in the model were identified through the analysis of patient-reported outcome data in MONALEESA-2, which collected health-related quality-of-life data using the EuroQoL 5-dimension (EQ-5D-5L) questionnaire. HSU values were calculated using the EQ-5D-5L UK social tariff reported by Devlin et al.Citation33.
HSU values in the progressed disease state were based on published data from a study by Hudgens et al.Citation34. In this study, health-related quality-of-life data were obtained by mapping the QLQ-C30 quality of life cancer questionnaire data from a phase III trial of 1,102 advanced, metastatic breast cancer patients with progressed disease to EQ-5D patient preferencesCitation34. As empirical mapping is considered a better approach than the vignette method for estimating disutility, Hudgens et al. was considered more appropriate than the estimates from Lloyd et al. that were used in the previous analysisCitation20,Citation35. Additionally, the Lloyd et al. study is now outdated, published in 2006, and therefore is not considered to be a relevant source to estimate health-related quality-of-life data in the metastatic state in today’s clinical practice; the source of progressed disease utility was therefore updated for this analysis. The HSU values used in the model are shown in .
Costs and resource use
The drug acquisition cost was calculated from the list price of medication and the mean total dose of therapy administered in each cycle. The list price of each drug was sourced from the British National FormularyCitation36. Drug dosing was modeled based on the posology stated in the summary of product characteristicsCitation16,Citation17. The unit costs for resource use were sourced from the NHS reference costs 2019–2020Citation37. Monitoring costs comprise biochemistry costs, complete blood counts and electrocardiograms, and the monitoring schedule was sourced from the summary of product characteristics for each drugCitation16,Citation17. As oral therapies, no drug administration costs were applied for either treatment; any additional costs associated with prescribing or dispensing the drugs would be the same for both treatments, and thus for simplicity were not considered in the analysis. Drug acquisition and monitoring costs used in the analysis are presented in .
Duration of treatment for ribociclib plus letrozole was modeled by fitting a parametric function (exponential) model to time-to-treatment discontinuation (TTD) data for ribociclib and letrozole from MONALEESA-2. For palbociclib plus letrozole, the trial reported median treatment duration of 20.1 months was used to generate the exponentially distributed TTD curveCitation27.
Dose reductions for ribociclib (from 600 mg to 400 mg, and 400 mg to 200 mg) were accounted for based on the latest clinical data from MONALEESA-2 from Month 1 to 79, and the dose distributions observed at the end of the study follow-up (e.g. Month 89) were applied to all months thereafter. Dose reductions for palbociclib (from 125 mg to 100 mg, and 100 mg to 75 mg) were likely to result in drug wastage and were based on dose reductions in PALOMA-2Citation12,Citation17.
Disease monitoring costs depend upon the progression-free and progressed disease status of the population (progressed disease is associated with a higher cost burden). Disease monitoring healthcare resource use data were informed by literature and costs used in the model are presented in .
As in the previous analysisCitation20, subsequent therapy costs for progressing patients were estimated by apportioning the patients entering progressed disease into two categories: those who receive second-line therapy and third-line therapy, further split into those who received endocrine therapies, chemotherapy, and no active treatment. This categorization was informed by data presented by BrufskyCitation38 and Das et al.Citation39. Data on the proportion of patients receiving endocrine or chemotherapy in the second and third-line settings were sourced from Lin et al.Citation40, and duration of treatment were sourced from Macalalad et al.Citation41. A mean monthly cost applied to all patients after progression was estimated by dividing these total costs by a post-progression survival of 20.5 monthsCitation42. Details of subsequent therapy costs are presented in .
End-of-life costs were aggregated across three categories of palliative support given to patients just before their death: hospital-based, hospice centre-based, and home-based (community support). These costs were sourced from Round et al.Citation43 and inflated to 2020. These were applied as one-off costs just before death to the number of patients dying across each cycle.
Sensitivity analyses
To account for the uncertainty associated with key parameters, both one-way deterministic and probabilistic sensitivity analyses were performed. In the one-way deterministic sensitivity analysis: discount rate was varied between 0–6%; costs for disease management, treatment acquisition, and monitoring were varied ±10%; progression-free and progressed disease HSU values were varied by their standard error; and HR applied for OS were varied by its 95% CI. Results are presented in the form of a Tornado plot.
Clinical parameters such as HRs, ORs, incidence rates of adverse events, and quality-of-life inputs such as HSU values were all included in the probabilistic sensitivity analysis. A multivariate normal distribution, with correlation between shape and scale, was applied to survival distributions for PFS and OS. Gamma distributions were applied to the costs for disease management, drug acquisition, monitoring, and adverse events. Beta distributions were applied to the utility weights assigned to progression-free and post-progression states. Results were presented on a cost-effectiveness plane and cost-effectiveness acceptability curve.
Results
Base-case results
The results of the deterministic analysis are presented in , which showed that treatment with ribociclib plus letrozole was less costly (incremental costs of −£3,273) and resulted in both a higher number of QALYs (incremental QALYs of 1.251) and life years ([LYs] incremental LYs of 1.597) compared with palbociclib plus letrozole. Consequently, ribociclib plus letrozole was dominant over palbociclib plus letrozole. A breakdown of the total costs is provided in .
Table 2. Results of the deterministic and probabilistic analyses.
Table 3. Costs breakdown of the deterministic results.
Probabilistic sensitivity analysis
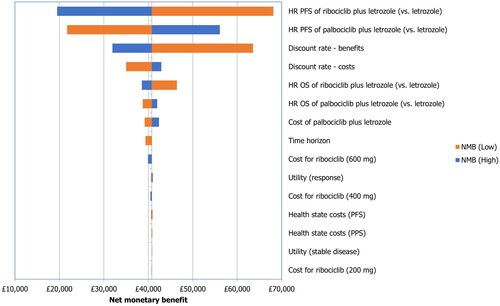
The results of the probabilistic analysis were similar to the results of the deterministic analysis (), with incremental costs, QALYs, and LYs of −£552, 1.376, and 1.761, respectively. The cost-effectiveness plane and cost-efficiency acceptability curve are shown in and , respectively. At a willingness-to-pay threshold of £30,000 per QALY, the probability of ribociclib plus letrozole being the cost-effective option was almost 100%.
Figure 1. Cost-effectiveness plane generated in the probabilistic sensitivity analysis. QALYs, quality-adjusted life years; WTP, willingness-to-pay.
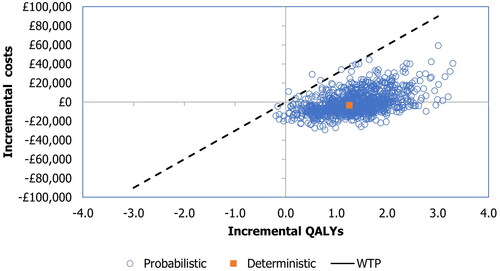
Figure 2. Cost-effectiveness acceptability curve generated in the probabilistic sensitivity analysis At a standard WTP threshold of £30,000 per QALY, the probability of ribociclib plus letrozole being the cost-effective option was almost 100%. QALY, quality-adjusted life-year; WTP, willingness-to-pay.
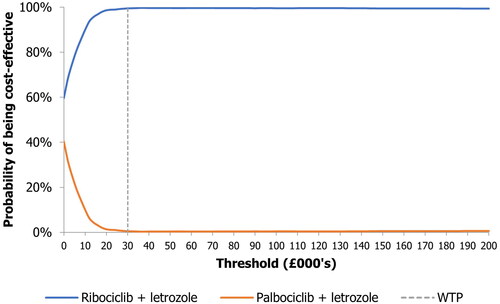
Deterministic sensitivity analysis
Results of the one-way deterministic sensitivity analysis are presented as a tornado plot in . This has been presented using change in the net monetary benefit rather than ICER due to the easier interpretation of scenarios with cost-savings and higher QALYs. The key model drivers were the PFS HRs of ribociclib plus letrozole and palbociclib plus letrozole, the discounting rate for benefits and costs, and the OS HRs of ribociclib plus letrozole and palbociclib plus letrozole. Nonetheless, in all scenarios the net monetary benefit was positive.
Discussion
The poor prognosis and survival rates of patients with ABC highlights the need for more effective hormonal therapies that extend life, slow disease progression, and avoid or delay the use of chemotherapy. While the cost-effectiveness of ribociclib plus letrozole versus palbociclib plus letrozole has been investigated previouslyCitation20, this analysis assessed the cost-effectiveness of ribociclib plus letrozole versus palbociclib plus letrozole from a UK NHS/PSS perspective using more mature trial data from the MONALEESA-2 and PALOMA-2 trials, confirming the findings from the previous analysis. It is important to note that this analysis has been conducted from a UK NHS/PSS perspective, so results should be interpreted within this setting; caution should be taken when extrapolating to other contexts.
Recently published PALOMA-2 OS data demonstrated no statistically significant benefit in OS for palbociclib plus letrozole versus placebo plus letrozoleCitation29. Indeed, ribociclib plus letrozole has a greater clinical benefit than palbociclib plus letrozole, with ribociclib plus letrozole having a significantly longer OS time when baseline characteristics were balanced between MONALEESA-2 and PALOMA-2 using a MAICCitation30. This greater clinical benefit with ribociclib plus letrozole led to an incremental QALY gain of 1.251 and incremental LY gain of 1.597 compared with palbociclib plus letrozole. Additionally, the analysis found ribociclib plus letrozole to be less costly than palbociclib plus letrozole, with incremental cost savings of £3,273. The majority of the cost savings resulted from savings in the 1L drug related-costs, with ribociclib plus letrozole being £17,156 cheaper than palbociclib plus letrozole over the lifetime horizon. The key driver of these savings was the lower drug cost (per mg) of ribociclib, which decreased with dose reductions compared with the fixed price of palbociclib irrespective of drug dose. The probabilistic sensitivity analysis demonstrates robustness of the results, which also found ribociclib plus letrozole to be dominant over palbociclib plus letrozole. At a willingness-to-pay threshold of £30,000 per QALY, there was an almost 100% probability of ribociclib plus letrozole being the cost-effective option. The conclusions are further strengthened by the deterministic sensitivity analysis; ribociclib plus letrozole remained cost-effective when HRs, utilities, ribociclib drug cost, and health state costs were varied.
The results of this analysis are consistent with the results of the previous analysis by Suri et al.Citation20, which concluded that ribociclib plus letrozole is both a cost-saving and cost-effective option compared with palbociclib plus letrozole. With more mature data from the MONALEESA-2 and PALOMA-2 trials, these conclusions have been strengthened.
Nevertheless, some limitations do remain in the present analysis. For instance, utility values are assumed to be the same for both ribociclib and palbociclib, rather than treatment-specific utilities being used. Additionally, this analysis utilised mature clinical trial data from the MONALESSA-2 and PALOMA-2 trials; to further strengthen the conclusions here, an analysis using real-world data could be conducted. Finally, due to the lack of head-to-head clinical trial data available for ribociclib versus palbociclib, an indirect treatment comparison was necessary for this analysis. While MAIC is a well-established and accepted method for providing comparative results in the absence of head-to-head data, there are some inherent limitations to this approachCitation44. While MAIC can address the observed differences between trials, there may be unmeasured and unobserved differences that remain; only a head-to-head randomized controlled trial can avoid unobserved confoundingCitation44. Despite the limitations associated with an indirect treatment comparison, the one-way sensitivity analysis presented here demonstrates the robustness of the results of this analysis when the HRs of PFS and OS are varied.
As mentioned above, another CDK4/6 inhibitor, abemaciclib, is also licensed and recommended for use in the same patient population as ribociclib and palbociclib in the UKCitation21,Citation45. A separate cost-effectiveness analysis of ribociclib versus abemaciclib has been conductedCitation22, which also found ribociclib to be both cost-saving and cost-effective options as compared to abemaciclib. The results of these cost-effectiveness analyses have implications for the treatment of HR+/HER2− ABC in the UK, in which the CDK4/6 inhibitors ribociclib, palbocilib, and abemaciclib are all approved for useCitation16,Citation17,Citation21. The results of both this analysis, and the separate analysis against abemaciclib, suggest that ribociclib is a cheaper and more effective treatment option compared with the other CDK4/6 inhibitors in the first-line endocrine setting.
Conclusions
The results of this cost-effectiveness analysis demonstrate that in postmenopausal women with HR+/HER2− ABC, 1L treatment with ribociclib plus letrozole is both cost-saving and cost-effective compared with palbociclib plus letrozole, from a UK NHS and PSS perspective. When considering more mature OS data, ribociclib shows evidence of a clear survival benefit, and this analysis demonstrates that the superior clinical efficacy is not associated with higher cost; indeed, ribociclib dominated palbociclib and is both cost-saving as well as cost-effective.
Transparency
Declaration of funding
This study was sponsored by Novartis Pharmaceuticals. Support for third-party writing assistance for this article, provided by Patrick Cox, BSc, and Fern Woodhouse, MChem, from Costello Medical, UK, was funded by Novartis Pharmaceuticals in accordance with Good Publication Practice (GPP 2022) guidelines (GPP 2022 [ismpp.org]).
Declaration of financial/other relationships
DCa: research funding from Exact Sciences, Novartis and Sanofi; consultant for Lilly, Novartis and Pfizer; VKS: employee of Novartis Healthcare Pvt Ltd, Hyderabad, India; CB: employee of Novartis Healthcare Pvt Ltd, Hyderabad, India; CC: employee of Novartis Pharmaceuticals UK Ltd, London, UK; DCh: employee of Novartis Services Inc., East Hanover, NJ, USA; PP: employee of Novartis Services Inc., East Hanover, NJ, USA.
Author contributions
Substantial contributions to study conception and design: DCa, VKS, CB, CC, DCh, PP; substantial contributions to material preparation, data collection and analysis: DCa, VKS, CB, CC, DCh, PP; drafting the article or revising it critically for important intellectual content: DCa, VKS, CB, CC, DCh, PP; final approval of the version of the article to be published: DCa, VKS, CB, CC, DCh, PP.
Reviewer disclosures
Peer reviewers on this manuscript have received an honorarium from JME for their review work but have no other relevant financial relationships to disclose.
Acknowledgements
The authors acknowledge Patrick Cox and Fern Woodhouse, from Costello Medical, UK, for medical writing and editorial assistance based on the authors’ input and direction.
References
- World Health Organization. Breast cancer. 2021. https://www.who.int/news-room/fact-sheets/detail/breast-cancer
- Cancer Research UK. Breast cancer incidence (invasive) statistics. 2021. https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/breast-cancer/incidence-invasive
- O'Shaughnessy J. Extending survival with chemotherapy in metastatic breast cancer. Oncologist. 2005;10(S3):20–29.
- Metastatic Breast Cancer Network. Incidence and incidence rates. 2022. http://mbcn.org/incidence-and-incidence-rates/
- DeKoven M, Bonthapally V, Jiao X, et al. Treatment pattern by hormone receptors and HER2 status in patients with metastatic breast cancer in the UK, Germany, France, Spain and Italy (EU-5): results from a physician survey. J Comp Eff Res. 2012;1(5):453–463.
- Howlader N, Altekruse SF, Li CI, et al. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst. 2014;106(5):dju055.
- NCI. Surveillance, epidemiology, and end results program. cancer stat facts: female breast cancer subtypes. 2023. https://seer.cancer.gov/statfacts/html/breast-subtypes.html
- Elfgen C, Bjelic-Radisic V. Targeted therapy in HR + HER2- Metastatic breast cancer: current clinical trials and their implications for CDK4/6 inhibitor therapy and beyond treatment options. Cancers. 2021;13(23):5994.
- Spring LM, Wander SA, Zangardi M, et al. CDK 4/6 inhibitors in breast cancer: current controversies and future directions. Curr Oncol Rep. 2019;21(3):25.
- Finn RS, Boer K, Bondarenko I, et al. Overall survival results from the randomized phase 2 study of palbociclib in combination with letrozole versus letrozole alone for first-line treatment of ER+/HER2- advanced breast cancer (PALOMA-1, TRIO-18). Breast Cancer Res Treat. 2020;183(2):419–428.
- Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16(1):25–35.
- Finn RS, Martin M, Rugo HS, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375(20):1925–1936.
- Hortobagyi GN, Stemmer S, Burris HA, et al. LBA17 overall survival (OS) results from the phase III MONALEESA-2 (ML-2) trial of postmenopausal patients (pts) with hormone receptor positive/human epidermal growth factor receptor 2 negative (HR+/HER2−) advanced breast cancer (ABC) treated with endocrine therapy (ET) ± ribociclib (RIB). Ann Oncol. 2021;32: s1290–S1291.
- Hortobagyi GN, Stemmer SM, Burris HA, et al. Ribociclib as First-Line therapy for HR-Positive, advanced breast cancer. N Engl J Med. 2016;375(18):1738–1748.
- Xu B, Hu X, Li W, et al. 228MO PALOMA-4: primary results from a phase III trial of palbociclib (PAL) + letrozole (LET) vs placebo (PBO) + LET in asian postmenopausal women with estrogen receptor-positive/human epidermal growth factor receptor 2-negative (ER+/HER2-) advanced breast cancer (ABC). Ann Oncol. 2021;32:s457.
- European Medicines Agency (EMA). Kisqali: summary of Product Characteristics. 2022. https://www.ema.europa.eu/en/documents/product-information/kisqali-epar-product-information_en.pdf
- European Medicines Agency (EMA). Ibrance: summary of Product Characteristics. 2022. https://www.ema.europa.eu/en/documents/product-information/ibrance-epar-product-information_en.pdf
- Slamon DJ, Neven P, Chia S, et al. Overall survival with ribociclib plus fulvestrant in advanced breast cancer. N Engl J Med. 2020;382(6):514–524.
- Turner NC, Slamon DJ, Ro J, et al. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med. 2018;379(20):1926–1936.
- Suri G, Chandiwana D, Lee A, et al. Cost-effectiveness analysis of ribociclib plus letrozole versus palbociclib plus letrozole in the United Kingdom. J Health Econ Outcomes Res. 2019;6(2):20–31.
- European Medicines Agency (EMA). Verzenios: summary of Product Characteristics. 2022. https://www.ema.europa.eu/en/documents/product-information/verzenios-epar-product-information_en.pdf
- Cameron D, Sharma V, Biswas C, et al. EE361 Cost-Effectiveness analysis of ribociclib Versus abemaciclib in the First-Line (1L) treatment of postmenopausal women With HR+/HER2- Advanced breast cancer (ABC). Value in Health. 2022;25(12S): s126.
- Study of Efficacy and Safety of LEE011 in postmenopausal women with advanced breast cancer (MONALEESA-2). 2022. https://clinicaltrials.gov/ct2/show/NCT01958021.
- Hortobagyi GN, Stemmer SM, Burris HA, et al. Overall survival with ribociclib plus letrozole in advanced breast cancer. N Engl J Med. 2022;386(10):942–950.
- Hortobagyi GN, Stemmer SM, Burris HA, et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann Oncol. 2018;29(7):1541–1547.
- Study of letrozole with or without palbociclib (PD-0332991) for the first-line treatment of hormone-receptor positive advanced breast cancer. 2022. https://clinicaltrials.gov/ct2/show/NCT00721409
- A study of palbociclib (PD-0332991) + Letrozole vs. Letrozole for 1st line treatment of postmenopausal women with ER+/HER2- advanced breast cancer (PALOMA-2). 2022. https://clinicaltrials.gov/ct2/show/NCT01740427
- A Study Of Palbociclib (PD-0332991) + Letrozole VS. Placebo + Letrozole for 1st line treatment of asian postmenopausal women with ER+/HER2- advanced breast cancer [PALOMA-4]. 2022. https://clinicaltrials.gov/ct2/show/NCT02297438
- Finn RS, Rugo HS, Dieras VC, et al. Overall survival (OS) with first-line palbociclib plus letrozole (PAL + LET) versus placebo plus letrozole (PBO + LET) in women with estrogen receptor–positive/human epidermal growth factor receptor 2–negative advanced breast cancer (ER+/HER2− ABC): analyses from PALOMA-2. J Clin Oncol. 40(17_suppl):LBA1003–LBA1003.
- Jhaveri K, O'Shaughnessy J, Fasching PA, et al. Matching adjusted indirect comparison of PFS & OS comparing ribociclib + letrozole vs palbociclib + letrozole as first-line treatment of HR+/HER2− ABC: analysis based on updated PFS & final OS results of MONALEESA-2 & PALOMA-2. Eur J Cancer. 2022;175:S2–S3.
- Latimer NR. Survival analysis For economic evaluations alongside clinical Trials - Extrapolation with Patient-Level data. NICE DSU technical support document 14. London: national Institute for Health and Care Excellence (NICE); 2013. https://www.ncbi.nlm.nih.gov/books/NBK395885/
- Rugo HS, Finn RS, Diéras V, et al. Palbociclib plus letrozole as first-line therapy in estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer with extended follow-up. Breast Cancer Res Treat. 2019;174(3):719–729.
- Devlin NJ, Shah KK, Feng Y, et al. Valuing health-related quality of life: an EQ-5D-5L value set for England. Health Econ. 2018;27(1):7–22.
- Hudgens S, Briggs A, Tremblay G, et al. Comparison of methods to estimate health state utilities in metastatic breast cancer (MBC). Value Health. 2014;17(7):A557.
- Lloyd A, Nafees B, Narewska J, et al. Health state utilities for metastatic breast cancer. Br J Cancer. 2006;95(6):683–690.
- National Institute for Health and Care Excellence. British National Formulary (BNF). 2022. https://bnf.nice.org.uk/
- National Health Service. 2019-20 reference costs. 2022. https://digital.nhs.uk/data-and-information/data-collections-and-data-sets/data-collections/reference-costs
- Brufsky AM. Delaying chemotherapy in the treatment of hormone Receptor-Positive, human epidermal growth factor receptor 2-Negative advanced breast cancer. Clin Med Insights Oncol. 2015;9:137–147.
- Das R, Cope S, Ouwens M, et al. Economic evaluation of fulvestrant 500 mg versus generic nonsteroidal aromatase inhibitors in patients with advanced breast cancer in the United Kingdom. Clin Ther. 2013;35(3):246–260.e5.
- Lin PL, Hao Y, Xie J, et al. Physician experiences and preferences in the treatment of HR+/HER2- metastatic breast cancer in the United States: a physician survey. Cancer Med. 2016;5(2):209–220.
- Macalalad AR, Hao Y, Lin PL, et al. Treatment patterns and duration in post-menopausal women with HR+/HER2- metastatic breast cancer in the US: a retrospective chart review in community oncology practices (2004-2010). Curr Med Res Opin. 2015;31(2):263–273.
- Saad ED, Katz A, Buyse M. Overall survival and post-progression survival in advanced breast cancer: a review of recent randomized clinical trials. J Clin Oncol. 2010;28(11):1958–1962.
- Round J, Jones L, Morris S. Estimating the cost of caring for people with cancer at the end of life: a modelling study. Palliat Med. 2015;29(10):899–907.
- Signorovitch JE, Sikirica V, Erder MH, et al. Matching-adjusted indirect comparisons: a new tool for timely comparative effectiveness research. Value Health. 2012;15(6):940–947.
- National Institute for Health and Care Excellence. Abemaciclib with an aromatase inhibitor for previously untreated, hormone receptor-positive, HER2-negative, locally advanced or metastatic breast cancer [TA563]. 2019. https://www.nice.org.uk/guidance/ta563