Abstract
Objective
The radiopharmaceuticals radium-223 and the pharmacy preparation 177Lu-PSMA-I&T are reimbursed in the Netherlands for metastatic castration-resistant prostate cancer (mCRPC) treatment. Although shown to be life-prolonging in patients with mCRPC, the treatment procedures associated with these radiopharmaceuticals can be challenging for both patients and hospitals. This study investigates the costs of mCRPC treatment in Dutch hospitals for currently reimbursed radiopharmaceuticals with a demonstrated overall survival benefit.
Methods
A cost model that calculated the direct medical per-patient costs of radium-223 and 177Lu-PSMA-I&T was developed, following clinical trial regimens. The model considered six 4-weekly administrations (i.e. ALSYMPCA regimen) of radium-223. Regarding 177Lu-PSMA-I&T, the model used both the VISION regimen (i.e. five 6-weekly administrations) and the SPLASH regimen (i.e. four 8-weekly administrations). Based on health insurance claims, we also estimated the coverage a hospital would receive for providing treatment. No fitting health insurance claim for 177Lu-PSMA-I&T is currently available; therefore, we calculated a break-even value for a potential health insurance claim that would exactly counterbalance the per-patient costs and coverage.
Results
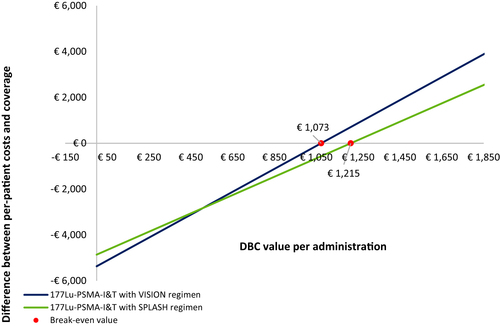
Radium-223 administration is associated with per-patient costs of €30,905, and these costs are fully covered by the coverage a hospital receives. The per-patient costs of 177Lu-PSMA-I&T range between €35,866 and €47,546 per administration period, depending on the regimen. Current healthcare insurance claims do not fully cover the costs of providing 177Lu-PSMA-I&T: hospitals must pay €4,414–€4,922 for each patient out of their own budget. The break-even value for the potential insurance claim covering 177Lu-PSMA-I&T administration with a VISION (SPLASH) regimen is €1,073 (€1,215).
Conclusion
This study shows that, without consideration of the treatment effect, radium-223 treatment for mCRPC leads to lower per-patient costs than treatment with 177Lu-PSMA-I&T. The detailed overview of the costs associated with radiopharmaceutical treatment provided by this study is relevant for both hospitals and healthcare insurers.
PLAIN LANGUAGE SUMMARY
Prostate cancer is the most common form of cancer among men in the Netherlands, and its treatment is increasingly expensive. Given the limited hospital budget, it is important to consider costs in the treatment of prostate cancer. Radiopharmaceuticals are one of the multiple treatment options for metastatic prostate cancer. The current study looked at the costs of two radiopharmaceuticals, radium-223 and 177Lu-PSMA-I&T, while using multiple treatment regimens.
The cost of radium-223 treatment is €30,905 per patient and is fully covered by insurance. The cost of 177Lu-PSMA-I&T treatment ranges from €35,866 to €47,546 per patient and is partially paid from the budget of the hospitals considering current reimbursement amounts. The study shows that, without consideration of the treatment effects, radium-223 treatment for prostate cancer leads to lower per-patient costs than treatment with 177Lu-PSMA-I&T. The detailed overview of the costs associated with radiopharmaceutical treatment provided by this study is relevant for both hospitals and healthcare insurers to manage prostate cancer treatment costs.
Introduction
Prostate cancer is the most prevalent cancer among males in the Netherlands, with over 12,000 cases diagnosed every year.Citation1 Some of these patients will have metastatic castration-resistant prostate cancer (mCRPC), an advanced form of prostate cancer that no longer responds to castration treatments (surgical or chemical [induced by hormones]).Citation2,Citation3 Despite several available treatment options, mCRPC has a poor prognosis – patients have an estimated overall survival of 14.0 months.Citation3 Moreover, skeletal-related events and severe pain associated with bone metastases impact a patient’s physical well-being and ability to perform the basic activities of daily life, which significantly reduces their quality-of-life.Citation4,Citation5 The economic impact of prostate cancer is also substantial – in 2019, the total healthcare expenses for prostate cancer in the Netherlands were estimated at 350 million euros. Hospital care and medicine use comprised 80% and 11% of these costs, respectively.Citation6
Life-prolonging drugs in mCRPC include chemotherapy, androgen receptor pathway inhibitors, poly(adenosine diphosphate-ribose) polymerase inhibitors, Sipuleucel-T, and radiopharmaceuticals.Citation7,Citation8 Although many options are available, not all treatments are effective in all patients. The selection of treatment for mCRPC is multifactorial and generally depends on previous treatments, quality of response, resistance, and patient characteristics.Citation8 Therefore, appropriate treatment management is critical. Several radiopharmaceuticals are used for the treatment of metastatic bone pain in mCRPC.Citation9–12 However, currently, only two with a demonstrated overall survival benefit are reimbursed for the treatment of mCRPC in the Netherlands: radium-223 and the pharmacy preparation 177Lu-PSMA-I&T.Citation9,Citation13
Radium-223 is a radiopharmaceutical that emits alpha radiation and targets bone metastases that has been on the Dutch market since 2013Citation9,Citation14,Citation15. 177Lu-PSMA-I&T is a pharmacy preparation with an almost similar mechanism of action to the commercial 177Lu-PSMA-617.Citation16 These 177Lu-PSMA therapies target cancer cells by binding to prostate-specific membrane antigen (PSMA) receptors (present in over 80% of mCRPC patients) and emitting beta radiation.Citation17 The commercialCitation16 177Lu-PSMA-617 has recently received approval from the European Commission for the treatment of progressive PSMA-positive mCRPC; however, the time frame in which 177Lu-PSMA-617 will receive reimbursement in the Netherlands is uncertain as the Dutch reimbursement process for hospital drugs varies in duration (median duration is 420 days).Citation18,Citation19 The SPLASH trial is currently studying the efficacy of 177Lu-PSMA-I&T compared with abiraterone or enzalutamide in chemo-naïve patients with mCRPC.Citation20 Although the European Medicines Agency (EMA) is yet to approve 177Lu-PSMA-I&T, the pharmacy preparation already received reimbursement in the Netherlands in 2021. Reimbursement is restricted to the treatment of mCRPC in adult men that are PSMA positive in the absence of a more suitable therapeutical.Citation12
The economic impact of metastatic prostate cancer is substantial and is escalating.Citation21 Although both radium-223 and 177Lu-PSMA-I&T are effective in the treatment of mCRPC, radiopharmaceuticals are also accompanied by additional treatment procedures (e.g. diagnostic scans and observation) that impact resource use and costs.Citation16,Citation22 As the introduction of new radiopharmaceuticals in metastatic prostate cancer continues (e.g. actinium-225-PSMA)Citation23 and the use of radiopharmaceuticals is expected to increase, the (economic) impact of radiopharmaceuticals on clinical practice in the Netherlands is still unclear. Radiopharmaceutical treatment is subsidized by the hospital budget and given the budget cap of hospitals and high mCRPC expenses,Citation6 it is important to consider the treatment procedures and associated cost impact in the use of radiopharmaceuticals. This study contributes to insight into the cost aspects of radiopharmaceutical treatment by investigating the integral per-patient costs of mCRPC treatment in Dutch hospitals for currently reimbursed radiopharmaceuticals with demonstrated overall survival benefit.
Methods
Model characteristics
A cost model was developed in Microsoft Excel 2016 (Redmond, WA) to calculate the per-patient costs of radium-223 and the pharmacy preparation 177Lu-PSMA-I&T in Dutch hospitals with a bottom-up approach following the Consolidated Health Economic Evaluation Reporting Standards 2022 (Supplementary Appendix 1).Citation24 In addition, to assess the impact of radiopharmaceutical treatment on the economic burden, the model estimated the difference between the per-patient costs and the coverage a hospital receives for the treatment based on the average claim to healthcare insurers. This insight into the per-patient costs and coverage which is of value for hospitals and healthcare insurance companies given the limited yearly hospital budget. Besides the average outcomes, we performed a scenario and breakeven analysis in which the impact of different treatment patterns and reimbursement values was determined.
The course of radium-223 treatment was based on its Summary of Product Characteristics (SmPC) and the ALSYMPCA trial.Citation9,Citation14 The course of 177Lu-PSMA-I&T treatment was based on the VISION and SPLASH trials.Citation16,Citation20 In the ALSYMPCA trial, radium-223 significantly improved median overall survival by 3.6 months (hazard ratio for death = 0.70 [0.58–0.83, p < 0.001]) compared with a placebo.Citation14 In the VISION trial, 177Lu-PSMA-617 was shown to significantly improve median overall survival by 4.0 months (hazard ratio for death = 0.62 [0.52–0.74, p < 0.001]) compared with standard care.Citation16 Although the overall survival benefits seem comparable,Citation14,Citation16,Citation25 no (in)direct treatment comparisons have been performed, and there are some differences in their targeted patient population. Radium-223 is approved as monotherapy or in combination with a luteinizing hormone-releasing hormone (LHRH) analogue in the treatment of adult mCRPC patients with symptomatic bone metastases and no known visceral metastases, who progressed after at least two prior lines of systemic therapy for mCRPC (other than LHRH analogues) or who are not eligible for available systemic mCRPC treatment.Citation9 177Lu-PSMA-I&T is indicated for PSMA-positive mCRPC patients as last resort treatment.Citation9,Citation16 Our analysis focused on the treatment costs for hospitals and healthcare insurers and did not encompass the treatment effect and patient characteristics reported in the clinical trials. The model structure and inputs were validated by two Dutch clinical experts.
Time horizon and perspective
Because our study focused on treatment costs that are relevant for hospitals and healthcare insurers, we used a Dutch healthcare payer’s perspective, which includes all direct medical costs related to mCRPC treatment with radiopharmaceuticals.Citation26 Only variable cost parameters were considered, and these consisted of medication, administration, clinic visits, imaging, hospital admission, and supportive care. The time horizon was equal to the administration period of radiopharmaceuticals because it covered all costs relevant to Dutch healthcare payers. Due to the short time horizon, no discount rate was used.Citation27
Healthcare utilization during radiopharmaceutical treatment
Our model based the number of injections, dosage, and regimen on clinical trial data (). The base case treatment regimen of radium-223 was based on the median number of injections in the ALSYMPCA trial.Citation14 The treatment regimen of 177Lu-PSMA-I&T in the base case was based on the median number of injections used in the VISION trial and the number of injections in the SPLASH trial.Citation16,Citation20 As of this writing, the efficacy of 177Lu-PSMA-617 was established in the VISION regimen,Citation16 but the efficacy of 177 LU-PSMA-I&T in the SPLASH regimen was yet to be determined.Citation20 Our analysis included both regimens to reflect the Dutch clinical practice as closely as possible.
Table 1. The healthcare utilization of radium-223 and 177Lu-PSMA-I&T.
Other healthcare utilization input was based on literature, hospital websites, and expert opinion. Prior to radium-223 treatment, the presence of bone metastases was recognized by a bone scan, the presence of visceral metastases was ruled out by a CT scan,Citation9 and an alkaline phosphatase assessment was performed before radium-223 administration.Citation9,Citation14 After each 177Lu-PSMA-I&T administration, an observation period of 6 hours was incorporated because of the beta radiation exposure.Citation28 Prior to 177Lu-PMSA-I&T treatment, the presence of PSMA receptors on the tumor cells was established using 68Ga-PSMA-PET/CT scans following Dutch clinical practice.Citation22
Patients can be admitted to a hospital in between or during their radiopharmaceutical injections. Diagnosis treatment combination (diagnose-behandelcombinatie [DBC]) registration data from 2020 suggest that approximately 12% of patients experience a hospital admission during or between radiopharmaceutical administrations.Citation29 The hospital admission was assumed to last 1 day, based on expert opinion. The use of bone health agents such as bisphosphonates or denosumab was considered during radium-223 treatment.Citation9 During 177Lu-PSMA treatment, the use of both bone health agents and anti-emetics was assumed.Citation16
Costs
summarizes all costs that were incorporated into the model. All costs were inflated to 2021 prices in euros using the published consumer price index from the Dutch Central Bureau of Statistics.Citation30 Treatment costs incorporated medication costs, administration costs, and outpatient visits for every injection.Citation31,Citation32 Medication costs were based on list prices retrieved from data on file, excluding value-added tax. For 177Lu-PSMA-I&T, the list price reflects the cost price of hospital preparation (i.e. raw materials, devices, and labor).Citation13,Citation33 Imaging, biomarker assessments, observation time, hospital admission, and supportive care costs were also incorporated (). Imaging costs were estimated on the average of the published rates of two Dutch hospitals.Citation34,Citation35 Biomarker assessment costs were based on the rates published by the Dutch Healthcare Authority.Citation36 Costs of observation and hospital admissions were determined following the Dutch cost manual.Citation32 The costs of bone health agents were calculated by using the average list prices of denosumab and the available bisphosphonates in the Netherlands (Supplementary Appendix 2). The costs of anti-emetics were based on an average of the list prices of dexamethasone.
Table 2. Overview of included cost inputs.
Difference in per-patient costs and coverage
After establishing the per-patient costs of treating mCRPC with radiopharmaceuticals, the model calculated the difference between the per-patient costs and the coverage that a hospital receives. In the Netherlands, a hospital covers healthcare expenses with claims to healthcare insurers. This coverage is retrieved via DBC codes for healthcare utilization and add-on codes for expensive medicines (i.e. >€1,000 per patient per year). DBC codes are standardized sets of care activities that are combined into one product.Citation29 When a care activity is performed, a healthcare provider can reimburse the costs for this activity by invoicing the corresponding DBC code at a health insurance company. In theory, DBC codes should cover all hospital expenses; however, the coverage may be below or above the actual expenses because expenses and coverage are based on the average patient and are hospital dependent.
The impact on the hospital budget was calculated by subtracting the calculated hospital expenses from the average coverage a hospital receives from health insurance claims in the form of DBC codes. The average DBC code values were obtained from OpenDIS ().Citation29 Currently, there is a dedicated DBC code for radium-223 administration, but no DBC code for 177Lu-PSMA-I&T administration. This dedicated DBC code for radium-223 is restricted to radium-233 administration; that is, hospitals cannot use the DBC code for radium-223 administration for 177Lu-PSMA-I&T.Citation29 Therefore, hospitals commonly use a more general, less restrictive DBC code for supportive care in prostate cancer to cover 177Lu-PSMA-I&T administration. The selection of the supportive care DBC codes was based on expert opinion.
Table 3. DBC codes used to calculate the coverage of hospitals for radiopharmaceutical administration.
Break-even price
As no dedicated DBC code is currently available for 177Lu-PSMA-I&T, a break-even value for a future DBC code for 177Lu-PSMA-I&T administration was calculated – defined as the DBC value that would exactly counterbalance the per-patient costs and the coverage. Instead of the implementation frequency of 120 days of the current DBC code for supportive care, the calculation assumed an implementation before every administration.Citation37
Univariate sensitivity and scenario analysis
To assess the impact of input parameters on the per-patient costs of radiopharmaceutical treatment, a univariate sensitivity analysis was performed. In the univariate sensitivity analysis, parameters were varied within a 25% interval.Citation27 Because no SmPC or guideline regarding 177Lu-PSMA-I&T treatment is available, the specific treatment protocols in hospitals differ, and several assumptions had to be made (Supplementary Appendix 3). A scenario analysis was performed to account for those differences. First, in the base case, our analysis based the regimens of radiopharmaceuticals on clinical trial regimens. However, the 177Lu-PSMA-I&T regimens in Dutch hospitals are ambiguous and may differ from those clinical trials.Citation22,Citation38,Citation39 Therefore, the scenario analysis estimated the per-patient costs of three 177Lu-PSMA-I&T administrations. The three administrations were based on expert opinion and given in a modified SPLASH trial regimen (i.e. 8-week intervals and a dosage of 6.8 GBq), the effectiveness of which has not yet been studied. Second, some hospitals use a CT whole body scan or 18 F-FDG PET/CT scan before and during 177Lu-PSMA-I&T treatment besides the diagnostic PET/CT scan at the start of treatment.Citation39 Therefore, the impact of including these scans was explored in the scenario analysis. Third, the duration of observation varies between hospitals; consequently, the effect of an observation period of 24 hours was explored.Citation22,Citation39
Results
Base case outcomes
shows the per-patient costs and coverage costs over the administration periods of radium-223 and 177Lu-PSMA-I&T. Radium-223 is associated with the lowest per-patient costs (€30,905). The coverage a hospital receives with radium-223 treatment (€34,806) is sufficient to cover the expenses made. With five 6-weekly injections of 7.4 GBq (VISION regimen), the per-patient costs of 177Lu-PSMA-I&T are €47,546, and the coverage costs are €42,624, resulting in a difference of −€4,922. With four 8-weekly injections of 6.8 GBq (SPLASH regimen), the per-patient costs of 177Lu-PSMA-I&T are €35,866, and the coverage costs are €31,452, resulting in a difference of −€4,414. For both treatments, regardless of the regimen, medication costs account for the largest part of the costs (i.e. 85–88% of the total costs).
Table 4. Per-patient costs and coverage in the administration period of radiopharmaceuticals.
Break-even analysis
The break-even analysis was performed to establish the tariff for a potential DBC code of 177Lu-PSMA-I&T that would exactly counterbalance the per-patient costs and the coverage (). When the VISION regimen was followed (i.e. five 6-weekly injections of 7.4 GBq), the break-even value per injection for the DBC code covering 177Lu-PSMA-I&T administration was €1,073. When a hospital followed the SPLASH regimen (i.e. four 8-weekly injections of 6.8 GBq), the break-even value per injection of the DBC code covering 177Lu-PSMA-I&T administration was €1,215.
Univariate sensitivity analysis
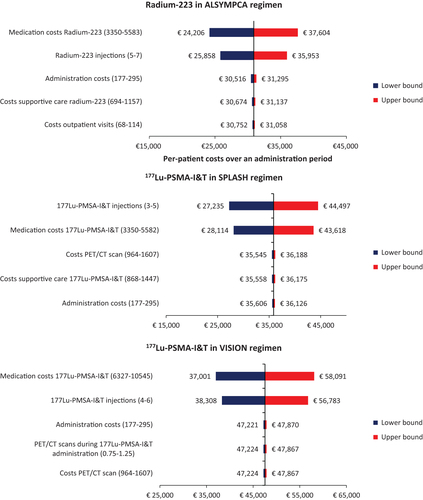
For both radium-223 and 177Lu-PSMA-I&T, the parameters with the most impact were the number of injections and medication costs (). Administration and supportive care expenditures had a substantial influence on the per-patient costs of radium-223. For 177Lu-PSMA-I&T in the VISION regimen, the costs of both the administration and frequency of PET/CT scans were also important contributors to the total expenses. In the SPLASH regimen, costs and frequency of PET/CT scans and costs of supportive care were important contributors to the per-patient costs.
Figure 2. Tornado diagrams presenting the five most influential parameters on the per-patient costs of radium-223 in the ALSYMPCA regimen, and the per-patient costs of 177Lu-PSMA-I&T in the VISION and SPLASH regimens. Abbreviations. CT, computed tomography; 177Lu-PSMA-I&T, lutetium-177-labeled-prostate-specific membrane antigen imaging and treatment.
Scenario analysis
The scenario analysis estimated the impact of a modified SPLASH regimen with three 177Lu-PSMA-I&T administrations (Scenario 1). and show that three administrations for 177Lu-PSMA-I&T led to lower per-patient costs than the per-patient costs of radium-223: 177Lu-PSMA-I&T incurred per-patient costs of €27,221 with a dosage of 6.8 GBq and 8-weekly administrations, and radium-223 incurred per-patient costs of €30,905 (). The coverage does not fully cover the costs of providing 177Lu-PSMA-I&T, but the difference is smaller than in the base case: hospitals have to pay €3,521 for each patient out of their budget. The break-even value per injection of the DBC code covering 177Lu-PSMA-I&T was €1,322 when three injections were used (Supplementary Appendix 4).
Figure 3. Per-patient costs for 177Lu-PSMA-I&T and radium-223 per administration period. Note, the modified SPLASH regimen consisted of three 8-weekly injections of 6.8 GBq. Abbreviations. 177Lu-PSMA-I&T, lutetium-177-labeled-prostate-specific membrane antigen imaging and treatment.
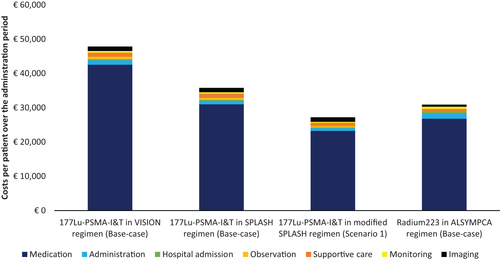
Table 5. The impact on and the difference between per-patient costs and coverage in the scenario analyses.
Both the 24-hour observation period after 177Lu-PSMA-I&T administration (Scenario 2) and the inclusion of extra PET/CT and 18-FDG PET/CT scans (Scenario 3) increased the per-patient costs of 177Lu-PSMA-I&T (). The 24-hour observation period after 177Lu-PSMA-I&T administration led to per-patient costs of €49,548 in the VISION regimen and €37,468 in the SPLASH regimen. The addition of more scans led to per-patient costs of €50,117 in the VISION regimen and €38,437 in the SPLASH regimen.
Discussion
The insights into the per-patient costs and coverage per administration period of radiopharmaceutical therapies that our study provides can help guide hospitals in the appropriate use of these treatments for mCRPC and contribute to the management of the escalating costs.Citation21 Our research shows that, among the radiopharmaceuticals currently reimbursed for the treatment of mCRPC patients, radium-223 treatment incurs lower per-patient costs (€30,905) than treatment with 177Lu-PSMA-I&T (VISION regimen: €47,546; SPLASH regimen: €35,866) when following clinical trial regimens. While the claims of the health insurance companies cover all expenses of radium-223 administration, hospitals pay €4,414–€4,922 out of their own budget per patient treated with 177Lu-PSMA-I&T.
Currently, there is no fitting DBC code for 177Lu-PSMA-I&T administration available, which forces hospitals to invoice DBCs that do not cover all costs. It is expected that a DBC code for 177Lu-PSMA-I&T will become available in the coming years;Citation37 therefore, our study calculated the break-even value that would be needed to reach equal per-patient costs and coverage for this DBC code. Our study indicates that the break-even value of this DBC code will be dependent on the regimen used (i.e. €1,073 in the VISION regimen and €1,215 in the SPLASH regimen).
Our study includes the radiopharmaceuticals that are reimbursed in the Netherlands for the treatment of mCRPC. Although 177Lu-PSMA-617 has recently received European Commission approval,Citation18 it is not yet reimbursed in the Netherlands and therefore not included in our analysis. It is expected that 177Lu-PSMA-617 will have a comparable regimen and treatment protocol to 177Lu-PSMA-I&T, but higher medication costs will result in higher per-patient costs.Citation40,Citation41 However, an update of this study after reimbursement is needed to confirm these expectations.
As of this writing, the efficacy of 177Lu-PSMA was determined in the VISION trial using five injections,Citation16 but the efficacy based on four injections, studied in the SPLASH trial, had not yet been established.Citation20 Although costs should be considered only when the treatment (regimen) has proven to be effective, we chose to include both regimens to reflect Dutch clinical practice as closely as possible; regimens differ between hospitals and every hospital negotiates its budget with healthcare insurers. Nevertheless, not all hospitals use the four to five cycles that are protocol in the VISION and SPLASH trial.Citation16,Citation20 Therefore, based on expert opinion, the scenario analysis further assessed the uncertainty in the 177Lu-PSMA-I&T treatment regimen. The costs of a modified SPLASH regimen (i.e. three 8-weekly 177Lu-PSMA-I&T injections of 6.8 GBq) were established, although the efficacy of this regimen has not yet been studied. This scenario resulted in lower per-patient costs than radium-223 per administration period (i.e. €27,221 for 177Lu-PSMA-I&T and €30,905 for radium-223).
It is important to relate clinical trial data to real-world evidence. Currently, several real-world data projects in prostate cancer are being conducted, which might help homogenize treatment patterns for patients with mCRPC.Citation42 Older Dutch real-world data suggest that the patient characteristics of mCRPC in ≥ third-line treatment slightly deviate from the clinical trial data (i.e. similar age but higher ECOG).Citation14,Citation16,Citation43 However, patient characteristics and treatment effects do not necessarily affect the treatment course and therefore, in our case, the study outcomes. To account for the possibility that subsets of patients receive different numbers of injections, we provided a detailed overview of the different cost parameters to make the results translatable to different clinical practices.
The treatment patterns assumed for radium-223 were based on clinical trial data and SmPC description. Moreover, the input for the resource use is in line with earlier Dutch health economic models focusing on the costs of radium-223.Citation31 However, the lack of SmPC or guidelines regarding 177Lu-PSMA-I&T treatment means hospitals differ in specific treatment protocols and thus assumptions were required for the model, resulting in uncertainty in the outcomes. Therefore, several scenarios were performed in which the impact of different treatment patterns was explored. First, the use of scans before and during 177Lu-PMSA-I&T treatment differs between hospitals.Citation22,Citation39 The base case scenario only considers the use of a diagnostic PET/CT scan. However, some hospitals use an additional PET/CT after the first administration and/or use a (diagnostic) F18-FDG PET/CT scan.Citation39 The scenario analysis showed that the inclusion of those scans increased costs by €2,571 for each patient per administration period. Second, the observation period differs between hospitals. The scenario analysis showed that an observation period of 24 hours increased the total per-patient costs by €2,002 in the VISION regimen and €1,602 in the SPLASH regimen.
Pharmacy preparation requires raw materials, labor, and devices. The list price of 177Lu-PSMA-I&T is estimated to be a reliable indicator of its total preparation and medication costs and, to further explain uncertainties in the list price, we account for variations in the univariate sensitivity analysis.Citation33 However, the list price does not consider investment and hospital capacity as it already requires facilities, equipment, and labor. In addition, hospitals need access to dedicated rooms and a sufficient workforce for the observation of patients who have been administered radiopharmaceuticals that emit beta radiation.Citation22,Citation44 It is a limitation of our study that it does not consider the investment costs of facilities or the impact on the capacity of a hospital, and that it is restricted to direct medical per-patient costs. Therefore, in a future study, it is important to also consider the impact of radiopharmaceutical treatment on hospital capacity.
Although our study includes the costs of radiopharmaceutical therapies, our analysis does not consider patient characteristics and treatment effectiveness. Selecting the most appropriate therapy for a patient should not be led by costs but rather by patient characteristics and patient preferences. In this respect, it is important to consider the suitability of radiopharmaceuticals by, for example, taking patient characteristics and preferences into account. Although both 177 LU-PSMA-I&T and radium-223 are used to treat mCRPC, the exact indications do not correspond. Differences in the indications include the type of metastases (i.e. radium-223 solely targets bone metastases) and PSMA status (i.e. 177Lu-PSMA-I&T only targets PSMA-positive cells).Citation14,Citation20 Current research and experience in daily practice are needed to determine which treatment is most appropriate for each patient.Citation42 To improve the clinical interpretability of our analysis, an update should be performed as soon as this long-term real-world data on treatment effect is available.
Given the limited hospital budget and capabilities combined with the high mCRPC expenses, it is important to consider the treatment procedures and associated economic impact in the use of radiopharmaceuticals. Therefore, the detailed overview of the costs and coverage associated with the radiopharmaceutical treatment of mCRPC produced by this study is highly relevant information that can be of use to Dutch hospitals and healthcare insurers to improve the treatment management of mCRPC patients. The findings inform stakeholders about the impact of different cost parameters of radiopharmaceutical treatment on the hospital budget and can be used in price negotiations.
Our study shows that, without consideration of the treatment effect, the per-patient costs of treating mCRPC are lower for radium-223 compared with 177Lu–PSMA-I&T when following clinical trial regimens. Furthermore, our study demonstrates that the current lack of a fitting DBC code results in insurance company claims that do not cover the expenses incurred during 177 Lu-PSMA-I&T administration. The results of this study can contribute important data for improving healthcare allocation in radiopharmaceutical treatments for mCRPC.
Transparency
Declaration of funding
This work was funded by Bayer B.V., Pharmaceuticals Division. The funder provided support in the form of payment to Asc Academics, of which SWQ, JHJP and RDF are employees.
Declaration of financial/other relationships
SWQ, JHP, DNJW, and RDF have no relationships to be declared in relation to the subject. JN has disclosures in the form of Research Grants, Consulting, Teaching, and Invited Talks for AAA/Novartis, ABX, Bayer AG, CURIUM, NRG, Pfizer, and POINT biopharma.
Author contributions
Conception and design: SWQ, JHJP, DNJW, JN
Analysis and interpretation of data: SWQ, JHJP, RDF
Drafting of the paper: SWQ
Revision of the paper: JHJP, DNJW, JN, RDF
Final approval of the paper: SWQ, JHJP, DNJW, JN, RDF
All authors are accountable for all aspects of the work
Reviewer disclosures
Peer reviewers on this manuscript have received an honorarium from JME for their review work but have no other relevant financial relationships to disclose.
Supplemental Material
Download MS Word (25.5 KB)Supplemental Material
Download MS Word (27.1 KB)Supplemental Material
Download MS Word (17 KB)Acknowledgements
The authors thank Roma Kwiatkiewicz and Magdalena Beynon of Asc Academics for providing their medical writing support.
Data availability statement
Most data are included in the manuscript and its supporting information files. The analyses were largely conducted based on publicly available information which is presented and referenced in the article and supplementary information files.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
References
- IKNL Nederland. [Prostate cancer in the Netherlands] [Internet]. 2022. [cited 2022 Dec 1]. Available from: https://iknl.nl/prostaatkanker-in-nederland.
- Zorginstituut Nederland. Verbetersignalement Zinnig gebruik van geneesmiddelen bij patiënten met castratie refractair prostaatcarcinoom [Improvement report Sensible use of medicines patients with castration refractory prostate carcinoma] [Internet]. 2016. [cited 2022 Dec 1]. Available from: https://www.zorginstituutnederland.nl/werkagenda/publicaties/rapport/2016/11/21/zinnige-zorg-verbetersignalement-zinnig-gebruik-van-geneesmiddelen-bij-patienten-met-castratie-refractair-prostaatcarcinoom.
- Kirby M, Hirst C, Crawford ED. Characterising the castration-resistant prostate cancer population: a systematic review. Int J Clin Pract. 2011;65:1180–1192.
- Ivanescu C, Phung D, Loriot Y, et al. Skeletal-related events (SRES) impact significantly the health-related quality of life (HRQOL) of chemo-naive men with metastatic castration resistant prostate cancer (MCRPC). Value in Health. 2014;17:A650–A651.
- Sraieb M, Hirmas N, Conrad R, et al. Assessing the quality of life of patients with metastatic castration-resistant prostate cancer with bone metastases receiving [223Ra]RaCl2 therapy. Medicine. 2020;99:e22287.
- VZInfo. Zorguitgaven prostaatkanker naar sector 2019 [Prostate cancer health care expenditure by sector 2019] [Internet]. [cited 2022 Oct 7]. Available from: https://www.vzinfo.nl/prostaatkanker/zorguitgaven.
- He L, Fang H, Chen C, et al. Metastatic castration-resistant prostate cancer: academic insights and perspectives through bibliometric analysis. Medicine (Baltimore). 2020; 99:e19760.
- European Association of Urology. Guidelines on prostate cancer [Internet]. 2022. [cited 2023 Feb 14]. Available from: https://d56bochluxqnz.cloudfront.net/documents/full-guideline/EAU-EANM-ESTRO-ESUR-ISUP_SIOG-Guidelines-on-Prostate-Cancer-2022_2022-04-25-063938_yfos.pdf.
- European Medicines Agency (EMA). Summary of product characteristics (SmPC) radium-223. 2013 [cited 2023 Jan 5]. Available from: https://www.ema.europa.eu/en/documents/product-information/xofigo-epar-productinformation_en.pdf
- de Klerk J, ter Heine R, Bloemendaal H. Richtlijn 89Sr chloride (Metastron®) [Guideline Richtlijn 89Sr chloride (Metastron®)] [Internet]. [cited 2022 Dec 6]. Available from: https://richtlijnendatabase.nl/gerelateerde_documenten/f/17268/89Sr%20Chloride.pdf
- Liepe K, Kropp J, Runge R, et al. Therapeutic efficiency of rhenium-188-HEDP in human prostate cancer skeletal metastases. Br J Cancer. 2003;89:625–629.
- Petersen LL, Lund L, Jonler M, et al. Samarium-153 treatment of bone pain in patients with metastatic prostate cancer. Dan Med Bull. 2010;57:A4154.
- Z-index 177Lu-PSMA-I&T (16995740). 2022.
- Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. New Eng J Med. 2013;369:213–223.
- Z-Index Radium-223 (16225643).
- Sartor O, de Bono J, Chi KN, et al. Lutetium-177–PSMA-617 for metastatic castration-resistant prostate cancer. New Eng J Med. 2021;385:1091–1103.
- Heidegger I, Kesch C, Kretschmer A, et al. Biomarkers to personalize treatment with 177Lu-PSMA-617 in men with metastatic castration-resistant prostate cancer - a state of the art review. Ther Adv Med Oncol. 2022;14:17588359221081922.
- Novartis. Novartis receives European Commission approval for Pluvicto® as the first targeted radioligand therapy for treatment of progressive PSMA–positive metastatic castration-resistant prostate cancer [Internet]. 2022. [cited 2023 Jan 5]. Available from: https://www.novartis.com/news/media-releases/novartis-receives-european-commission-approval-pluvicto-first-targeted-radioligand-therapy-treatment-progressive-psma-positive-metastatic-castration-resistant-prostate-cancer.
- BS Health Consultancy. Toelatingslandschap Geneesmiddelen Nederland: hoepels & knelpunten [Licensing landscape Medicines Netherlands: hoops & bottlenecks]. 2020.
- Study Evaluating mCRPC Treatment Using PSMA [Lu-177]-PNT 2002. Therapy after second-line hormonal treatment (SPLASH). [cited 2022 Oct 7]; Available from: https://clinicaltrials.gov/ct2/show/NCT04647526.
- Martini A, Mottet N, Montorsi F, et al. A plea for economically sustainable evidence-based guidelines. Eur Urol. 2022;82:449–451.
- Meander Hospital. Lutetium-177-PSMA-therapie [Lutetium-177-PSMA-therapy] [Internet]. [cited 2022 Dec 1]. Available from: https://www.meandermc.nl/zorg/behandelingen-onderzoeken/lutetium-177-psma-uitzaaiingen-prostaatkanker/.
- ErasmusMC. Productievergunning voor nieuw radioactief geneesmiddel [Production licence of new radioactive medication] [Internet]. 2021. Available from: https://amazingerasmusmc.nl/radiologie/productievergunning-voor-nieuw-radioactief-geneesmiddel/.
- Husereau D, Drummond M, Augustovski F, et al. Consolidated health economic evaluation reporting standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. BMC Med. 2022;20:23.
- Stichting Dutch Ur-Oncology studygroup (DUOS). Uitkomst VISION -177Lutetium-PSMA-617 (177Lu-PSMA)- helaas geen grote vooruitgang [Outcome VISION -177Lutetium-PSMA-617 (177Lu-PSMA)- unfortunately no major progress] [Internet]. 2021. [cited 2023 Jan 30]. Available from: https://stichtingduos.nl/top-5-meest-gelezen-nieuwsberichten-stichting-duos-juni-2021/.
- Zorginstituut Nederland. Guideline for economic evaluations in healthcare [Internet]. 2016. [cited 2020 Dec 1]. p. 1–45. Available from: https://english.zorginstituutnederland.nl/publications/reports/2016/06/16/guideline-for-economic-evaluations-in-healthcare.
- Briggs A, Claxton K, Sculpher M. Decision modelling for health economic evaluation. Oxford: Oxford University Press; 2006.
- NVNG NN, NVKF, NVS, et al. Blootstelling van derden bij therapie met 177Lu [Third party exposure in therapy with 177Lu] [Internet]. [cited 2022 Dec 1]. Available from: https://richtlijnendatabase.nl/richtlijn/therapeutische_doses_radionucliden/blootstelling_van_derden_bij_therapie_met_177lu.html#:∼:text=Indien%20op%20basis%20van%20het,blijft%2C%20kan%20ontslag%20eerder%20plaatsvinden.
- OpenDIS data. [cited 2023 Jan 5]. Available from: opendisdata.nl
- Dutch statistics (CBS). Consumer price index [Internet]. [cited 2022 Dec 1]. Available from: https://opendata.cbs.nl/statline/#/CBS/nl/dataset/83131ned/table?fromstatweb.
- Peters ML, de Meijer C, Wyndaele D, et al. Dutch economic value of radium-223 in metastatic Castration-Resistant prostate cancer. Appl Health Econ Health Policy. 2018;16:133–143.
- Hakkaart-van Roijen L, van der Linden N, Bouwmans C, et al. Kostenhandleiding: Methodologie van kostenonderzoek en referentieprijzen voor economische evaluaties in de gezondheidszorg. [Cost manual: Methodology of cost studies and reference prices for health care economic evaluations.] Dutch National Health Care Institute. 2016;1–73. [cited 2023 Jan 5]. Available from: https://www.zorginstituutnederland.nl/over-ons/werkwijzen-en-procedures/adviseren-over-en-verduidelijkenvan-het-basispakket-aan-zorg/beoordeling-van-geneesmiddelen/richtlijnen-voor-economische-evaluatie.
- Nederlandse Zorgautoriteit (NZA). Kostprijsaanlevering add-on/ozp apotheekbereiding [Cost price delivery add-on/ozp pharmacy preparation] [Internet]. [cited 2023 Jan 24]. Available from: https://puc.overheid.nl/nza/doc/PUC_628654_22/1/.
- Erasmus MC. Tarieven ODV per 1 maart 2021 [Tarriffs ODV per 1 March 2021]. [Internet]. 2021 [cited 2022 Dec 1]. Available from: Erasmusmc.nl.
- Antoni van Leeuwenhoek. Passantenprijslijst DBC zorgproducten en overige zorgproducten 2021 [Passantenprijslijst DBC zorgproducten en overige zorgproducten 2021] [Internet]. [cited 2022 Dec 1]. Available from: https://www.avl.nl/media/4346/passantenprijzen-zorgproducten-avl-2021-versie-tbv-publicatie.pdf.
- Nederlandse Zorgautoriteit (NZA). Tarieven diagnostisch onderzoek [Tarrifs diagnostic screening] [Internet]. 2020. [cited 2020 Dec 10]. Available from: https://puc.overheid.nl/nza/doc/PUC_21659_22/1/.
- Nederlandse Zorg authoriteit. Overzicht wijzigingsverzoeken medisch-specialistische zorg [Overview of change requests for specialist medical care] [Internet]. 2022. [cited 2022 Nov 24]. Available from: https://puc.overheid.nl/nza/doc/PUC_259733_22/1/.
- Utrecht UMC. Behandeling prostaatkanker met Lutetium-177-PSMA [Treatment prostate cancer with Lutetium-177PSMA] [Internet]. [cited 2022 Dec 2]. Available from: https://www.umcutrecht.nl/nl/behandeling/behandeling-prostaatkanker-met-lutetium-177-psma.
- Radboud UMC. Behandeling met radioactief lutetium-177-PSMA [Treatment with radioactive Lutetium-177-PSMA] [Internet]. [cited 2022 Oct 24]. Available from: https://www.radboudumc.nl/patientenzorg/behandelingen/behandeling-met-radioactief-lutetium-177-psma.
- Horizonscan. 177Lu-PSMA-617 [Internet]. 2021. [cited 2022 Oct 24]. Available from: https://www.horizonscangeneesmiddelen.nl/geneesmiddelen/lutetium-177lu-vipivotide-tetraxetan-oncologie-prostaatkanker/versie2?lang=nl.
- National Institute for Health and Care Excellence [NICE]. Lu vipivotide tetraxetan for treating PSMA-positive hormone-relapsed metastatic prostate cancer after 2 or more therapies [Internet]. 2022. Available from: https://www.nice.org.uk/guidance/indevelopment/gid-ta10730.
- Omar MI, Roobol MJ, Ribal MJ, et al. Introducing PIONEER: a project to harness big data in prostate cancer research. Nat Rev Urol. 2020;17:351–361.
- Kuppen MCP, Westgeest HM, van der Doelen MJ, et al. Real-world outcomes of radium-223 dichloride for metastatic castration resistant prostate cancer. Future Oncol. 2020;16:1371–1384.
- Meander hospital. Ontwikkeling van radioactief geneesmiddel Lutetium [Development of radioactive medication Lutetium] [Internet]. [cited 2022 Oct 24]. Available from: https://www.meandermc.nl/zorg/specialismen-afdelingen/apotheek/top-10-innovaties-2021/ontwikkelling-van-radioactief-geneesmiddel-lutetium/