Abstract
Aims
To assess the cost-effectiveness of adjuvant atezolizumab in the treatment of early-stage NSCLC patients (stage II–IIIA) with expression PD-L1 ≥ 50% without mutations in EGFR or ALK rearrangements in Spain.
Materials and methods
A 5-states Markov model (DFS, locoregional recurrence, 1 L-metastatic recurrence, 2 L-metastatic recurrence, and death states) was adapted to the Spanish setting. Demographic characteristics of the hypothetical cohort, transition probabilities from the DFS state, and safety parameters were obtained from IMpower010 study (GO29527). Transition probabilities from locoregional and metastatic health states were obtained from the literature. The usual clinical practice in Spain (use of health resources, management of the disease, etc.) was obtained from a previous analysis carried out by the authors of this study. A societal perspective was considered so both direct and indirect costs were included (expressed in € of 2021). A lifetime horizon was used, so costs and health outcomes were discounted at 3% per year. Sensitivity analyses were performed to evaluate uncertainty.
Results
Over a lifetime horizon, treatment with adjuvant atezolizumab provided greater effectiveness (+2.61 life years [LY] and +1.95 quality-adjusted life years [QALY]) and higher cost (€+22,538) than BSC. The incremental cost-effectiveness ratio (ICER) and incremental cost-utility ratios (ICUR) of the analysis were €8,625/LY gained and €11,583/QALY gained, respectively. Robustness of these base-case results was confirmed by the sensitivity analyses performed. In the probabilistic sensitivity analysis, 90% of the simulations performed showed that adjuvant atezolizumab is cost-effective versus BSC, considering a threshold of €30,000/QALY.
Conclusions
Our results showed that adjuvant treatment with atezolizumab in patients with early-stage resected NSCLC with overexpression of PD-L1 and without EGFR and ALK mutations is cost-effective versus BSC as the ICERs and ICURs obtained are below the cost-effectiveness thresholds commonly considered in Spain, thus offering a new treatment alternative for these patients.
Introduction
Lung cancer is the leading cause of cancer-related death worldwide.Citation1,Citation2 An estimated 2.52 million new cases of lung cancer are expected globally in 2025,Citation2 of which about 13% of all will be small cell lung cancer (SCLC) and 84% non-small cell lung cancer (NSCLC).Citation3
In recent years, immunotherapy has emerged as a treatment option for advanced/metastatic NSCLC and can result in robust and durable treatment responses in a subset of patients.Citation4 In this sense, pembrolizumab plus chemotherapy demonstrated response and survival improvements with manageable safety in comparison with chemotherapy alone in PD-L1-negative advanced/metastatic NSCLC, and it is a standard-of-care first-line therapy for patients with advanced NSCLC, regardless of PD-L1 expression.Citation5 Other immunotherapies such as atezolizumabCitation6 have shown improvements in overall survival (OS) as compared to chemotherapy alone, and both are approved by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) as first-line therapy in advanced NSCLC.
Surgery is the primary treatment and best curative option for patients with resectable early-stage non-small cell lung cancer (NSCLC).Citation7 In certain patients (stage II or III), adjuvant platinum-based doublet chemotherapy, as a surgery complement, is the standard of care after resection to reduce the risk of recurrence and prolong disease-free survival (DFS).Citation7 However, based on evidence, adjuvant chemotherapy may offer only modest survival benefits (∼5% improved overall survival (OS) at 5 years).Citation8
Regarding the cost-effectiveness of treatment intervention (chemotherapy, surgery, and/or radiotherapy) for these patients with localized disease, adjuvant chemotherapy appears to be more cost-effective than observation, even though there are few published data.Citation9
Since their introduction in the 2000s, very few therapeutic advances have been made in chemotherapy regimens used in the adjuvant settings. Thus, there is considerable room for improvement, which is particularly relevant given the high risk of recurrence associated with stage I–III NSCLC (50% of recurrence within 5 years of treatment initiation) and the ensuing poor prognosis when recurrence occurs.Citation7,Citation8 Since there is only one cancer immunotherapy (CITs) approved for the adjuvant treatment of resectable early-stage NSCLC, and because current available therapies are associated with limited benefits, there is an unmet medical need for more effective therapies that delay or prevent the onset of disease relapse and improve survival outcomes. Also, economic evaluations of CITs in the adjuvant setting of NSCLC published to date are still very scarce.
Atezolizumab (ATZ) is a humanized immunoglobulin G1 (IgG1) monoclonal antibody that binds to PD-L1 on tumor-infiltrating immune cells (ICs) or tumor cells (TCs).Citation10 A randomized, multicenter, open-label, phase 3 study in patients with early‐stage (II–IIIA) resected NSCLC (IMpower010) showed a DFS benefit with atezolizumab versus best supportive care (BSC) after adjuvant chemotherapy, with pronounced benefit in the subgroup whose tumors expressed PD-L1 on 1% or more of tumor cells, and no new safety signals.Citation11 Thus, considering the clinical activity of atezolizumab in previously treated NSCLC and the unmet medical need to improve upon survival for patients with resected NSCLC, treatment with atezolizumab following adjuvant platinum-based chemotherapy may offer the potential for better efficacy outcomes and a manageable tolerability profile in patients with completely resected stage IB–IIIA NSCLC.
The objective of this study is to assess the cost-effectiveness of atezolizumab monotherapy as adjuvant treatment following resection and platinum-based chemotherapy for adult patients with non-small cell lung cancer whose tumors have overexpression of PD-L1 based in analysis sources evidence from the phase III IMpower010 studyCitation11 and other external sources.
Materials and methods
The cost-effectiveness analysis was performed using a global model adapted to the Spanish settings according to guidelines for health technology assessment in Spain and international recommendations.Citation12,Citation13 To this end, a panel of experts (composed of four oncologists and four hospital pharmacists from different Spanish regions) validated the assumptions made, the model parameters, and the clinical feasibility of the results through a two-round consensus process (first round of questionnaire completion and second round of virtual meeting).
Model structure
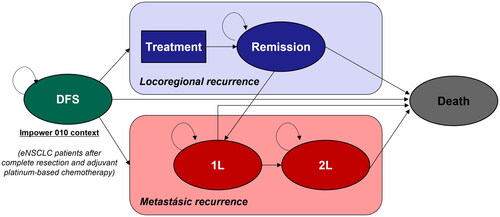
A 5-health states (disease-free survival, locoregional recurrence, 1 L-metastatic recurrence, 2 L metastatic recurrence, and death) Markov model with monthly cycles was used to assess the long-term clinical and economic outcomes associated with early NSCLC ().
Figure 1. Diagram of the Markov model. DFS, disease-free survival; eNSCLC, early non-small cell lung cancer; 1L, first line; 2L, second line.
A lifetime horizon was used in the base case in order to allow the appropriate capture of the patient’s health outcomes. Therefore, a discount rate of 3% was applied both for costs and for future effects, in accordance with the published recommendations on health technology assessment in Spain.Citation14,Citation15
The present analysis compares ATZ versus BSC, both in the adjuvant setting after resection and platinum-based chemotherapy. So, in the DFS state patients received up to 16 cycles of ATZ or received BSC. The demographic characteristics of the hypothetical cohort of patients replicate the subgroup of patients from the IMpower010 study (GO29527) defining the indication finally approved by the EMA: specifically, those with stage II–IIIA NSCLC with overexpression of PD-L1 (defined as tumor proportion score [TPS] ≥ 50%), without sensitizing mutations in EGFR or alterations in ALK.Citation11 Mean age was 61 years, nearly 67% of the patients were men and the demographic characteristics of the patient cohort considered in the model were mean weight (74 kg), mean height (169 cm), and mean body surface area (BSA, 1.85 m2).Citation11 Mortality of the general population was adapted as per the Spanish mortality information from the National Institute of Statistics (INE).
The analysis was performed from a social perspective, considering both direct and indirect health costs.
Clinical inputs
Transition probabilities
DFS state
The probability of remaining in the DFS state in each model cycle, as well as the probability of death or event (locoregional or metastatic relapse) was calculated based on the DFS curves of IMpower010. If an event occurs, after accounting for the probability of transitioning to the death state, the distribution between locoregional or metastatic relapse as first event was obtained also from both arms of the IMpower010 study, separately (ATZ: 61.9% locoregional, 31.8% metastatic; BSC: 35.0% locoregional, 65.0% metastatic).
Since the median follow-up of the trial was 32 months at the latest data cut-off (21 January 2021), DFS has been extrapolated with data from IMpower010. Various parametric distributions (Exponential, Weibull, Log-Logistic, Log-Normal, Gompertz, Generalized Gamma, and Gamma) were fitted separately to the atezolizumab and BSC arms of the trial. Goodness of fit according to the Akaike and Bayesian Information Criteria (AIC and BIC) was assessed (Table S1, Supplementary Material), and international therapeutic area experts were consulted on the clinical plausibility of the extrapolations. Based on these criteria, the log-logistic model was selected as base case (parameters in Table S2, Supplementary Material, as it provides – within clinically plausible models – more conservative estimates on the benefit that atezolizumab may have on long-term DFS. Visual adjustment of the log-logistic model is shown in Figures S1 and S2, Supplementary Material.
Finally, three adjustments were applied over the DFS curves to ensure that it predicted proportions of patients in a health state over time that reflect reality:
Cure rate: the proportion of patients who are not at risk for a DFS event increase linearly from year 2 to a maximum of 91.5% at year 5.
Mortality adjustment: in these “cured” patients (not at risk for a DFS event), an excess mortality (1.25 standardized mortality ratio) is conservatively applied.
Treatment effect adjustment: atezolizumab effect ceases at year 5 (same year at which the proportion of cured patients reaches its maximum).
Locoregional recurrence and metastatic recurrence
Since no post-relapse information is available in the IMp010 study, the transition probabilities from recurrences states (locoregional and metastatic) were extracted from the literature and validated by an international group of experts and agreed upon by the Spanish panel of experts ().
Table 1. Transition probabilities.
In the locoregional recurrence state, for patients treated with curative intent (62.1% of locoregional recurrences), the model applied different transition probabilities depending on the local treatment received (chemo-radiotherapy or only radiotherapy, both in addition to surgery). Transition probabilities were calculated based on the evidence from the literature on progression-free survival (PFS) of patients who had locoregional relapse after treatment for early NSCLC as per the study by Nakamichi et al.Citation16 For palliative treatment or untreated patients (37.9% of locoregional recurrences), the probability of moving from the locoregional recurrence state under palliative treatment to death has been obtained from the study by Kruser et al.Citation17
For patients with 1 L metastatic recurrence, the model drew evidence from another literature sourceCitation18 on the PFS and OS of patients who had metastatic relapse after treatment for early NSCLC to calculate the probabilities of transitioning probabilities to 2 L metastatic treatment and death health states. In the case of 2 L metastatic recurrence, the evidence reported by the OAK study was used to calculate the monthly probabilities of transitioning to death.Citation19
Regarding metastatic recurrence health states (1 L and 2 L), two premises were agreed upon by the panel of experts: use of a conservative approach of 1 year waiting to immunotherapy re-challenging after previous treatment with atezolizumab, and a maximum duration of 2 years of treatment with immunotherapy.
Safety
Treatment-related adverse events (AEs) of grade ≥3, reported with a frequency ≥5% in the clinical trials of the treatments included in the model were incorporated into the model. Treatment-related AEs only affects the model results in terms of costs since no disutilities were considered.
Utilities
IMpower010 does not collect patient reported outcomes, therefore the model sources information on health state utility values from the literature. In the DFS state, 0.76 from Jang et al.Citation20 was considered since it provides more conservative values. In the locoregional recurrence state, 0.73 from Chouaid et al.Citation21 and 0.62 from van den Hout et al.Citation22 were considered for curative and treatment intent, respectively. In the metastatic states (1 L and 2 L) utilities from IMpower150 (0.71 and 0.69 for 1 L and 2 L, respectively) were used from patients on active treatment (data on file). The model also sources a utility value of 0.62 from van den Hout et al.Citation22 for patients who are not actively treated in the metastatic context.
Economic parameters (healthcare resource utilization and unit costs)
Both direct and indirect costs were considered (expressed in € of 2022). Direct costs include drug acquisition costs and costs of intravenous administration, costs of local treatments such as radiotherapy or surgery, costs associated with the management of the disease, costs associated with treatment-related AEs, and end-of-life care. Indirect costs were those associated with loss of patient productivity.
Direct costs
Pharmacological costs (fully covered by the Spanish national health system) were expressed as the ex-factory price considering (when appropriate) the corresponding deductions according to Royal Decree Law (RDL) 08/2010.Citation23 Vial sharing was considered for drugs where the dose is dependent on body weight or body surface area.
In the DFS state, treatment with atezolizumab (1,200 mg every 3 weeks for up to 16 cycles) was compared to best standard of care (BSC).
In locoregional state, four cycles of cisplatin plus vinorelbine were the chemotherapy (CT) received for both curative and palliative intent. Patients on curative treatment also receive radiotherapy and undergo a surgical procedure, according to the percentages reported in de Castro et al.Citation24 Consolidation therapy with durvalumab in stage III patients receiving chemoradiotherapy were also explored in scenarios analyses.
In the metastatic relapse states (1 L and 2 L), the distribution of treatments differs depending on whether immunotherapy has been received within the previous year or not. If disease progression occurs 12 months after ATZ treatment, patients would be candidates for immunotherapy again. On the other hand, if the progression is before 1 year, patients cannot be re-challenged with immunotherapy.
shows the distribution of treatments in each health state of the model.
Table 2. Treatments distribution in each health state.
Healthcare resource consumption was obtained from the two-round consensus panel, as reported also in the cost analysis by de Castro et al.Citation24 In the DFS health state, recommendations of the European Society for Medical Oncology (ESMO)Citation25 were followed (one visit to the oncologist, complete blood and CT scan every 6 months for the first 2 years, and annually thereafter until year 5). In the recurrence states, the health resource use obtained from the experts panel and reported in the study of de Castro et al.Citation24 is shown in Table S3 (Supplementary Material).
Unit costs for healthcare resources and treatment-related AEs were obtained from the literatureCitation26 and the Spanish healthcare database (e-Salud).Citation27
Lastly, a one-off cost of €14,297,Citation28 corresponding to the care received by the patient prior to death, was included in the model.
Indirect costs
The human-capital approach was used to account in the model for the costs associated to productivity loss. A retirement age of 65 years was assumed, and an unemployment rate of 13.4% was considered for the working age population, which was used to adjust the average annual salary of these patients.Citation29
Conservatively, it is assumed that patients in DFS state continue to work if an event does not occur. After a locoregional relapse, only 33% of patients were considered to return to work, while no patient returns to work after a metastatic relapse (indefinite sick leave).
Sensitivity analysis
Both deterministic (univariate and alternative scenarios) and probabilistic sensitivity analysis were performed to evaluate the uncertainty of the variables used in the model and determine the robustness of the results obtained.
Several alternative scenarios to the base case were explored. Shorter time horizons, alternative distributions for DFS parametric curves, modifications to DFS adjustments (cure rate, excess mortality adjustment, and treatment effect adjustment), and costing assumptions (vial wastage, consolidation therapy with durvalumab in stage III patients after chemoradiotherapy), were some of the alternative scenarios proposed. In the one-way sensitivity analysis, demographic characteristics were individually modified by ±10% with respect to the base case values, and the rest of the model variables were individually modified by ±20% with respect to the base case values.
In the probabilistic sensitivity analysis, 1,000 simulations were performed using the Monte-Carlo method, in line with the recommendations in the literature.Citation30 Distributions between treatments were studied using Dirchlet; transition probabilities were modified using beta and normal distributions; costs and frequencies were modified using gamma distribution; and utility values were modified using beta distribution.
Results
Base case
shows the base case results of the cost-effectiveness analysis.
Table 3. Base case results.
The results of the cost-effectiveness analysis show that atezolizumab as an adjuvant treatment for early NSCLC (stage II–IIIA), with overexpression of PD-L1 (TPS ≥ 50%), provides more LY and QALYs than BSC, but at a higher cost. The ICERs and ICURs are below the traditionally considered cost-effectiveness threshold of 20,000–30,000/QALY in Spain.Citation31,Citation32
Sensitivity analysis
Among the alternative scenarios to the base case explored as sensitivity analyses, none of them exceeded the cost-effectiveness threshold. Shortening the time horizon to 10 years increases the ICUR to €23,016/QALY. Scenarios where the assumptions of the DFS adjustments are modified have a more moderate effect on the ICUR. Allowing re-challenging with immunotherapy at 3 years instead of 1 year decreases the ICUR to €7,069/QALY, while variations in the cure rate or the excess of mortality barely affect the ICUR of the base case. Using log-normal or gamma models instead of the log-logistic model for DFS extrapolation also shows moderate base case ICUR changes (€10,894/QALY and €12,499/QALY with log-normal or gamma models, respectively). Finally, regarding cost scenarios, considering vial wastage instead of vial sharing, leads to an ICUR of €5,203/QALY, and considering consolidation therapy with durvalumab in stage III patients after chemoradiotherapy leads to an ICUR of €11,662/QALY.
Results of the one-way sensitivity analysis are represented by a tornado diagram (), showing how individual changes in each variable modifies the base case ICUR (€11,583/QALY).
Figure 2. Tornado diagram. DFS, disease-free survival; ICUR, incremental cost-utility ratio; Ind, indirect costs; IV, intravenous; QALY, quality-adjusted life year; 1L, first line; 2L, second line.
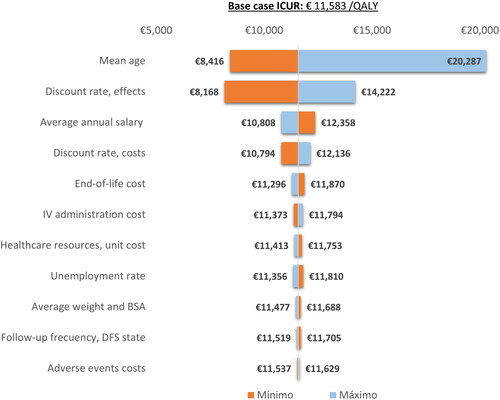
The mean age of the hypothetical cohort has the greatest impact on the ICUR of the base case, especially in its maximum value, given that in this case the mean age exceeds 65 and consequently the indirect costs have less influence on the results.
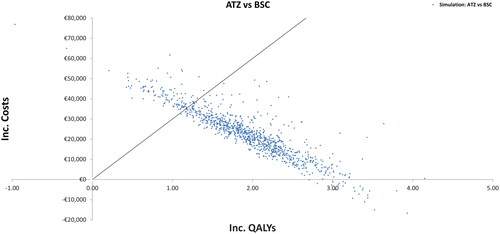
The probabilistic sensitivity analysis results are represented by an incremental cost-effectiveness plot (), where the ordinate axis represents the long-term incremental cost of atezolizumab monotherapy versus BSC, while the abscissa axis represents incremental long-term QALYs of atezolizumab in monotherapy versus BSC. Approximately 90% of the simulations showed that it is cost-effective versus BSC considering a threshold of €30,000/QALY, and ATZ was dominant in 2.4% of the simulations.
Discussion
The landscape of resectable NSCLC has changed dramatically in recent years, with the advent of adjuvant and neoadjuvant chemotherapy, and subsequently the imminent arrival of immunotherapies and targeted therapies in both adjuvant and neoadjuvant settings.Citation33 Many patients suffer from postoperative recurrence due to the heterogeneous prognosis of early stages disease; thus, postoperative adjuvant chemotherapy can reduce the onset of recurrence and metastasis in NSCLC.Citation34 However, despite the use of post-operative chemotherapy, roughly half of patients with stage IB to roughly three-quarters of patients with stage IIIA lung cancer relapse with metastatic disease.Citation35
The advent of immunotherapy has revolutionized the treatment of patients with advanced NSCLC and generated unprecedented increases in survival outcomes.Citation36 In recent years, researchers are gradually turning their attention to neoadjuvant and adjuvant immunotherapy, hoping that immunotherapy can benefit more patients with early-stage NSCLC. Immunotherapy is relatively well tolerated compared to cisplatin-based chemotherapy and offers the potential for durable, long-lasting anti-tumor response.Citation36 Based on this, several studies were designed to explore the efficacy of adjuvant immunotherapy after surgery for early-stage NSCLC.Citation37
In the setting of early-stage NSCLC, the first positive results from a phase III trial in the adjuvant setting were provided by IMpower010, which showed that adjuvant atezolizumab significantly improved survival after adjuvant chemotherapy in patients with resected NSCLC compared to best supportive care.Citation11,Citation38 Other phase III clinical trials in the adjuvant setting are currently ongoing.Citation38,Citation39 PEARLS/KEYNOTE-091 tested pembrolizumab against placebo in 1,177 stage IB–IIIA NSCLC patients, after standard chemotherapy; results from an interim analysis have shown a DFS benefit in the experimental arm.Citation38,Citation40 Similarly, in the ANVIL study 1 year of treatment with nivolumab is compared to observation after standard CT, and the study completion date is estimated to be July 2024.Citation39,Citation41 Atezolizumab, pembrolizumab, and nivolumab also have their respective studies in the neoadjuvant setting, such as IMpower 030, KEYNOTE 671, or CheckMate 816, respectively.Citation42–44 Clinical trials with other immunotherapy agents such as durvalumab, toripalimab, or canakinumab are currently ongoing in this neoadjuvant/adjuvant setting.Citation38 Also, recent development of novel therapeutic approaches, such as tyrosine kinase inhibitors (TKI) like osimertinib, has changed the management of patients with certain subtypes of NSCLC.Citation39
Results of the cost analysis show that, in Spain, relapses in NSCLC entail a substantial economic burden, with an average cost of €84,814 for metastatic relapses and four lines of treatment.Citation24 It is furthermore important to note that the costs of relapses in the metastatic setting are considerably higher than the costs associated with a locoregional relapse. Therefore, this estimate of the economic burden of relapses highlights the need for effective treatments in order to delay, and even prevent, relapses in early NSCLC.
To date, two pharmacoeconomic studies have been identified, which analyzed the cost-effectiveness of atezolizumab in the context of adjuvant therapy for early NSCLC. On the one hand, Das et al.Citation45 evaluated the cost-effectiveness of atezolizumab versus BSC following adjuvant chemotherapy in resected patients with Stage II–IIIA PD-L1+ NSCLC from a US commercial payer perspective. On the other hand, Chen et al.Citation46 analyzed the cost-effectiveness of atezolizumab versus BSC as adjuvant therapy after platinum-based chemotherapy for stage IB–IIIA resectable NSCLC from the perspective of the Chinese health care system. The results of these studies are contradictory, due to the considerable methodological differences and major differences in the healthcare systems of the countries.
This present study is the first to evaluate, from a societal perspective in a European country, the cost-effectiveness of atezolizumab monotherapy as adjuvant treatment following resection and platinum-based chemotherapy for adult patients with NSCLC whose tumors have PD-L1 expression of ≥ 50% of tumor cells and no alterations in EGFR or ALK versus BSC. As described in this article, several clinical parameters have been obtained from the IMpower010 clinical trial, in the same way as the two aforementioned cost-effectiveness studies, which offers a clearer and more transparent comparison of the results between studies.
The results of our cost-effectiveness analysis show that adjuvant treatment with atezolizumab for 16 cycles in patients with stage II–IIIA NSCLC with overexpression of PD-L1 and without mutations in EGFR and ALK is cost-effective compared to BSC. However, there are important differences between different regions, which are exemplified by the results of the study carried out by Chen et al.Citation46 In this study, the results shown by the authors reflected that the use of atezolizumab as adjuvant therapy after platinum-based chemotherapy resulted in a ICER above the willingness-to-pay (WTP) threshold of three times the per capita gross domestic product of China in 2022 for all subpopulations analyzed, making atezolizumab less likely to be cost-effective in patients after postoperative platinum-based chemotherapy for early NSCLC. This study extrapolates the OS of the IMpower010 study, despite its immaturity, which represents an important methodological difference with respect to the Das et al.Citation45 study or our own.
On the other hand, the study from Das et al.Citation45 showed that, from the US commercial payer perspective, atezolizumab was cost-effective versus BSC at $46,859 per QALY in the base case, considering a WTP threshold of $150,000, for the adjuvant treatment of resected patients with PD-L1+ Stage II–IIIA NSCLC. It is important to note that WTP threshold used by study authors are different than the WTP threshold habitually considered in Spain, which are estimated around €30,000 per QALY; however, the results of this study were robust and continued to show that atezolizumab was cost-effective both in the proposed scenario analysis (using Medicare-specific costs) and in the 91% iterations performed in the probabilistic sensitivity analysis.Citation45
The ratios obtained in our study (ICER of €8,625/LY and ICUR of €11,583/QALY) well below the cost-effectiveness thresholds commonly considered in our country,Citation31,Citation32 show that gains attained with atezolizumab in both LY and QALYs (+2.61 and +1.95, respectively) compensate for the higher cost associated with treatment with atezolizumab (€22,538 of incremental cost, with the pharmacological cost of atezolizumab being the most important item).
As in most cost-effectiveness analyses, some limitations are inherent to these types of models, that stem from their structural rigidity hindering a complete representation of usual clinical practice, in addition to limitations related the long-term projections of the clinical trial data. In this regard, it should be noted that, although the results of the IMpower010 study have already reached statistical significance for the main variable, the data are not fully mature. So, the extrapolation of DFS across time was based on 32 months of median follow-up data, which leads to uncertainty around the incremental benefit of the intervention after the trial follow-up period.
Another structural limitation arising from not using a partitioned survival model is that the transition probabilities after recurrence remain time invariant, which may not reflect reality. Also, it was assumed that death is the only available transition for patients being treated with palliative treatment or no treatment, and that all second recurrences after initial locoregional recurrence are metastatic recurrences. Similarly, since the IMpower010 study does not collect information after patient relapses, external sources had to be used to estimate the probabilities of transition between locoregional relapse, metastatic relapse (1 L and 2 L), and death. Thus, the use of such studies is not without some uncertainty and could lead to the use of incorrect input values, and subsequently affect the results. However, it is unclear as to how this may affect the results of the model. In the same way, IMpower010 does not collect patient reported outcomes, so utilities were derived from the published literature, which may have introduced bias related to differences between underlying study populations or countries.
Finally, there are also some limitations related to the adaptation of the model to the Spanish setting, where healthcare resources consumption and treatment pathways have been obtained from a panel of experts. So, it is a lower level of evidence than a retrospective or observational study.
In any case, the sensitivity analyses carried out have shown the uncertainties to be manageable and the results of the analysis to be robust, since even under the most conservative assumptions atezolizumab remains cost-effective compared to BSC, considering the cost-effectiveness thresholds commonly considered in Spain.
Conclusion
In summary, the analysis shows that atezolizumab offers a new cost-effective treatment for patients with stage II–IIIA NSCLC with overexpression of PD-L1 and without EGFR and ALK mutations after complete resection and adjuvant platinum-based chemotherapy.
Transparency
Declaration of funding
This work was supported by Roche Farma S.A. Roche Farma S.A played no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Declaration of financial/other relationships
VE-V received advisory fees from Astellas, Bristol-Myers Squibb, GlaxoSmithKline, Janssen, Merck Sharp & Dohme, Novartis, and Pfizer outside the submitted work. RC-B received advisory fees from Boehringer-Ingelheim, Janssen, Merck Sharp & Dohme, Hoffmann-La Roche, Amgen Inc, GlaxoSmithKline, Novartis, and Pfizer outside the submitted work. JdC received advisory fees from Merck Sharp & Dohme, Hoffmann-La Roche, Bristol-Myers Squibb, AstraZeneca, Pfizer, PharmaMar, Boehringer Ingelheim, Takeda, and Tesaro outside the submitted work. AI received advisory fees from Hoffmann-La Roche, Pfizer, BMS, Sanofi, Amgen, and AstraZeneca outside the submitted work. AM-M received advisory fees from Bristol-Myers Squibb, Hoffmann-La Roche, Merck Sharp & Dohme, Pfizer, Boehringer Ingelheim, Merck Sharp & Dohme, and AstraZeneca outside the submitted work. IS received advisory fees from Hoffmann-La Roche outside the submitted work. AM reports a relationship with University Hospital Mútua Terrassa that includes consulting or advisory. EF has no conflict of interest outside the submitted work. DC is an employee of Hygeia Consulting which received funding from Roche to conduct the analysis. NA is an employee of Roche.
Author contributions
All authors contributed to the development of the study. NA and DC designed the analysis and performed the model adaptation. VE-V, RC-B, JdC, AI, AM-M, EF, IS, and AM formed the panel of experts that collected and validated model data and contributed to results interpretation. The first draft of the manuscript was written by DC and all authors commented on previous versions of the manuscript. All the authors read and approved the final manuscript.
Reviewer disclosures
A reviewer on this manuscript has disclosed that they were involved as an expert with Roche France on this topic. All the peer reviewers on this manuscript have received an honorarium from JME for their review work.
Previous presentations
The preliminary results of our work have been presented in a poster at ISPOR 2022 held in Vienna (7–9 November 2022) (https://www.ispor.org/heor-resources/presentations-database/presentation/euro2022-3567/121386)
In addition, similar adaptations of the model for France and Finland were presented at the same congress. (https://www.ispor.org/heor-resources/presentations-database/presentation/euro2022-3566/120313) (https://www.ispor.org/heor-resources/presentations-database/presentation/euro2022-3565/120278)
Supplemental Material
Download MS Word (59 KB)Acknowledgements
No assistance in the preparation of this article is to be declared.
Data availability statement
Qualified researchers may request access to individual patient level data through the clinical study data request platform (https://vivli.org/). Further details on Roche’s criteria for eligible studies are available here (https://vivli.org/members/ourmembers/). For further details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here (https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm).
References
- World Health Organization. Fact sheets: cancer 2018. World Health Organization; 2018. https://www.who.int/en/news-room/fact-sheets/detail/cancer
- World Health Organization. Cancer Today. World Health Organization; 2022. https://gco.iarc.fr/today/home
- American Cancer Society. Lung cancer statistics | How common is lung cancer?. American Cancer Society; 2022. https://www.cancer.org/cancer/lung-cancer/about/key-statistics.html
- Paz-Ares L, Luft A, Vicente D, KEYNOTE-407 Investigators, et al. Pembrolizumab plus chemotherapy for squamous Non-Small-Cell lung cancer. N Engl J Med. 2018;379(21):2040–2051.
- Borghaei H, Langer CJ, Paz-Ares L, et al. Pembrolizumab plus chemotherapy versus chemotherapy alone in patients with advanced non-small cell lung cancer without tumor PD-L1 expression: a pooled analysis of 3 randomized controlled trials. Cancer. 2020;126:4867–4877.
- Horn L, Mansfield AS, Szczęsna A, IMpower133 Study Group, et al. First-Line atezolizumab plus chemotherapy in Extensive-Stage Small-Cell lung cancer. N Engl J Med. 2018;379(23):2220–2229.
- Postmus PE, Kerr KM, Oudkerk M, ESMO Guidelines Committee, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl_4):iv1–iv21.
- Pignon JP, Tribodet H, Scagliotti G V, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE collaborative group. J Clin Oncol. 2008;26:3552–3559.
- Chouaid C, Atsou K, Hejblum G, et al. Economics of treatments for non-small cell lung cancer. Pharmacoeconomics. 2009;27:113–125.
- European Medicines Agency. Annex 1 – summary of product characteristics – atezolizumab 2021. European Medicines Agency; 2021.
- Felip E, Altorki N, Zhou C, IMpower010 Investigators, et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet. 2021;398(10308):1344–1357.
- Husereau D, Drummond M, Augustovski F, et al. Consolidated health economic evaluation reporting standards (CHEERS) 2022 explanation and elaboration: a report of the ISPOR CHEERS II good practices task force. Value Health. 2022;25:10–31.
- Ortega Eslava A, Marín Gil R, Fraga Fuentes MD, et al. Incluye actualización del área económica del programa madre 4.0. proyecto de investigación financiado mediante las ayudas a los grupos de la sefh 2014-15. Autores: guía Práctica. Grupos de trabajo SEFH SociedaEspañola de Farmacia Hospitalaria (SEFH). 2016.
- López Bastida J, Oliva J, Antoñanzas F, et al. Propuesta de guía Para la evaluación económica aplicada a las tecnologías sanitarias. Gac Sanit. 2010;24:154–170.
- Puig-Junoy J, Oliva-Moreno J, Trapero-Bertrán M, et al. Guía y recomendaciones Para la realización y presentación de evaluaciones económicas y análisis de impacto presupuestario de medicamentos en el ámbito del CatSalut. Barcelona: Generalitat de Catalunya Departament de Salut Servei Català de la Salut; 2014.
- Nakamichi S, Horinouchi H, Asao T, et al. Comparison of radiotherapy and chemoradiotherapy for locoregional recurrence of non-small-cell lung cancer developing after surgery. Clin Lung Cancer. 2017;18:e441–e448.
- Kruser TJ, McCabe BP, Mehta MP, et al. Reirradiation for locoregionally recurrent lung cancer: outcomes in small cell and non-small cell lung carcinoma. Am J Clin Oncol. 2014;37(1):70–76.
- Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for First-Line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378:2288–2301.
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255–265.
- Jang RW, Isogai PK, Mittmann N, et al. Derivation of utility values from european organization for research and treatment of cancer quality of Life-Core 30 questionnaire values in lung cancer. J Thorac Oncol. 2010;5(12):1953–1957.
- Chouaid C, Agulnik J, Goker E, et al. Health-related quality of life and utility in patients with advanced non-small-cell lung cancer: a prospective cross-sectional patient survey in a real-world setting. J Thorac Oncol. 2013;8:997–1003.
- van den Hout WB, Kramer GWPM, Noordijk EM, et al. Cost-utility analysis of short- versus long-course palliative radiotherapy in patients with non-small-cell lung cancer. J Natl Cancer Inst. 2006;98(24):1786–1794.
- Consejo General de Colegios Oficiales de Farmacéuticos (CGCOF). Base de datos del Consejo General de Colegios Oficiales de Farmacéuticos. 2022. www.portalfarma.com
- De Castro J, Insa A, Collado-Borrell R, et al. Economic burden of locoregional and metastatic relapses in resectable early-stage non-small cell lung cancer in Spain. BMC Pulm Med. 2023;23(1):69.
- Remon J, Soria JC, Peters S, ESMO Guidelines Committee. Electronic address: [email protected]. Early and locally advanced non-small-cell lung cancer: an update of the ESMO clinical practice guidelines focusing on diagnosis, staging, systemic and local therapy. Ann Oncol. 2021;32(12):1637–1642.
- Ortega-Joaquin N, Echave M, Oyagüez I, et al. Cost-Analysis for toxicity management in advanced squamous Non-Small cell lung cancer: nivolumab vs docetaxel. Value in Health. 2016;19:a722.
- Gisbert R, Brosa M. Healthcare cost database eSalud. Barcelona; 2022. http://www.oblikue.com/bddcostes/
- Nuño-Solinís R, Herrera Molina E, Librada Flores S, et al. Care costs and activity in the last three months of life of cancer patients who died in the Basque Country (Spain). Gac Sanit. 2017;31:524–530.
- INE. Instituto Nacional de Estadística 2022. https://ine.es/
- Briggs AH. Handling uncertainty in cost-effectiveness models. Pharmacoeconomics. 2000;17(5):479–500.
- Vallejo-Torres L, García-Lorenzo B, Serrano-Aguilar P. Estimating a cost-effectiveness threshold for the Spanish NHS. Health Econ. 2018;27:746–761.
- Sacristán JA, Oliva J, Campillo-Artero C, et al. ¿Qué es una intervención sanitaria eficiente en españa en 2020? Gac Sanit. 2020;34:189–193.
- Loh J, Wijaya ST, Sooi K, et al. Resectable non-small cell lung cancer: an evolving landscape. Transl Lung Cancer Res. 2022;11:1241–1246.
- Zhou K, Zhao Y, Liang L, et al. Adjuvant chemotherapy may improve long-term outcomes in stage IB non-small cell lung cancer patients with previous malignancies: a propensity score-matched analysis. Front Oncol. 2022;12:938195.
- Mithoowani H, Febbraro M. Non-Small-Cell lung cancer in 2022: a review for general practitioners in oncology. Curr Oncol. 2022;29:1828.
- Shukla N, Hanna N. Neoadjuvant and adjuvant immunotherapy in Early-Stage Non-Small cell lung cancer. Lung Cancer. 2021;12:51–60.
- Duan J, Tan F, Bi N, et al. Expert consensus on perioperative treatment for non-small cell lung cancer. Transl Lung Cancer Res. 2022;11:1247–1267.
- de Scordilli M, Michelotti A, Bertoli E, et al. Targeted therapy and immunotherapy in Early-Stage Non-Small cell lung cancer: current evidence and ongoing trials. Inter J Mol Sci. 2022;23:7222.
- Szeto CH, Shalata W, Yakobson A, et al. Neoadjuvant and adjuvant immunotherapy in Early-Stage Non-Small-Cell lung cancer, past, present, and future. J Clin Med. 2021;10(23):5614.
- Paz-Ares L, O’Brien MER, Mauer M, et al. VP3-2022: pembrolizumab (pembro) versus placebo for early-stage non-small cell lung cancer (NSCLC) following complete resection and adjuvant chemotherapy (chemo) when indicated: randomized, triple-blind, phase III EORTC-1416-LCG/ETOP 8-15–PEARLS/KEYNOTE-091 study. Ann Oncol. 2022;33:451–453.
- Chaft JE, Dahlberg SE, Khullar O V, et al. EA5142 adjuvant nivolumab in resected lung cancers (ANVIL). 2018;36:TPS8581–TPS8581.
- Peters S, Kim AW, Solomon B, et al. IMpower030: phase III study evaluating neoadjuvant treatment of resectable stage II-IIIB non-small cell lung cancer (NSCLC) with atezolizumab (atezo) + chemotherapy. Ann Oncol. 2019;30:II30.
- Tsuboi M, Luft A, Ursol G, et al. 1235TiP perioperative pembrolizumab + platinum-based chemotherapy for resectable locally advanced non-small cell lung cancer: the phase III KEYNOTE-671 study. Ann Oncol. 2020;31:S801–S802.
- Forde PM, Spicer J, Lu S, CheckMate 816 Investigators, et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med. 2022;386(21):1973–1985.
- Das M, Ogale S, Johnson A, et al. EP04.01-017 Cost-Effectiveness of atezolizumab for adjuvant treatment of patients with stage II-IIIA PD-L1+ non-small cell lung cancer. J Thorac Oncol. 2022;17: S253.
- Chen P, Yang Q, Li Y, et al. Cost-effectiveness analysis of adjuvant therapy with atezolizumab in chinese patients with stage IB-IIIA resectable NSCLC after adjuvant chemotherapy. Front Oncol. 2022;12:4678.