Abstract
Aims
Approximately 75% of bladder cancer (BC) cases present as non-muscle-invasive BC (NMIBC). In patients with high-risk NMIBC, the mainstay treatment is intravesical Bacillus Calmette-Guérin (BCG), with immediate radical cystectomy (RC) as an alternative treatment option. The aim of the present study was to evaluate the cost-utility of BCG versus RC in patients with high-risk NMIBC from the UK healthcare payer perspective.
Materials and methods
A six-state Markov model was developed that covered controlled disease, recurrence, progression to muscle-invasive BC, metastatic disease, and death. The model included adverse events of BCG and RC and monitoring and palliative care. Drug costs were obtained from the British National Formulary. Intravesical delivery, RC, and monitoring costs were sourced from the National Tariff Payment System and the literature. Utility data were obtained from the literature. Analyses were run over a 30-year time horizon, with future costs and effects discounted at 3.5% per annum. One-way and probabilistic sensitivity analyses were performed.
Results
The base case analysis comparing BCG with RC showed that BCG would increase life expectancy by 0.88 years versus RC, from 7.74 to 8.62 years. BCG resulted in an increase of 0.76 quality-adjusted life years (QALYs) versus RC, from 5.63 to 6.39 QALYs. Patients incurred lower lifetime costs if treated with BCG (£47,753) than with RC (£64,264). Cost savings were mainly driven by the lower cost of BCG versus RC, and palliative care costs. Sensitivity analyses showed that results were robust to assumptions.
Limitations
The evidence base informing efficacy estimates of BCG is heterogeneous as different BCG administration schedules were reported in the literature, while incidence and cost data on some BCG-associated adverse events were sparse.
Conclusions
Intravesical BCG led to increased QALYs and reduced costs versus RC for patients with high-risk NMIBC from the UK healthcare payer perspective.
PLAIN LANGUAGE SUMMARY
Intravesical Bacillus Calmette-Guérin (BCG) is a recommended immunotherapy option for patients with high-risk non-muscle invasive bladder cancer (NMIBC) who have undergone transurethral resection of bladder tumor (TURBT). BCG is known for its high efficacy and good safety profile. However, current evidence on its effectiveness against other comparators such as radical cystectomy (RC) is limited, mainly because different BCG schedules are used, particularly with regard to maintenance therapy. Given this lack of evidence, we conducted the first cost-utility analysis which considers adequate BCG therapy relative to RC for the UK. We developed a Markov model that captured the effects of the intravesical BCG and RC on NMIBC, in addition to incidences of adverse events associated with either treatment. Costs of the two treatments, their administration, maintenance, and of treatments for adverse events were modelled alongside the quality-of-life effects of NMIBC, adverse events, and palliative care. We used published clinical data and UK cost data to inform our model. Our results show that BCG was associated with higher quality-adjusted life expectancy than RC and lower total costs from the healthcare system perspective. These results imply that adequate BCG immunotherapy is likely valuable for the National Healthcare System in terms of its effects on patients and indeed cost-saving relative to RC.
Introduction
Bladder cancer was the 12th most common cancer worldwide in 2020.Citation1 Non-muscle-invasive bladder cancer (NMIBC) accounts for between 70–75% of bladder cancers at the time of initial diagnosis,Citation2 and can be further classified into low-, intermediate-, high-, or very high-risk disease.Citation3 Treatment guidelines for high-risk NMIBC recommend patients undergo transurethral resection of bladder tumor (TURBT), followed by intravesical immunotherapy with Bacillus Calmette-Guerin (BCG).Citation3,Citation4 BCG is a live attenuated strain of Mycobacterium bovis that is administered into the bladder using a catheter and left in the bladder for 2 h, with weekly treatments for the first 6 weeks and, ideally, subsequent maintenance therapy, and with follow-up cystoscopies for the remainder of patient lifetimes.Citation5 As an alternative to intravesical BCG, immediate radical cystectomy (RC) may be considered.Citation3,Citation5,Citation6 This surgical procedure involves the complete removal of the bladder and surrounding tissues, followed by the creation of an ileal conduit (urostomy) or (orthotopic) neobladder to allow storing and passing of urine.Citation7
Intravesical BCG is the mainstay of NMIBC treatment.Citation3,Citation8,Citation9 Despite its acknowledged success, including relative to comparators such as mitomycin-C for intermediate-risk NMIBC,Citation10 the evidence base supporting BCG is of mixed quality and often inconsistent. An important challenge is the use of different BCG schedules, specifically for BCG maintenance therapy. Differences in treatment schedules, as well as high rates of discontinuation, also make it difficult to assess if treatment failures are due to a lack of BCG efficiency or inadequate therapy, including insufficient TURBT quality, lack of repeat TURBT in T1 tumors, and absence of detrusor muscle in resected Ta high-grade specimen.Citation3,Citation11,Citation12 Definitions of “adequate BCG therapy” have been providedCitation13,Citation14 and adequate BCG therapy has been shown to have excellent real-world outcomes.Citation15 However, these definitions were too recent to be in widespread use in the literature at the time of writing.
Demonstrating the value of BCG to healthcare payers is therefore as challenging as it is important. The aim of the present study was the development of a cost-utility model for the evaluation of the clinical, cost, and quality of life effects of adequate BCG therapy in the treatment of high-risk NMIBC, from the perspective of the English National Health Service (NHS).
Methods
Markov model overview
A Markov model was used to evaluate the decision problem.Citation16 The conceptualization of the model was informed by the European Association of Urology (EAU) guidelines on NMIBCCitation3 and muscle-invasive bladder cancer (MIBC),Citation17 respectively, and by prior health economic models.Citation18–20 Based on these recommendations, adequate BCG was the intervention of interest in the model. The comparator was immediate RC. As recommended by the EAU, all patients were assumed to have undergone TURBT initially to confirm the diagnosis of NMIBC and completely remove visible lesions,Citation3 and the analysis focused on treatment with either BCG or RC after the initial TURBT.
Costs were evaluated from the perspective of NHS England (and ultimately therefore the perspective of the Department of Health and Social Care) and presented in 2022 Great British Pounds (£), using a 30-year time horizon and a 3-monthly cycle length for a population with a mean age of 70 years. Future costs and effects were discounted at 3.5% per annum.Citation21
Markov model structure
The Markov model () was developed from treatment recommendations in EAU guidelines and informed by earlier models of NMIBC. Patients entered the Markov model in a controlled disease state in the first model cycle, in which their assigned treatment began. Patients could stay in the controlled disease state, transition to a post-cystectomy state following immediate RC, or transition to either a progression or a recurrence state. A death state was also included as an absorbing state, reachable from all other states in the model.
Figure 1. Markov model schematic. Regular Markov states are colored in blue and tunnel states are in grey. The “Death” state is accessible from every other state in the model and is itself an absorbing state. Immediate radical cystectomy is indicated by the arrow pointing from “Controlled disease” to “Post-cystectomy”. Abbreviations. CHT, Chemohyperthermia; MIBC, Muscle-Invasive Bladder Cancer.
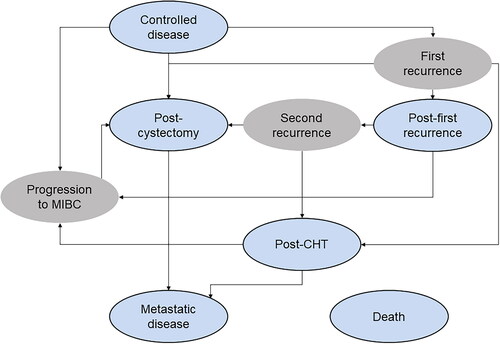
Progression was defined as transitioning from NMIBC to MIBC.Citation18,Citation20 This state was a one-cycle tunnel state, during which all patients, in line with EAU guidelines on MIBC, were assumed to undergo RC.Citation17 Recurrence was understood to be the same or lower-stage disease as before the initial TURBT.Citation20 After cystectomy, patients entered a post-cystectomy state.
Model assumptions
Several key assumptions underpinned the model. No difference in efficacy or safety by BCG strain was assumed, in line with recent EAU guidelines,Citation3 which implied that studies using different strains were used to inform different data points in the model. Failure of BCG within 6 months of initiating treatment was labelled as “BCG-unresponsive”.Citation13,Citation14 Any recurrence beyond this point was not considered to indicate BCG-unresponsiveness and a second round of BCG – specifically, induction BCG – was possible. A proportion of patients eligible for RC after progression was assumed to refuse RC or not undergo surgery for other reasons,Citation22 and it was assumed that these patients would receive chemohyperthermia with mitomycin C.
Model outcomes
Costs, life expectancy (in life years), quality-adjusted life expectancy (in quality-adjusted life years [QALYs]), and incremental cost-utility ratios (ICURs) were the main outcomes of the model. Net monetary benefit (NMB) was calculated using willingness-to-pay (WTP) thresholds of £20,000 and 30,000 per QALY gained, respectively.Citation23
Clinical data
The risks of recurrence, progression, and death in high-risk patients treated with adequate BCG were obtained from Matulay et al.Citation15 Matulay et al. reported findings from a real-world US study, which included patients treated between 2004 and 2018 with adequate BCG as defined by the Food and Drug Administration (FDA) and the International Bladder Cancer Group.Citation13,Citation14 One-year estimates for recurrence-free, progression-free, and overall survival from this study for high-risk patients, respectively, were converted to 3-month transition probabilities, assuming a constant rate of events ().Citation24
Table 1. Transition probabilities.
The risk of disease following a first recurrence was affected only by the treatment given for the repeat recurrence. This was based on a finding by Simon et al.,Citation25 who observed no association between the number of recurrences and risk of progression in low- and intermediate-risk patients; in the present analysis, the progression risk following the first recurrence in high-risk disease was also assumed to depend only on the treatment given.
The efficacy of repeat BCG was calculated by applying risk ratios for second induction BCG versus first induction BCG from Daniels et al.,Citation26 which were not statistically significant. No difference was assumed for the effect on mortality. The risk of death following repeat recurrence was assumed to equal that following the first recurrence.
The risk of developing metastatic disease after cystectomy and the risk of death following RC were obtained, in the absence of UK data, from a cohort of patients with high-risk NMIBC who had undergone early RC at a French hospital between 2001 and 2020.Citation27 Over a median follow-up of 65 months, thirty-two patients developed metastases. Five-year overall survival was reported as 68.4%. The study did not report mortality specific to patients with metastatic disease so the corresponding transition probability was calculated from a UK cohort comprising 216 patients with locally advanced or metastatic disease, which reported median overall survival of 16.2 months and 1-year overall survival of 67.5%.Citation28
Transition probabilities following chemohyperthermia were derived from a study of hyperthermic-intravesical chemotherapy (HIVEC) in patients with high-risk NMIBC, which had a median follow-up of 18 months and reported rates of progression to muscle infiltration, metastasis, and death.Citation29
Mortality
For RC, the literature showed that the use of neoadjuvant chemotherapy was associated with an overall survival benefit of 5–8% over 5 years relative to not using neoadjuvant chemotherapy.Citation17,Citation30 In the base case, the midpoint of this interval (6.5%) was used to model the mortality benefit of neoadjuvant chemotherapy.
Background mortality was modelled using published lifetables for the UK (2018–2020).Citation31 General population mortality was adjusted using net survival for bladder cancer in patients aged 65–74 years from Public Health England.Citation32 As net survival estimates were only available for up to 5 years after diagnosis, the last observation (for 5 years) was carried forward and assumed to apply to the remaining years.
Health-related quality of life data
Health-related quality of life data including utility and disutility values () were sourced primarily from the BOXIT trial,Citation33 which provided a recent UK source for the target population (data in this trial pertained predominantly to high-risk patients).
Table 2. Utility and disutility values.
Several values for post-cystectomy states were available from the literature but were either implausibly high or based on unclear evidence. Given these concerns, the post-cystectomy value was assumed to be equal to the post-recurrence value reported in the BOXIT trial.Citation33 This value was closely aligned with values used in prior economic models.Citation6,Citation18
The health state utility value for metastatic disease was sourced from Kulkarni et al.,Citation34 as the value for metastatic disease responsive to chemotherapy, which was originally derived from the breast cancer literature.
The effects of adverse events on quality of life were captured by applying a one-off disutility for the entire duration of the cycle in which the event occurred. Disutilities came from a range of articles, including for conditions other than bladder cancer ().Citation35–47 This is in line with previous models in the field that also found a sparsity of relevant and directly applicable (dis-) utility data for adverse events associated with bladder cancer treatment.Citation18,Citation48 To capture the quality-of-life impact of intravesical administration events, a utility decrement of 0.02Citation48 was applied in each cycle during which BCG was administered as either first-line therapy or for repeat induction.
Adverse events
The selection of adverse events for BCG was based on available data and the summary of product characteristics for intravesical BCG.Citation49 If cumulative frequencies rather than incidence rates were reported, they were converted to risks adjusted for the 3-month cycle length.Citation24
For RC and neoadjuvant chemotherapy, adverse events and their probabilities came from a model by Magee et al.Citation50 and the sources therein. A full overview of the adverse events included for each treatment option, as well as their respective probability estimates, can be found in the supplementary material (Tables S1–S3). Note that adverse events for BCG and RC were included regardless of their frequency, i.e. no minimum frequency was specified for inclusion, to allow capturing rare events.
Cost and resource use
The main source of costs for delivery of intravesical BCG and cystectomy was the 2022/2023 National Tariff Payment System.Citation51 Costs of medications were captured from the NHS England perspective and, where necessary, inflated to 2022 values using the consumer price index for medical services.Citation52
BCG
BCG dosing was based on the SWOG schedule, which is currently recommended in the BCG treatment guidelines.Citation53 This schedule involves six once-weekly instillations as induction therapy. While the SWOG schedule specifies 3-year maintenance therapy for high-risk patients, the base case was modeled as 1-year maintenance therapy, given that in clinical practice maintenance therapy is usually given from 1 year up to 3 years and many patients fall on the lower end of this time span.Citation6,Citation15 Maintenance therapy included three instillations each at 3, 6, and 12 months. A scenario analysis using a 3-year maintenance treatment, with an additional three instillations each at 18, 24, 30, and 36 months, was conducted as a post-hoc scenario analysis.
Intravesical BCG was assumed to be adequate, as per definitions advanced by the FDACitation13 and Kamat et al.,Citation14 and in line with efficacy data from Matulay et al.Citation15 that informed transition probabilities in the BCG arm of the model.
Costs for BCG were modelled based on the 2015 National Institute for Health and Care Excellence (NICE) guideline,Citation6 including costs for delivery and drug acquisition. Drug costs came from the British National Formulary (BNF), and, in the base case analysis, BCG was priced at £150 per vial.
Per-instillation intravesical delivery costs for BCG were £163 per delivery.Citation51 In line with the 2015 NICE guideline, the model incorporated a calculation of the average weighted delivery cost by specifying the proportion of patients undergoing outpatient versus combined day case/ordinary elective spell administration procedures. The proportions of patients undergoing such procedures were determined based on those outlined in the NICE guidelineCitation6 (57% day case).
Radical cystectomy
One RC was modelled for each patient transitioning into the post-cystectomy state. Additionally, and in line with NICE guidanceCitation6 and guidelines on the management of bladder cancer from the West Midlands Expert Advisory Group for Urological Cancer,Citation54 follow-up after RC was modelled as three consultations with a urologist, one cystoscopy per year, two blood tests (to test for metabolic acidosis, B12, and folate deficiency) per year, one glomerular filtration rate test per year, and three CT scans (guidance specifies to have CT scans 6, 12, and 24 months after RCCitation54 or annually as per the NICE guidanceCitation6).
RC was costed in line with the NICE 2015 guideline,Citation6 with 2022/23 National Tariff costs by complication score weighted by 2019/2020 activity data from the NHS 2019–20 national schedule of costs (the latest version of these data to be made publicly available based on pre-COVID-19 data).Citation51 This yielded a cost of £10,883.19. Costs also included follow-up procedures associated with RC and their respective costs (per procedure). These procedures comprised cystoscopy (£251), CT scan (£83), glomerular filtration rate (GFR) testing (£257), blood test (£3.26), and follow-up with a urologist/consultant (£90).
Neoadjuvant chemotherapy
In the base case, it was assumed that all patients were eligible for cisplatin-based neoadjuvant chemotherapy. Based on a previous study,Citation55 16.5% of patients were modelled to receive cisplatin-based neoadjuvant chemotherapy, of whom 90% received gemcitabine plus cisplatin and 10% received accelerated M-VAC.Citation6
A single cycle of gemcitabine plus cisplatin included 1000 mg of gemcitabine per square meter of body surface area (BSA) on days 1, 8, and 15, and 70 mg/m2 of cisplatin on day 2.Citation6,Citation56 Three cycles were modelled for neoadjuvant chemotherapy.Citation6
A single cycle of accelerated M-VAC included 30 mg/m2 methotrexate, 3 mg/m2 vinblastine, 30 mg/m2 adriamycin/doxorubicin, and 70 mg/m2 cisplatin, all given on day 2 of a cycle.Citation6 As for gemcitabine plus cisplatin, neoadjuvant chemotherapy with accelerated M-VAC included three cycles. Granulocyte colony-stimulating factors (G-CSF) were assumed to be used in 25.2% of patients undergoing neoadjuvant chemotherapy, to prevent febrile neutropenia.Citation55,Citation57 For dosing based on BSA, a mean BSA of 1.71 m2 for women and 1.79 m2 for men was used, based on retrospective data for adult cancer patients in the UK.Citation58 The mean weights in kilogram for women (71.8 kg) and men (85.9 kg) as reported by the Office for National Statistics for 2019 to 2020 were used for weight-based dosing.Citation59
Palliative care
Some patients dying from the metastatic disease were assumed to have received palliative care. Although no data for the UK were identified, a US study reported that 4.1% of patients with advanced bladder cancer (T4, N+, M+) received palliative care,Citation60 and this value was used in the base case analysis.
In line with the NICE guidance on bladder cancer,Citation6 palliative care was assumed to consist of 3.1 admissions, on average, each with an average length of stay of 11.4 d. The entire cost of palliative care was applied to 4.1% of patients upon transitioning from metastatic disease to death.
Costs of palliative care were calculated based on a daily cost obtained from the Unit Costs of Health and Social Care 2021,Citation61 which reported an average inpatient specialist palliative care cost of £419.98 per bed day.
Costs associated with adverse events and additional costs
Costs associated with the treatment of adverse events and additional costs came from a variety of documents, primarily the 2022–2023 NHS tariff.Citation51 Where multiple tariff codes were available for different degrees of comorbidity, average costs across these comorbidity categories were calculated as averages weighted by activity in each category as per activity data from the NHS 2019–20 national schedule of costs. A full description of these costs can be found in Table S4.
Sensitivity analysis
One-way and probabilistic sensitivity analyses (PSA) were conducted to evaluate the robustness of the model and the base-case results. Key model parameters were sampled during PSA to investigate the effects of uncertainty surrounding the model inputs; PSA consisted of 10,000 Monte Carlo iterations. For the one-way sensitivity analyses, individual model parameters were altered to investigate the magnitude of their effect on model outcomes, and a tornado diagram was used to summarize NMB outcomes.
Results
Base case
The base case analysis comparing BCG with RC showed that BCG would increase life expectancy by 0.88 years versus RC, from 7.74 to 8.62 years (). BCG also resulted in an increase of 0.76 QALYs versus RC, from 5.63 to 6.39 QALYs, yielding an NMB of £31,748 for a WTP threshold of £20,000 and of £39,366 for a WTP of £30,000 per QALY gained. Patients treated with BCG incurred lower costs than those treated with RC, with costs of £64,264 relative to £47,753 over patient lifetimes. Cost savings were mainly driven by the lower cost of BCG versus RC, with palliative care also leading to further cost savings (£28,638 for RC and £18,713 for BCG). A full breakdown of costs for each treatment can be found in . Based on these results, BCG was the dominant intervention, improving quality-adjusted life expectancy and reducing costs versus RC.
Table 3. Base case results and cost breakdown.
Probabilistic sensitivity analyses
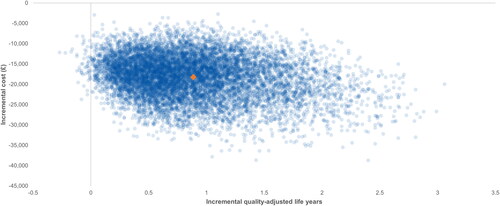
In most PSA iterations, ICURs fell within the south-eastern quadrant of the cost-utility plane, indicating that the use of BCG resulted in an increase of QALYs and cost savings (). At all WTP thresholds between GBPP 20,000 and £100,000, there was a 100% likelihood of adequate BCG therapy being considered a cost-effective strategy.
One-way sensitivity analysis
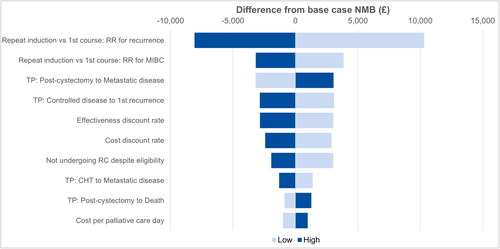
For the one-way sensitivity analyses (OWSA), the ten parameters with the largest impact on the final NMB are presented in . The relative risk for a first recurrence following repeat induction relative to a first course of BCG was the model parameter associated with the greatest variation in NMB when compared with the original base case results. Specifically, when the risk of a recurrence following repeat induction of BCG was three times higher than after a first course, the NMB decreased by more than £8000 while a relative risk of 0 was associated with an NMB increase of more than £10,300. Other relatively influential parameters were the relative risk for muscle-invasive disease following a repeat versus first induction with BCG and the probability of transitioning from the post-cystectomy state to the metastasis state. Notably, the NMB remained positive, favoring BCG over RC, in all one-way sensitivity analyses.
Figure 3. Tornado diagram for one-way sensitivity analyses. The ten most influential drivers of cost-utility results are presented. Abbreviations. CHT, Chemohyperthermia; MIBC, Muscle-Invasive Bladder Cancer; NMB, Net Monetary Benefit; RC, Radical Cystectomy; RR, Relative Risk; TP, Transition Probability.
A BCG maintenance period of 3 years was associated with cost savings of £29,000 relative to RC and NMBs of £62,453 and £79,179 at willingness-to-pay thresholds of £20,000 and £30,000 per QALY gained, respectively.
Discussion
The present study sought to determine the cost-utility of adequate BCG therapy in comparison to RC for treating patients with high-risk NMIBC. To our knowledge, this is the first study that attempts to evaluate the cost-utility of BCG in a patient population where only adequate BCG therapy has been administered.
The analysis showed that adequate BCG is cost-effective compared to RC in patients with high-risk NMIBC. Specifically, BCG resulted in 0.76 additional QALYs versus RC, while reducing costs over patient lifetimes. Cost savings with BCG versus RC were driven by factors such as acquisition and palliative care costs. The PSA demonstrated that, at all WTP thresholds between £20,000 and £100,000, adequate BCG therapy was highly likely to be considered cost-effective, with most PSA iterations suggesting that BCG would lead to cost-savings and QALY gains when compared to RC. The OWSA showed that patients not undergoing RC despite their eligibility had the greatest impact on the final NMB.
There were several limitations to this study, with the first being that there were limited relevant health outcomes data available in the literature, particularly concerning adequate BCG therapy. Given these limitations, assumptions were made concerning the applicability of similar data, considering the treatment setting and the risk group of patients. For example, where UK study data were unavailable, results from US studies informed proxy clinical inputs for the economic model, and, in the case of progression following prior recurrence, no data specific to high-risk NMIBC could be identified so data from intermediate-risk NMIBC was assumed to apply. This is particularly relevant for the clinical efficacy data, which were taken from a single-center US studyCitation15 owing to the current sparsity of data on the efficacy of adequate BCG therapy for high-risk NMIBC. It should be noted that the real-world recurrence and progression data used for the analysis are closely aligned with those for standard frequency BCG administration from the European multi-center NIMBUS trial in patients with high-grade NMIBC.Citation62 While there are some differences between adequate therapy and the reference therapy used in the NIMBUS trial, the comparability of results, which point to the excellent efficacy of induction followed by maintenance BCG therapy, suggest that the clinical efficacy estimates informing the present analysis can be considered robust. However, the use of UK-specific data, once available, would likely be preferable.
Similar to clinical efficacy data, if data for the risks surrounding the development of adverse events were unavailable (e.g. vascular infections from BCG therapy), assumptions and calculations were made. These calculations were based on data such as the number of cases reported in the literature, as well as incidence categories outlined in the summary of product characteristics.Citation49 Although these calculations were based on data on the intervention in question, a risk of incorrect estimation remained for the final figures obtained, which affects the generalizability of the results.
A second limitation was that several assumptions were made regarding the incorporation of adverse events, due to the limitations of the Markov model design. An example of this was incorporating multiple CT scans and follow-ups with a consultant during the first year after treatment with RC. Guidance for CT scans states that patients should receive scans at 6, 12, and 24 months after RC, or three annually according to NICE guidelines.Citation6 However, the Markov model structure could not fully account for time-in-state. As a result, an informed assumption was made to include three CT scans and three consultant follow-ups for the first year following RC, based on the existing literature. The specific assumptions were made to best reflect the most important aspects of patient experiences concerning treatment with RC, and subsequent health outcomes and costs. However, these figures also potentially represent an underestimation of the true number of scans and follow-ups (and therefore costs) that RC patients may experience in their first-year post-treatment. Consequently, the final cost-utility results may in turn represent an underestimation of the final cost savings associated with BCG.
A potential further limitation is that data on the clinical outcomes of adequate BCG therapy were taken from a study conducted at a high-volume reference center in the US, which may not reflect standard treatment.Citation15 However, no other data source of comparable quality and sample size could be identified so the data by Matulay et al.Citation15 were used despite concerns about their potential generalizability. It is noteworthy that the European NIMBUS trial reported comparable results,Citation63 which may also not be considered fully generalizable to standard care given the clinical trial setting but should alleviate concerns regarding country-specific differences.
This study has highlighted limitations in the BCG outcomes evidence base, particularly regarding adequate BCG therapy. Although the clinical effectiveness and safety of BCG have been well-established, the clinical literature on the effectiveness and safety of BCG is heterogeneous in terms of the BCG dosing schedules used, the populations under investigation and the outcomes reported. These differences can be linked to shortcomings in therapy based on how BCG is used in clinical practice, in particular the inconsistent use and scheduling of maintenance therapy. While existing clinical guidelines describe what constitutes adequate BCG therapy, clinical practice has been found to deviate frequently from guideline recommendations.Citation11,Citation64,Citation65 This non-adherence to guidelines can be considered one of the major residual problems in NMIBC care and is compounded by challenges in completing therapy, including a shortage of BCG and, recently, treatment interruptions due to the COVID-19 pandemic.Citation66–68 The BCG shortage has led to rationing or foregoing treatment with BCG and investigations of reduced BCG dosing. Additionally, alternatives to BCG are now being considered due to supply shortages, which is already reflected in recommendations to consider using intravesical chemotherapy in intermediate-risk NMIBC patients.Citation3,Citation69
A further limitation is the lack of comparative data for BCG relative to RC. Such data will likely be difficult to generate, however, as patient numbers available for RC are generally small – while NICE guidance and the NHS specify that immediate RC is an alternative treatment option for high-risk NMIBC,Citation5,Citation6 clinicians and patients may prefer to not use RC early in the disease course and consider RC only for muscle-invasive disease. In a recent trial of photodynamic diagnosis-guided TURBT in patients with intermediate- and high-risk NMIBC from the UK, less than 5% of all patients underwent immediate RC.Citation70 Similar findings were obtained by the BRAVO-Feasibility study, which compared patients with high-risk NMIBC treated with maintenance BCG and RC in the UK.Citation71 The study authors found recruitment to be challenging – many patients expressed a treatment preference, in most cases in favor of BCG over RC, and 20% of patients assigned to RC withdrew from the study as they favored BCG (conversely, only 8% of patients assigned to BCG withdrew due to a preference for RC). Note, however, that the more limited use of immediate RC in the UK does not affect the comparative health economic argument made in the present study, with its focus on value for money.Citation72
Limited evidence is available for the quality-of-life impact of BCG relative to RC. Results on quality of life in the BRAVO-Feasibility were underpowered, but the quality of life outcomes at 12 months were similar for BCG and RC after a rebound in quality of life from 3 months with RC, while treatment decision regret was higher in the BCG relative to the RC arm.Citation71 Observational studiesCitation73,Citation74 and reviewsCitation75 also reported that quality of life may recover within 12–24 months after RC but stated that sexual function and body image may not fully recover, or recover more slowly, and remain below values in the general population. Additional evidence to fill current gaps in patient-reported outcomes and treatment perceptions in NMIBC would be highly welcome and might contribute to improved communication with patients regarding their concerns around their disease and potential treatment sequelae.Citation71,Citation76,Citation77
While our study demonstrates that adequate BCG therapy is a cost-effective treatment option relative to immediate RC for NMIBC in the UK, current real-world practices show that inconsistent use of BCG and non-adherence to treatment guidelines remain important barriers to effective therapy. For the NHS to see maximal utility gains from adequate BCG therapy, more work is required to ensure uniformity of dosing and scheduling, as well as improve patient adherence.
Conclusion
Adequate intravesical BCG led to QALY gains and reduced costs for patients with high-risk NMIBC from the perspective of NHS England when considering RC as a comparator. Further work investigating the effects of adequate BCG treatment on patient health outcomes could be beneficial in strengthening the evidence base for future cost-effectiveness studies.
Transparency
Author contributions
KGH, CH, JP, and RFP conceived and designed the analysis. JP developed the simulation model and conducted the statistical and health economic analyses in collaboration with RFP. WA prepared the first draft of the manuscript, which was revised critically for intellectual content by all authors. All authors approved the final version to be published. All authors agree to be accountable for all aspects of the work.
Reviewer disclosures
A reviewer on this manuscript has disclosed that they have undertaken paid consultancy for a medical device manufacturer that operates within the bladder cancer field. Their technology, however, is of no direct relevance to either BCG treatment or radical cystectomy. The other peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Previous presentations
The model was previously presented at ISPOR Europe 2022 (Vienna, November 6 to November 9, 2022) as poster EE563.
Supplemental Material
Download MS Word (70 KB)Acknowledgements
None reported.
Declaration of funding
medac funded the development of the health economic model and analysis, the preparation of the manuscript, and the article processing charge for the manuscript.
Disclosure statement of financial/other relationships
KGH and CH are full-time employees of medac GmbH, which manufactures and markets a BCG strain. RFP is a full-time employee, director, and shareholder in, and JP and WA are full-time employees of, Covalence Research Ltd, which received consultancy fees from medac to develop the Markov model and analysis and prepare the manuscript. IO has no conflicts of interest to disclose.
References
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249.
- Kamat AM, Hahn NM, Efstathiou JA, et al. Bladder cancer. Lancet. 2016;388:2796–2810.
- Babjuk M, Burger M, Capoun O, et al. European Association of Urology guidelines on non-muscle-invasive bladder cancer (Ta, T1, and carcinoma in situ). Eur Urol. 2022;81:75–94.
- Chang SS, Boorjian SA, Chou R, et al. Diagnosis and treatment of non-muscle invasive bladder cancer: AUA/SUO guideline, 2016. Amended 2020 [Internet]. 2020 [cited 2023 Feb 3]. Available from: https://www.auanet.org/documents/education/clinical-guidance/Non-Muscle-Invasive-Bladder-Cancer.pdf
- NHS. Bladder cancer – Treatment [Internet]. 2017. [cited 2023 Feb 3]. Available from: https://www.nhs.uk/conditions/bladder-cancer/treatment/.
- NICE. Bladder cancer: diagnosis and management NICE guideline [NG2] [Internet]. 2015; [cited 2023 Mar 8]. Available from: https://www.nice.org.uk/guidance/ng2
- Leeds Teaching Hospitals. Urology, bladder problems [Internet]. [cited 2023 Feb 3]. Available from: https://www.leedsth.nhs.uk/a-z-of-services/urology/common-urological-conditions/bladder-cancer/.
- Kamat AM, Flaig TW, Grossman HB, et al. Expert consensus document: consensus statement on best practice management regarding the use of intravesical immunotherapy with BCG for bladder cancer. Nat Rev Urol. 2015;12:225–235.
- Lobo N, Brooks NA, Zlotta AR, et al. 100 Years of Bacillus Calmette–Guérin immunotherapy: from cattle to COVID-19. Nat Rev Urol. 2021;18:611–622.
- Schmidt S, Kunath F, Coles B, et al. Intravesical Bacillus Calmette-Guérin versus mitomycin C for Ta and T1 bladder cancer. Cochrane Database Syst Rev. 2020;1(1):CD011935.
- Mori K, Miura N, Babjuk M, et al. Low compliance to guidelines in nonmuscle-invasive bladder carcinoma: a systematic review. Urol Oncol. 2020;38:774–782.
- Mariappan P, Johnston A, Padovani L, et al. Enhanced quality and effectiveness of transurethral resection of bladder tumour in non-muscle-invasive bladder cancer: a multicentre real-world experience from Scotland’s quality performance indicators programme. Eur Urol. 2020;78:520–530.
- Food and Drug Administration. BCG-unresponsive nonmuscle invasive bladder cancer: developing drugs and biologics for treatment: guidance for industry [Internet]. Silver Spring (MD): US Department of Health and Human Services Food and Drug Administration; 2018 [cited 2023 Feb 3]. Available from: https://www.fda.gov/media/101468/download
- Kamat AM, Sylvester RJ, Böhle A, et al. Definitions, end points, and clinical trial designs for non-muscle-invasive bladder cancer: recommendations from the international bladder cancer group. J Clin Oncol. 2016;34:1935–1944.
- Matulay JT, Li R, Hensley PJ, et al. Contemporary outcomes of patients with nonmuscle-invasive bladder cancer treated with Bacillus Calmette-Guérin: implications for clinical trial design. J Urol. 2021;205:1612–1621.
- Sonnenberg FA, Beck JR. Markov models in medical decision making: a practical guide. Med Decis Making. 1993;13:322–338.
- Witjes J, Bruins H, Carrión A, et al. EAU guidelines on muscle-invasive and metastatic bladder cancer [Internet]. Amsterdam: European Association of Urology; 2022 [cited 2023 Mar 8]. Available from: https://d56bochluxqnz.cloudfront.net/documents/full-guideline/EAU-Guidelines-on-Muscle-Invasive-And-Metastatic-Bladder-Cancer-2022.pdf
- ICER. Nadofaragene firadenovec and oportuzumab monatox for BCG-unresponsive, non-muscle invasive bladder cancer: effectiveness and value: final report [Internet]. Boston (MA): Institute for Clinical and Economic Review; 2020 [cited 2023 Mar 8]. Available from: https://icer.org/wp-content/uploads/2020/08/ICER_Bladder_Cancer_Final_Report_07292022.pdf
- Sharma V, Wymer KM, Borah BJ, et al. Cost-effectiveness of maintenance bacillus Calmette-Guérin for intermediate and high risk nonmuscle invasive bladder cancer. J Urol. 2020;204:442–449.
- Mossanen M, Wang Y, Szymaniak J, et al. Evaluating the cost of surveillance for non-muscle-invasive bladder cancer: an analysis based on risk categories. World J Urol. 2019;37:2059–2065.
- NICE. Guide to the methods of technology appraisal 2013: process and methods (PMG9) [Internet]. London, Manchester: National Institute for Health and Care Excellence; 2013 [cited 2022 Dec 5]. Available from: https://www.nice.org.uk/process/pmg9/resources/guide-to-the-methods-of-technology-appraisal-2013-pdf-2007975843781
- Elshabrawy A, Wang H, Satsangi A, et al. Correlates of refusal of radical cystectomy in patients with muscle-invasive bladder cancer. Urol Oncol. 2021;39:236.e9–236.e20.
- NICE. NICE health technology evaluations: the manual (PMG36) [Internet]. London (UK): National Institute for Health and Care Excellence; 2022 [cited 2023 Mar 8]. Available from: https://www.nice.org.uk/process/pmg36
- Gidwani R, Russell LB. Estimating transition probabilities from published evidence: a tutorial for decision modelers. Pharmacoeconomics. 2020;38:1153–1164.
- Simon M, Bosset P-O, Rouanne M, et al. Multiple recurrences and risk of disease progression in patients with primary low-grade (TaG1) non–muscle-invasive bladder cancer and with low and intermediate EORTC-risk score. PLOS One. 2019;14:e0211721.
- Daniels MJ, Barry E, Schoenberg M, et al. Contemporary oncologic outcomes of second induction course BCG in patients with nonmuscle invasive bladder cancer. Urol Oncol. 2020;38:5.e9–5.e16.
- Diamant E, Roumiguié M, Ingels A, et al. Effectiveness of early radical cystectomy for high-risk non-muscle invasive bladder cancer. Cancers. 2022;14:3797.
- Cheeseman S, Thompson M, Sopwith W, et al. Current treatment and outcomes benchmark for locally advanced or metastatic urothelial cancer from a large UK-based single centre. Front Oncol. 2020;10:167.
- Doisy L, Cimier A, Adypagavane A, et al. Efficacy of HIVEC in patients with high-risk non-muscle invasive bladder cancer who are contraindicated to BCG and in patients who fail BCG therapy. Int J Hyperthermia. 2021;38:1633–1638.
- Advanced Bladder Cancer (ABC) Meta-analysis Collaboration. Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. Eur Urol. 2005;48:202–205. discussion 205–206.
- ONS. National life tables – life expectancy in the UK: 2018 to 2020 [Internet]. Newport: Office for National Statistics; 2021 [cited 2022 Aug 30]. Available from: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/datasets/nationallifetablesunitedkingdomreferencetables
- Public Health England. Cancer survival in England for patients diagnosed between 2014 and 2018, and followed up until 2019 [Internet]. GOV.UK. 2020 [cited 2023 Mar 8]. Available from: https://www.gov.uk/government/statistics/cancer-survival-in-england-for-patients-diagnosed-between-2014-and-2018-and-followed-up-until-2019
- Cox E, Saramago P, Kelly J, et al. Effects of bladder cancer on UK healthcare costs and patient health-related quality of life: evidence from the BOXIT trial. Clin Genitourin Cancer. 2020;18:e418–e442.
- Kulkarni GS, Finelli A, Fleshner NE, et al. Optimal management of high-risk T1G3 bladder cancer: a decision analysis. PLOS Med. 2007;4:e284.
- Mohiuddin S, Reeves BC, Smart NJ, et al. A semi-Markov model comparing the lifetime cost-effectiveness of mesh prophylaxis to prevent parastomal hernia in patients undergoing end colostomy creation for rectal cancer. Colorectal Dis. 2021;23:2967–2979.
- Betts MB, Rane P, Bergrath E, et al. Utility value estimates in cardiovascular disease and the effect of changing elicitation methods: a systematic literature review. Health Qual Life Outcomes. 2020;18:251.
- Fujii H, Ueda Y, Hirose C, et al. Pharmaceutical intervention for adverse events improves quality of life in patients with cancer undergoing outpatient chemotherapy. J Pharm Health Care Sci. 2022;8(1):8.
- Stevenson SM, Danzig MR, Ghandour RA, et al. Cost-effectiveness of neoadjuvant chemotherapy before radical cystectomy for muscle-invasive bladder cancer. Urol Oncol. 2014;32:1172–1177.
- Hagiwara Y, Shiroiwa T, Shimozuma K, et al. Impact of adverse events on health utility and health-related quality of life in patients receiving first-line chemotherapy for metastatic breast cancer: results from the SELECT BC study. Pharmacoeconomics. 2018;36:215–223.
- Hawe E, McBride D, Balp M-M, et al. EQ-5D utilities in chronic spontaneous/idiopathic urticaria. Pharmacoeconomics. 2016;34:521–527.
- Lloyd A, Nafees B, Narewska J, et al. Health state utilities for metastatic breast cancer. Br J Cancer. 2006;95:683–690.
- Polotti C, Tan B, Borglum N, et al. Relationship between the Wisconsin stone quality of life (WISQOL) and preference-based/health utility measures of health-related quality of life (HRQoL) in kidney stone patients. Urology. 2020;141:33–38.
- Liem YS, Bosch JL, Hunink MGM. Preference-based quality of life of patients on renal replacement therapy: a systematic review and meta-analysis. Value Health. 2008;11:733–741.
- Li B, Cairns JA, Fotheringham J, et al. Understanding cost of care for patients on renal replacement therapy: looking beyond fixed tariffs. Nephrol Dial Transplant. 2015;30:1726–1734.
- Armstrong N, Büyükkaramikli N, Penton H, et al. Avatrombopag and lusutrombopag for thrombocytopenia in people with chronic liver disease needing an elective procedure: a systematic review and cost-effectiveness analysis. Health Technol Assess. 2020;24:1–220.
- National Clinical Guideline Centre (UK). Cost-effectiveness analysis – botulinum toxin type a versus augmentation cystoplasty [Internet]. London: Royal College of Physicians; 2012 (Report No. 148). [cited 2023 Mar 8]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK132839/
- Tawiah AK, Sayah A, Ohinmaa F, et al. Discriminative validity of the EQ-5D-5 L and SF-12 in older adults with arthritis. Health Qual Life Outcomes. 2019;17:68.
- Kulkarni GS, Alibhai SMH, Finelli A, et al. Cost-effectiveness analysis of immediate radical cystectomy versus intravesical Bacillus Calmette-Guerin therapy for high-risk, high-grade (T1G3) bladder cancer. Cancer. 2009;115:5450–5459.
- emc. OncoTICE powder for instillation fluid for intravesical use - summary of product characteristics (SmPC) [Internet]. 2021. [cited 2022 Jun 23]. Available from: https://www.medicines.org.uk/emc/product/1049
- Magee D, Cheung D, Hird A, et al. Trimodal therapy vs. radical cystectomy for muscle-invasive bladder cancer: a Markov microsimulation model. Can Urol Assoc J. 2022;16: E197–E204.
- NHS England and NHS Improvement. 2022/23 National tariff payment system [Internet]. London: NHS England and NHS Improvement; 2022 [cited 2022 May 26]. Available from: https://www.england.nhs.uk/publication/national-tariff-payment-system-documents-annexes-and-supporting-documents/
- Office for National Statistics. CPI INDEX: medical services [Internet]. 2022 [cited 2022 Jun 26]. Available from: https://www.ons.gov.uk/economy/inflationandpriceindices/timeseries/dkc3/mm23
- Lamm DL, Blumenstein BA, Crissman JD, et al. Maintenance bacillus Calmette-Guerin immunotherapy for recurrent TA, T1 and carcinoma in situ transitional cell carcinoma of the bladder: a randomized Southwest Oncology Group Study. J Urol. 2000;163:1124–1129.
- West Midlands Expert Advisory Group for Urological Cancer. Guidelines for the management of bladder cancer [Internet]. West Midlands Clinical Networks and Clinical Senate; 2019. [cited 2022 May 27]. Available from: https://www.england.nhs.uk/mids-east/wp-content/uploads/sites/7/2018/05/guidelines-for-the-management-of-bladder-cancer.pdf
- Niedersüss-Beke D, Puntus T, Kunit T, et al. Neoadjuvant chemotherapy with gemcitabine plus cisplatin in patients with locally advanced bladder cancer. Oncology. 2017;93:36–42.
- emc. Gemcitabine 38 mg/ml concentrate for solution for infusion - summary of product characteristics (SmPC) [Internet]. 2022 [cited 2022 May 30]. Available from: https://www.medicines.org.uk/emc/product/4483/smpc
- West Midlands Cancer Alliance. Guidelines for G-CSF use [Internet]. Birmingham: West Midlands Cancer Alliance; 2021 [cited 2022 Nov 24]. Available from: https://wmcanceralliance.nhs.uk/images/WMCA_Guideline_for_Use_of_GCSF_Final_v1.0.pdf
- Sacco JJ, Botten J, Macbeth F, et al. The average body surface area of adult cancer patients in the UK: a multicentre retrospective study. PLOS One. 2010;5:e8933.
- Office for National Statistics. UK health indicators [Internet]. 2022 [cited 2022 Jun 27]. Available from: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/healthandlifeexpectancies/datasets/healthindicatorsfortheunitedkingdomanditsconstituentcountriesbasedonthe2013to2014europeanhealthinterviewsurveywave2
- Hugar LA, Lopa SH, Yabes JG, et al. Palliative care use amongst patients with bladder cancer. BJU Int. 2019;123:968–975.
- Jones KC, Burns A. Unit costs of health and social care 2021. Kent (UK): Personal Social Services Research Unit; 2021.
- European Association Of Urology Research Foundation Türkeri LN, Babjuk MM. Treatment of high grade non-muscle invasive bladder carcinoma by standard number and dose of intravesical BCG instillations versus reduced number and dose of intravesical BCG instillations. A European Association of Urology Research Foundation randomised phase III clinical trial. Amsterdam: European Association of Urology Research Foundation; 2021.
- Grimm M-O, van der Heijden AG, Colombel M, et al. Treatment of high-grade non-muscle-invasive bladder carcinoma by standard number and dose of BCG instillations versus reduced number and standard dose of BCG instillations: results of the European Association of Urology Research Foundation randomised phase III clinical trial “NIMBUS”. Eur Urol. 2020;78:690–698.
- Jeglinschi S, Schirmann A, Durand M, et al. Factors affecting guideline adherence in the initial treatment of non-muscle invasive bladder cancer: retrospective study in a french peripheral hospital. Prog Urol. 2020;30:26–34.
- Choo SH, Nishiyama H, Kitamura H, et al. Practice pattern of non-muscle invasive bladder cancer in Japan, Korea and Taiwan: a web-based survey. Int J Urol. 2019;26:1121–1127.
- Ferro M, Giudice D, Carrieri F, et al. The impact of SARS-CoV-2 pandemic on time to primary, secondary resection and adjuvant intravesical therapy in patients with high-risk non-muscle invasive bladder cancer: a retrospective multi-institutional cohort analysis. Cancers. 2021;13:5276.
- Culpan M, Keser F, Acar HC, et al. Impact of delay in cystoscopic surveillance on recurrence and progression rates in patients with non-muscle-invasive bladder cancer during the COVID-19 pandemic. Int J Clin Pract. 2021;75:e14490.
- Bandari J, Maganty A, MacLeod LC, et al. Manufacturing and the market: rationalizing the shortage of Bacillus Calmette-Guérin. Eur Urol Focus. 2018;4:481–484.
- Balasubramanian A, Gunjur A, Weickhardt A, et al. Adjuvant therapies for non-muscle-invasive bladder cancer: advances during BCG shortage. World J Urol. 2022;40:1111–1124.
- Heer R, Lewis R, Duncan A, et al. Photodynamic versus white-light-guided resection of first-diagnosis non-muscle-invasive bladder cancer: PHOTO RCT. Health Technol Assess. 2022;26:1–144.
- Catto JWF, Gordon K, Collinson M, et al. Radical cystectomy against intravesical BCG for high-risk high-grade nonmuscle invasive bladder cancer: results from the randomized controlled BRAVO-Feasibility study. J Clin Oncol. 2021;39:202–214.
- Office for Health Improvement and Disparities. Cost utility analysis: health economic studies [Internet]. GOV.UK. 2020. [cited 2023 Mar 8]. Available from: https://www.gov.uk/guidance/cost-utility-analysis-health-economic-studies
- Clements MB, Atkinson TM, Dalbagni GM, et al. Health-related quality of life for patients undergoing radical cystectomy: results of a large prospective cohort. Eur Urol. 2022;81:294–304.
- Jung A, Nielsen ME, Crandell JL, et al. Health-related quality of life among non-muscle-invasive bladder cancer survivors: a population-based study. BJU Int. 2020;125:38–48.
- Yang LS, Shan BL, Shan LL, et al. A systematic review and meta-analysis of quality of life outcomes after radical cystectomy for bladder cancer. Surg Oncol. 2016;25:281–297.
- Rutherford C, Patel MI, Tait M-A, et al. Patient-reported outcomes in non-muscle invasive bladder cancer: a mixed-methods systematic review. Qual Life Res. 2021;30:345–366.
- Tan WS, Teo CH, Chan D, et al. Exploring patients’ experience and perception of being diagnosed with bladder cancer: a mixed-methods approach. BJU Int. 2020;125:669–678.
- Taylor JM, Feifer A, Savage CJ, et al. Evaluating the utility of a preoperative nomogram for predicting 90-day mortality following radical cystectomy for bladder cancer. BJU Int. 2012;109:855–859.