Abstract
Background
Seasonal influenza may result in severe outcomes, resulting in a significant increase of hospitalizations during the winter. To improve the protection provided by the standard dose influenza quadrivalent vaccine (SDQIV), a high-dose vaccine (HDQIV) has been developed specifically for adults aged 60 and older who are at higher risk of life-threatening complications,
Objectives
The aim of this study was to determine the cost-effectiveness of HD QIV vs. SD-QIV in the recommended population of three European countries: Belgium, Finland and Portugal.
Methods
A cost-utility analysis comparing HDQIV vs. SDQIV was conducted using a decision tree estimating health outcomes conditional on influenza: cases, general practitioner and emergency department visits, hospitalizations and deaths. To account for the full benefit of the vaccine, an additional outcome—hospitalizations attributable to influenza—was also evaluated. Demographic, epidemiological and economic inputs were based on the respective local data. HDQIV relative vaccine efficacy vs. SDQIV was obtained from a phase IV efficacy randomized clinical trial. The incremental cost-effectiveness ratios (ICER) were computed for each country, and a probabilistic sensitivity analysis (1,000 simulations per country) was performed to assess the robustness of the results.
Results
In the base case analysis, HDQIV resulted in improved health outcomes (visits, hospitalizations, and deaths) compared to SDQIV. The ICERs computed were 1,397, 9,581, and 15,267 €/QALY, whereas the PSA yielded 100, 100, and 84% of simulations being cost-effective at their respective willingness-to-pay thresholds, for Belgium, Finland, and Portugal, respectively.
Conclusion
In three European countries with different healthcare systems, HD-QIV would contribute to a significant improvement in the prevention of influenza health outcomes while being cost-effective.
1. Introduction
Influenza is an acute respiratory infection caused by an RNA virus of the Orthomyxoviridae family. Usually, flu starts suddenly with a high fever, muscular soreness, headache, severe fatigue, general discomfort, and respiratory symptoms, such as dry cough, rhinorrhea. The illness lasts for about a week, but fatigue is frequently felt for two weeks or moreCitation1. A dry cough can persist for two weeks. Although benign in most cases, influenza may lead to severe complications or an aggravation of an already existing chronic disease (such as diabetes, chronic obstructive pulmonary disease, heart failure, chronic kidney diseaseCitation2). Evidence shows that influenza increases the risk of pneumonia by 100 in the week following the infection, due to the influenza virus implication in the pathogenesis of several respiratory bacterial pathogens commonly associated with pneumoniaCitation3. In addition, a large number of published papers suggests that laboratory-confirmed influenza infection may be associated with an increased risk of acute myocardial infarction estimated as six times higherCitation4. In a case-crossover analysis using California data, among 36,975 hospitalized ischemic strokes, the risk of stroke after an Influenza‐like illness (ILI) episode increased by almost three times in the days following the flu infectionCitation5.
Worldwide, World Health Organization (WHO) estimates that influenza annual epidemics result in about 3–5 million cases of severe illness, among which 290,000–650,000 respiratory deathsCitation6. Each year, between 300,000 and 900,000 people visit their GP because of influenza-like illness in Belgium (Belgique en bonne santé website), with a total population of about 11.5 million. Among these, the annual number of influenza infections is estimated between 116,000 and 472,000 (Belgique en bonne santé website). In Finland, with population of 5.5 million, the number of influenza cases reported to the National Infectious Diseases Register progressed from 6,428 in 2012 to 19,829 in 2019, respectivelyCitation7. In Portugal, with a population of 10.3 million, an incidence rate of 933 per 100,000 resulted in ∼96,000 influenza cases estimated in 2019Citation8, while another studyCitation9 estimated that annual influenza-associated respiratory and cardiovascular hospitalizations were 199.6 per 100,000.
Due to immunosenescence and the increasing prevalence of chronic conditions with age, people aged 65 and over have a high susceptibility to the virus and are at greater risk of developing complications from influenza. In recent years, ∼88% of seasonal flu-related deaths have occurred in people 65 years and olderCitation10. While flu seasons vary in severity, during most seasons people 65 years and older bear the greatest burden of severe flu disease. As a result, WHO recommends that elderly people be vaccinated against seasonal influenzaCitation11.
The burden attributable to influenza is often underestimated due to the unspecific nature of influenza symptoms and diagnosisCitation6,Citation12. Accurate estimation of hospitalizations and deaths related to influenza is essential to measure the burden of influenza, however, high variability in published rates of influenza demonstrates the lack of adapted methodology, data sources and case definitions to appropriately estimate influenza-associated hospitalization burdenCitation13. Influenza virus infections are rarely confirmed systematically by laboratory diagnosis, and thus influenza hospitalizations and/or deaths might be attributed to other comorbid conditions or secondary infectionsCitation14. The overall underestimation of influenza burden can also be explained by the behavior of patients, who are seeking medical care late in their illness when influenza can no longer be detected from respiratory samplesCitation15. For these reasons, the estimations of influenza-related deaths and hospitalizations underestimate the true morbidity and mortality burden caused by influenza.
Influenza vaccination is viewed as the primary tool for the prevention of seasonal influenza disease. Influenza antivirals (oseltamivir and zanamivir) are also authorized in the European Union (EU), often only recommended as treatment for influenza in those at risk of severe complications due to the risk of developing resistance to these drugs and the risk of side effectsCitation16. Traditionally, inactivated influenza vaccines were trivalent to protect against influenza A(H3N2), pandemic A(H1N1) and one out of two circulating influenza B lineage viruses. Since 2017/8, quadrivalent vaccines protecting against both influenza B lineage viruses have been approved, but vaccines effectiveness remains suboptimal. An additional improvement specifically designed to protect the most vulnerable 65 years and older population was made with the “high dose” (HD) influenza vaccine, meaning that it contains four times the amount of antigen (60 μg of hemagglutinin) per strain than a standard dose (SD) vaccine. The HD influenza vaccine was first developed in a trivalent form (HD-TIV, available only in the US), and then continued with the quadrivalent formulation (HD-QIV, licensed also in numerous European countries since 2020)Citation17. The HD influenza vaccine has shown greater immunogenicity, efficacy, and effectiveness than standard-dose vaccines in adults 65 years old and olderCitation18–20 while having an acceptable safety profile.
Evidence of clinical superior efficacy of HD influenza vaccine was demonstrated in the FIM12 pivotal trial, a phase IIIb–IV, multicenter, randomized, double-blind, active-controlled trial to compare HD-TIV with standard dose trivalent, inactivated influenza vaccine (SD-TIV) in adults 65 years of age or older. A total of 31,989 participants were enrolled from 126 research centers in the United States and Canada. Incremental efficacy of a new vaccine is usually measured as its relative vaccine efficacy (rVE), meaning in this case the fraction of people protected by HD-TIV among those that remained unprotected by SD-TIV. HD-TIV demonstrated a rVE compared to SD-TIV of 24.2% (95% confidence interval [CI], 9.7–36.5%) to prevent laboratory confirmed influenza caused by any viral type or subtype associated with a protocol- defined influenza-like illness. A complementary analysis of study FIM12Citation21, documented the benefit of HD-TIV in the prevention of serious adverse events potentially linked to influenza. It showed that HD-TIV was significantly more effective than SD-TIV in preventing cardio-respiratory events possibly related to influenza overall (rVE, 17.7%; 95% confidence interval [CI], 6.6–27.4%) and serious pneumonia (rVE, 39.8%; 95% CI, 19.3–55.1%). In addition to HD-TIV superiority in the prevention of cases of laboratory-confirmed influenza vs. SD-TIV, the study shows that HD-TIV was also more effective to prevent other influenza-related complications, such as pneumonia, influenza-related cardiovascular and respiratory events, and hospitalizations for any causeCitation19.
Moreover, a systematic review was conducted for studies assessing the rVE of HD-QIV compared to SD-QIV against probable/laboratory-confirmed influenza-like illness (ILI), hospital admissions, and death in adults 65 years old and older. Seven studies (five RCTs and two observational studies) were included in a meta-analysis, resulting in a relative efficacy in preventing influenza hospitalization of 17.8% for influenza cases, 24.3% for respiratory complications and 18.2% for cardiorespiratory complications. The results of this study demonstrated that HD-QIV was more effective than SD-QIV at reducing hospital admission associated with influenza infection in older adultsCitation20.
The current study focused on the cost-effectiveness of switching from SD-QIV to HD-QIV vaccine in Belgium, Finland and Portugal, three countries having national recommendations to promote influenza vaccination among persons 65 years of age and olderCitation22–24 and where standard dose (SD) vaccine is currently the standard of care. HD-QIV was granted marketing authorization in April 2020 in Belgium, May 2020 in Portugal, and June 2020 in Finland according to a decentralized procedure. Reimbursement was granted in Belgium for 65+ people living in healthcare institutions (https://webappsa.riziv-inami.fgov.be/SSPWebApplicationPublic/fr/Public/ProductSearch), and has been requested in Portugal. The national immunization program for influenza is being reviewed in Finland.
2. Methods
2.1. Structure of the model
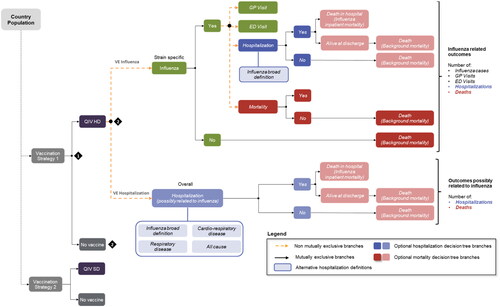
A static model with decision tree, in accordance with WHO recommendationsCitation25,Citation26 was used. See analogous to previously published similar analysisCitation27,Citation28. The model allows to define the target population for vaccination and the vaccination strategy to be tested. A decision tree is used to estimate the outcomes of interest in adults aged 65 years or older vaccinated with respectively HD-QIV and SD-QIV. Costs and consequences were estimated over the horizon of an average influenza season (November to April), apart from premature deaths due to influenza, for which we captured all quality-adjusted life years (QALYs) lost up to life expectancy. Given that horizon, no discounting was applied to costs, and the country-specific discounting rate was applied to premature death outcomes (1.5% in Belgium, 3% in Finland, 4% in Portugal).
The decision tree computes the possible events during an influenza season as followed: influenza cases, general practitioner (GP) and emergency department (ED) visits conditional on influenza, influenza-related hospitalizations and deaths. The aim of estimating influenza-related hospitalizations was to accurately reflect the burden of influenza. To that effect, two distinct methods were developed in the model: either hospitalizations were directly related to influenza cases, or they were assumed to be possibly related to an influenza case. For the first method, the probability of hospitalization was calculated from ICD-10 codes specific to influenza: J9, J10, J11. The description of each ICD-10 codes is provided in Supplementary Table S1. Regarding the second method, three options were derived from the methodology used in the FIM12 complementary analysis. Based on SAE diagnostic categories that were reported during the study, three health outcomes categories were identified by the investigators as possibly related to influenza, starting with influenza broad definition (influenza and pneumonia, ICD-10 codes: J09–J18), followed by respiratory disease (ICD-10 codes: J09–J18, J40–J45, and J96), and ending with cardio-respiratory events (ICD-10 codes: J09–J18, J40–J45, J96, I20, I21, I24, I25, I50, I60–I63, I65, I66, I69, G45, G46).
Table 1. Summary of unit costs (in €) utilized in the model.
A way to address the underestimation of influenza mortality consists in modelling studies that capture excess of deaths that are attributable to influenza, but are not necessarily reported as such. In this model we estimated both, excess deaths conditional on developing influenza and those attributable to influenza, and we applied the global rate to influenza cases. Influenza-related deaths and deaths from other causes were separated in the model. People who survived the influenza season were at risk of death in subsequent years based on the all-cause mortality rate.
The model calculates the incremental cost-effectiveness ratio (ICER) based on incremental costs and outcomes (QALYs). In accordance with the respective guidelines of each country, the perspective of the analyses corresponds to the total payer’s perspective (including patient copayment) in Belgium and Finland and to the National Health System (NHS) perspective (i.e. excluding copayment) in Portugal. In Portugal, the National Health System perspective corresponds to 37% of the published price.
2.2. Model inputs common to all countries
2.2.1. Attack rate
The number of influenza cases was estimated based on the attack rate, vaccine coverage and vaccine efficacy for each vaccine strategy. The attack rate among unvaccinated population aged 65 and older was assumed to be 7.2% in all three countriesCitation29. The reported value of 7.2% is consistent with the WHO estimation which reports an attack rate estimated between 5 and 10%Citation26.
2.2.2. Vaccine efficacies
Vaccines’ efficacies were obtained from RCTs and meta-analyses, as recommended by WHO in their guidelines on economic evaluations of infectious diseasesCitation26. Moreover, the robustness of these efficacies values was confirmed by well recognized health technology assessment bodiesCitation16,Citation30. Both rated with a GRADE A the evidence on the relative efficacy of HD vs. SD influenza vaccines, mostly based on an efficacy RCT ran over two consecutive influenza seasonsCitation19. Furthermore, the German STIKO and the USA ACIP, both recognized the benefits of HD-QIV vaccine over SD for the 65+ peopleCitation31,Citation32.
The vaccine efficacy for SD QIV was estimated based on current vaccination program efficacy against A and B strains. The first step in estimating the efficacy of SD QIV against influenza is to establish the efficacy by influenza strain. The efficacy of the vaccine was calculated as a weighted average of the vaccine efficacy estimates with the corresponding average influenza strain proportions. Annual reports by the Finnish Institute for Health and Welfare (THL) were utilized to determine the efficacy of current vaccination program to influenza cases. Influenza cases by strain were extracted from Finnish National Infectious Diseases Register. The relative efficacy of HDQIV compared to SDQIV was assumed to be the same as measured in the studyCitation19 for trivalent formulations (i.e. 24.2%Citation19) for all the outcomes considered (influenza hospitalization and cardiorespiratory events). This equivalence was justified by an immune-bridging clinical trialCitation33, an approach that has been approved by the European Medicines Agency.
2.3. Model inputs specific to each country
2.3.1. Costs
Country-specific cost data are presented in . Vaccination costs for HD-QIV and SD-QIV were obtained from the publicly available tariff list of each country. It was assumed that a general practitioner or a nurse would administer the influenza vaccine during a consultation in Belgium and Finland, respectively. In Portugal, it was assumed that flu vaccination would be performed in pharmacies and that any costs inherent to the act are supported by the patient. Medications costs were applied to all influenza cases and were calculated or extracted from the literature. The cost of visit to the GP were obtained from the national health service of each country. Influenza-related emergency department (ED) presentations were extracted from the KCE report 178 in Belgium and from the national health service in PortugalCitation44; in Finland, ED visits were assumed to be included in the cost of hospitalization and therefore no separate cost was assumed for ED visits.
2.3.2. Health-related quality of life
Country-specific health-related quality of life (HRQoL) data are presented . Each country elicited HRQoL values with the EQ-5D questionnaire using local tariffs or a proxy country when local tariffs were not available. The same disutility values presented were applied in all three countries per day of influenza and per hospitalization episode, values were obtained from the literatureCitation38,Citation39.
Table 3. Summary of epidemiological and clinical efficacy data.
2.3.3. Other country-specific parameters
2.3.3.1. Belgium
All inputs applied in the model base case are described in (HRQoL inputs) and . Considering the underestimation of influenza-related hospitalizations, and because influenza virus may cause pneumoniaCitation55, some assumptions were made for the model base case to include both influenza- and pneumonia-related hospitalizations when estimating the hospitalization rate potentially linked to influenza. This definition refers to “influenza broad definition” in the following paragraphs. Therefore, hospitalizations related to serious events potentially link to influenza, such as pneumonia was used to estimate the number of hospitalizations in the base case. The only clinical trial assessing the efficacy of the influenza vaccine in the population of subjects 65 years of age and older, reports an efficacy of 50%Citation52. In the absence of robust absolute VE estimates of SD-QIV in Belgium, the efficacy of SD-QIV was assumed to be 50% and applied for both strain A and B. The relative efficacy in hospitalization prevention of HD-QIV vs. SD-QIV was based on the meta-analysisCitation20 utilizing the influenza endpoint. The probability of influenza-related death was conditional on developing influenza and applied to influenza cases. The number of influenza-related deaths in the population aged 65 and over was estimated with excess of death that were attributable to influenza and reported in the 2017–2018 Sciensano reportCitation45. The probability of GP visit was calculated by dividing the number of medical consultations for influenza cases by the estimated number of influenza cases (Data from Belgian institute Sciensano). The HRQoL data used in Belgium reflects the French reference tariffCitation35.
Table 2. Quality of life data.
2.3.3.2. Finland
All inputs applied in the model base case are described in (HRQoL inputs) and . The hospitalization rate was estimated with similar ICD-10 codes as the ones selected in Belgium, which corresponds to the “influenza broad definition”, but most of the cases captured were due to pneumonia. However, the relative efficacy in hospitalization prevention of HD-QIV vs. SD-QIV was based on Lee et al.Citation20 utilizing the pneumonia endpoint. The proportion of influenza cases for strain A and B in the population aged 65 and over was 74 and 26%, respectively. It was estimated as an average over 2015–2020 seasons. Vaccine efficacy of influenza vaccines is monitored annually in Finland and reported in annual reports by the Finnish Institute for Health and Welfare (THL). Efficacy of SD-QIV in Finland over 2015–2019 seasons was used: 23.8% for strain A and 22.7% for strain B resulting in an average weighted efficacy of 23.5%. The probability of GP visit was assumed to be associated with every diagnosed influenza case. ED visits were assumed to be included in the cost of hospitalization and therefore no separate cost is assumed for ED visits. The probability of influenza-related death was conditional on hospitalization, by assuming that any influenza-related death occurred in a hospital setting. This probability was defined and validated by expert opinion. The HRQoL data used in Finland reflects the Swedish reference tariffCitation36.
2.3.3.3. Portugal
All inputs applied in the model base case are described in (HRQoL inputs) and . Influenza burden related to hospitalization was characterized in the Burden of Acute Respiratory Infections (BARI) study, a retrospective descriptive national epidemiological study. This study described the hospitalizations for influenza in Portugal over a period of 10 years (2008/2009–2017/2018 flu seasons). Only hospitalizations directly linked to influenza were selected to estimate the probability of hospitalization in the base case. The efficacy of SD-QIV was obtained from Fleming et al.Citation54. The SD-QIV efficacy was assumed to be equal for both influenza strains. The relative efficacy in hospitalization prevention of HD-QIV vs. SD-QIV was based on Lee et al.Citation20 utilizing the influenza endpoint. The probabilities of having one GP or one ED visit, respectively were calculated by dividing the number of GP and ED visits due to influenza by the number of influenza cases predicted by the modelCitation47. The number of influenza-related deaths in the population aged 65 and over was estimated with excess of death that were attributable to influenza and reported in the 2018–2019 Portuguese flu surveillance agency report (Programa Nacional de Vigilância da Gripe)Citation8. The HRQoL data used in Portugal reflects the Portuguese reference tariffCitation37.
2.4. Probabilistic sensitivity analysis
The model includes a probabilistic sensitivity analysis (PSA) to evaluate the stochastic parameters uncertainty, with 1,000 simulations per country.
In order to undertake the PSA, parameters in the model were assigned a probability distribution, reflecting both the central estimate (mean) of that parameter, its variance (standard error) and the anticipated shape of the data around its mean. The main outcome of the PSA is the % of simulations yielding an ICER below the assumed willingness-to-pay (WTP) threshold.
3. Results
The results from the base case analysis were summarized for all three countries in . The incremental cost per QALY gained using HD-QIV was 1,397, 9,581, and 15,267 €/QALY compared to the strategy with SD-QIV vaccine in the base case analysis for Belgium, Finland, and Portugal, respectively.
Table 4. Base case results.
Table 5. Breakdown of costs/clinical outcomes.
In Belgium, switching from a vaccination strategy with SD-QIV to a vaccination program with HD-QIV in 65+ would cost €3,178,842, or €1.46 for every vaccinated individual. Differences in healthcare costs were driven by higher vaccination costs for HD-QIV, which was partially compensated by savings attributable to reductions in primary and secondary healthcare resources required for influenza treatment. The switch from SD-QIV to HD-QIV prevented 10,103 cases of influenza, 4,253 GP visits, 81 ED visits, 1,849 episodes of hospitalization due to influenza or pneumonia, and 260 deaths due to influenza. Two scenario analyses including a broader hospitalization definition, lead to a greater incremental hospitalization cost, the vaccination strategy with HD-QIV would save €33.17 for every vaccinated individual with the respiratory hospitalization definition and €45.01 with the cardio-respiratory one, making HD-QIV a dominant strategy in Belgium. These results confirm that choosing the influenza diagnosis definition constitute the most conservative option. The results of the deterministic sensitivity analysis show that the key drivers of the model results were the rVE against influenza-associated hospitalization for HD-QIV compared to SD-QIV, cost of Influenza-related hospitalization and hospitalization rate. The probabilistic sensitivity analysis (PSA) shows that HD-QIV was cost-effective in all simulations with the willingness-to-pay (WTP) of 35,000€ and also less costly (dominant) in 39% of the iterations.
In Finland, the additional cost of a vaccination programme with HD-QIV represented €6,687,578 or €5.43 for every vaccinated individual. Similarly, differences in healthcare costs were driven by higher vaccination costs for HD-QIV, also partially compensated by reductions in primary and secondary healthcare resources. The switch from SD-QIV to HD-QIV prevented 8,125 cases of influenza, 8,125 GP visits, 1,532 episodes of hospitalization due to influenza or pneumonia, and 77 deaths due to influenza. The results of the deterministic sensitivity analysis were robust to most parameters, the key drivers of the model results were the efficacy against influenza-associated hospitalization for HD-QIV compared to SD-QIV, hospitalization rate and the probability of death conditional on being hospitalized. The PSA shows that HD-QIV has a probability of being cost-effective of 100% considering a WTP of €23,000 per QALY.
In Portugal, the additional cost of a vaccination programme with HD-QIV represented €18,632,383 or €8.47 for every vaccinated individual. The incremental difference between HD-QIV and SD-QIV in terms of total hospitalization costs was smaller than in the other two countries due to the lower hospitalizations costs per patient and the more conservative methodology (influenza specific hospitalization definition) used to estimate hospitalizations rates resulting in lower hospitalizations rates. Low influenza hospitalization rates decrease the positive impact of HD-QIV on the total costs saved. Still with this conservative approach, the switch from SD-QIV to HD-QIV prevented 12,259 cases of influenza, 1,078 GP visits, 466 ED visits, 108 episodes of hospitalization due to influenza or pneumonia, and 76 deaths due to influenza. A scenario analysis including hospitalizations for respiratory diseases leads to a greater difference in terms of QALY, as it allows the additional quality of life gains with the reduction of hospitalization episodes with HD-QIV. On the other hand, it also allows the reduction of the incremental cost, considering a greater reduction in hospitalization costs. In this scenario, the use of HD-QIV was associated with an incremental cost-effectiveness ratio (ICER) of €10,602 per QALY. The ICER was €4,310 per QALY in the scenario with hospitalizations for cardio-respiratory events. The results of the deterministic sensitivity analysis were robust to most parameters, being sensitive to estimates of the relative efficacy of HD-QIV and excess mortality from influenza, as well as the choice of perspective. The PSA shows that HD-QIV has a probability of being cost-effective of 84% considering a WTP of €25,000 per QALY.
When selecting the broader approach using the respiratory definition for Portugal, the ICER become more cost-effective from 15,267 to 10,602 €/QALY. Similarly, when using an even broader definition of “potentially related to influenza” and including “cardio-respiratory pathologies”, the ICER become even more cost-effective, from 10,602 to 4,310 €/QALY. A similar trend was observed for Belgium, where the use of a broader hospitalization definition made HD-QIV the dominant strategy.
4. Discussion and conclusion
The results from the base case analysis showed an incremental cost per QALY gained using HD-QIV of 1,397, 9,581, and 15,267 €/QALY compared to the strategy with SD-QIV vaccine in the base case analysis for Belgium, Finland, and Portugal, respectively. The cost-effectiveness acceptability curves (CEAC) performed for each country showed that100% of the simulations were cost-effective considering a WTP of €35,000 per QALY in Belgium, 100% considering a WTP of €23,000 per QALY in Finland, and 84% for a WTP of €25,000 per QALY in Portugal.
HD-QIV is now indicated for the active immunization against influenza in persons 60 years of age and older in Europe. Belgium, Finland and Portugal have published national recommendations to promote influenza vaccination among persons 65 years of age and older. In Belgium, reimbursement of HD-QIV was granted for persons 65 years of age and older living in long-term healthcare facilities, and it has been requested in Portugal. The national immunization program for influenza is being reviewed in Finland.
Higher ICER in Portugal is the results of a smaller incremental difference between HD-QIV and SD-QIV in terms of total hospitalization costs. Given the limitation in the screening and identification of influenza cases, a wider approach on the estimation of the economic burden of influenza is needed. As expected, the current study illustrates well how a narrower definition of hospitalizations (as the one used for Portugal) underestimates the inpatient costs, affecting therefore the ICER. Comparatively, the broader approach of hospitalization used for Finland and Belgium closes that gap by denoting an improved ratio cost to utility closer to the reality. The lower hospitalization rates in Portugal could be explained because only the hospitalizations explicitly associated with a documented diagnosis of influenza were considered, whereas in Belgium and Finland the probability of hospitalization included a wider range of lower tract respiratory infections, such as pneumonia. In order to address the underestimation of influenza related hospitalizations two distinct modeling approaches were developed, from the most conservative approach which only takes into account hospitalizations resulting from an influenza case, to the broader approach which also takes into account hospitalizations related to serious events potentially related to influenza, such as respiratory or cardio-respiratory events. The second approach differs from many previous influenza models in that it explicitly considers the impact of vaccination on hospitalization for potentially influenza-related reasons regardless of a history of influenza infection. This approach is justified by the absence of a systematic test for the detection of influenza viruses in Belgium, which means that hospitalizations due to influenza or its complications are not systematically identified as such. In Finland the second approach was applied similarly to previous analyses, such as Jack et al. to account for underestimation of influenza cases. This later approach stays robust in the context of HD cost effectiveness analysis—considering the available evidence on HD rVE over SD against broader hospitalization definition from the supplemental analysis of FIM12 pivotal trial. The definition used in the base case for Belgium and Finland were based on the influenza broad definition (influenza and pneumonia).
The high variability in the valorization of hospitalization costs between Belgium and the other countries is mainly driven by the length of stay for one influenza-related hospitalization. The mean hospitalization length of stay was estimated to be 20 days in Belgium compared to 6 days in Finland and 11 days in Portugal.
The greater efficacy of HD-QIV compared to SD-QIV allows to decrease the number of hospitalizations and consequently HD-QIV emerges as an effective tool to help controlling the overall winter pressure on health care services, which is now increased by the COVID-19 concomitant hospitalizations.
Several economic evaluations on high-dose influenza vaccines (HD-QIV and HD-TIV) are available in the literature, conducted with different methods and settingsCitation27,Citation28,Citation56–59. In agreement with these publications, the results presented here consistently show that the switch from SD-QIV to HD-QIV could be a cost-effective strategy for the prevention of influenza in older adults, for the three healthcare settings considered.
Some limitations need to be mentioned for this study. First the rVE for HD-QIV vs. SD-QIV in the prevention of influenza cases was assumed to be identical to that of HD-TIV vs. SD-TIV. This assumption was considered reasonable as adding a second B strain in the HD-QIV vaccine does not compromise the protection against the other strains and hence the rVE should remain the same, as suggested by the immunobridging studyCitation33. Second, it was difficult to accurately estimate the influenza burden. As previously stated, influenza virus infections are not confirmed systematically by a laboratory diagnosis, and thus influenza deaths and hospitalizations might be attributed to other comorbid conditions or secondary infectionsCitation14. This could lead to an underestimation of influenza-related deaths and hospitalizations when assessing the clinical and economic burden associated with influenza. To mitigate this risk, the approach taken in this study consisted in including “influenza-attributable” complications, such as pneumonia in the hospitalization rate estimations in Belgium and Finland; and in running scenario analysis using an alternative definition of hospitalizations that takes into account influenza related complications, based on the outcomes of a randomized clinical trialCitation21. Another limitation was the use of a static model, instead of a dynamic transmission model, which could lead to an underestimation of the vaccine value . Even if this approach does not account for the potential indirect benefits from vaccines, WHO guidelines indicate that decision trees are suitable both, for assessing the costs and consequences of an intervention over a short period, and for targeting older adults, as it is the case of vaccination against influenza with HD-QIVCitation60. In Belgium, the efficacy for the standard dose influenza vaccine was based on the publication by Govaert et al. without specifying whether it applies to strains A and/or B. The model therefore considers that the efficacy resulting from the publication applies to both strains. To mitigate this assumption a scenario analysis was performed by assuming a lower efficacy of 35%Citation61. The impact of this parameter on the results was very limited. Lastly, given the country to country heterogeneity of the methodologies applied to measure the influenza burden, as well as the seasonal, this work did not intend to compare the results obtained for the three countries.
This study has shown that across several countries with different healthcare systems, switching from SD-QIV to HD-QIV would contribute to significant improvement in terms of public health (i.e. lower number of flu cases, GP and ED visits, hospitalizations, and deaths) while being a cost-effective option. Further, it demonstrates that the protection granted by quadrivalent vaccines goes beyond the flu episode itself, as it also decreases the burden associated with influenza on the longer term (by avoiding influenza-related complications, such as pneumonia, cardiovascular or respiratory events or hospitalizations for any causes).
Transparency
Declaration of funding
This work was funded by Sanofi Pasteur. Sanofi Pasteur were involved in the design of the economic model, analysis, and interpretation of results as well as the writing of the manuscript.
Declaration of financial/other relationships
FPA, HB, CM, AS, CP, and CC are employees of Sanofi, which manufactures HD-QIV. They may/may not hold shares in the company.
PC, MB, and TS received consulting fees from Sanofi to conduct the country adaptations of the economic model. PC and MB received consulting fees from Sanofi to produce the manuscript.
Author contributions
FPA, HB, and CC were involved in the conception and design, analysis and interpretation of all the data.
PC, MB, and CP conducted the Belgian economic evaluation; TS and AS conducted the Finish economic evaluation; CM conducted the Portuguese economic evaluation.
MB and PC drafted the manuscript. All authors revised it critically and approved the version submitted.
All authors agree to be accountable for all aspects of the work.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplemental Material
Download MS Word (18.9 KB)Acknowledgements
None reported.
References
- John Hopkins University. Conditions and diseases → influenza [cited 2023 Mar 13]. Available from: https://www.hopkinsmedicine.org/health/conditions-and-diseases/influenza
- Macias AE, McElhaney JE, Chaves SS, et al. The disease burden of influenza beyond respiratory illness. Vaccine. 2021;39(Suppl 1):A6–A14.
- Shrestha S, Foxman B, Berus J, et al. The role of influenza in the epidemiology of pneumonia. Sci Rep. 2015;5:15314.
- Kwong JC, Schwartz KL, Campitelli MA, et al. Acute myocardial infarction after laboratory-confirmed influenza infection. N Engl J Med. 2018;378(26):2540–2541.
- Boehme AK, Luna J, Kulick ER, et al. Influenza‐like illness as a trigger for ischemic stroke. Ann Clin Transl Neurol. 2018;5(4):456–463.
- World Health Organization. Fact sheet: influenza (seasonal); 2018 [cited 2020 Nov 6]. Available from: https://www.who.int/en/news-room/fact-sheets/detail/influenza-(seasonal)
- Finnish Institute for Health and Welfare (THL); 2020. Available from: https://thl.fi/en/web/infectious-diseases-and-vaccinations/surveillance-and-registers/infectious-diseases-in-finland-publications
- Pechirra P, Cristóvão P, Costa I, et al. Programa Nacional de Vigilância da Gripe: relatório da época 2018/2019. Lisboa: Instituto Nacional de Saúde Doutor Ricardo Jorge IP (INSA); 2019.
- Froes F, Carmo M, Lopes H, et al. Excess hospitalizations and mortality associated with seasonal influenza in Portugal, 2008–2018. BMC Infect Dis 2022;22: 726.
- Paget J, Iuliano AD, Taylor RJ, et al. Estimates of mortality associated with seasonal influenza for the European Union from the GLaMOR project. Vaccine. 2022;40(9):1361–1369.
- World Health Organization. 2013. Available from: https://www.euro.who.int/en/health-topics/communicable-diseases/influenza/vaccination/seasonal-vaccination-policies-and-coverage-in-the-european-region
- Carrat F, Vergu E, Ferguson NM, et al. Timelines of infection and disease in human influenza: a review of volunteer challenge studies. Am J Epidemiol. 2008;167(7):775–785.
- Roguski KM, Rolfes MA, Reich JS, et al. Variability in published rates of influenza-associated hospitalizations: a systematic review, 2007–2018. J Glob Health. 2020;10(2):020430.
- Iuliano AD, Roguski KM, Chang HH, et al. Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. Lancet. 2018;391(10127):1285–1300.
- Centers for Disease Control and Prevention. Frequently asked questions about estimated flu burden: deaths; 2020 [cited 2021 Feb 2]. Available from: https://www.cdc.gov/flu/about/burden/faq.htm
- European Centre for Disease Prevention and Control. Systematic review of the efficacy, effectiveness and safety of newer and enhanced seasonal influenza vaccines for the prevention of laboratory-confirmed influenza in individuals aged 18 years and over. ECDC; 2020.
- Pepin S, Nicolas JF, Szymanski H, et al. QHD00011 study team. Immunogenicity and safety of a quadrivalent high-dose inactivated influenza vaccine compared with a standard-dose quadrivalent influenza vaccine in healthy people aged 60 years or older: a randomized phase III trial. Hum Vaccin Immunother. 2021;17(12):5475–5486.
- Falsey A, Treanor J, Tornieporth N, et al. Randomized, double-blind controlled phase 3 trial comparing the immunogenicity of high-dose and standard-dose influenza vaccine in adults 65 years of age and older. J Infect Dis. 2009;200(2):172–180.
- DiazGranados CA, Dunning AJ, Kimmel M, et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N Engl J Med. 2014;371(7):635–645.
- Lee JKH, Lam GKL, Shin T, et al. Efficacy and effectiveness of high-dose versus standard-dose influenza vaccination for older adults: a systematic review and meta-analysis. Expert Rev Vaccines. 2018;17(5):435–443.
- DiazGranados CA, Robertson CA, Talbot HK, et al. Prevention of serious events in adults 65 years of age or older: a comparison between high-dose and standard-dose inactivated influenza vaccines. Vaccine. 2015;33(38):4988–4993.
- Conseil Supérieur de la Santé. Avis DU Conseil Superieur DE LA Sante N° 9625; 2021.
- Finnish Institute for Health and Welfare (THL). Influenza vaccine. Available from: https://thl.fi/en/web/infectious-diseases-and-vaccinations/vaccines-a-to-z/influenza-vaccine
- Direçao Geral de Saúde. Normas e circulares normativas. Vacinação contra a gripe. Época 2021/2022; 2021. Available from: https://www.dgs.pt/normas-orientacoes-e-informacoes/normas-e-circulares-normativas/norma-n-0062021-de-25092021.aspx
- World Health Organization. Guidance on the economic evaluation of influenza vaccination; 2016.
- World Health Organization. WHO guide on standardization of economic evaluations of immunization programmes; 2019 [cited 2020 May 1]. Available from: https://www.who.int/immunization/documents/who_ivb_19.10/en/
- Redondo E, Drago G, López-Belmonte JL, et al. Cost-utility analysis of influenza vaccination in a population aged 65 years or older in Spain with a high-dose vaccine versus an adjuvanted vaccine. Vaccine. 2021;39(36):5138–5145.
- Mattock R, Gibbons I, Moss J, et al. Cost-effectiveness of high dose versus adjuvanted trivalent influenza vaccines in England and Wales. J Med Econ. 2021;24(1):1261–1271.
- Somes MP, Turner RM, Dwyer LJ, et al. Estimating the annual attack rate of seasonal influenza among unvaccinated individuals: a systematic review and meta-analysis. Vaccine. 2018;36(23):3199–3207.
- National Advisory Committee on Immunization. An Advisory Committee Statement (ACS). Literature review update on the efficacy and effectiveness of high-dose (Fluzone® high-dose) and MF59-adjuvanted (Fluad®) trivalent inactivated influenza vaccines in adults 65 years of age and older; 2018.
- Michaelis K, Scholz S, Buda S, et al. Beschluss und Wissenschaftliche Begründung der Ständigen Impfkommission (STIKO) für die Aktualisierung der Influenza-Impfempfehlung für Personen im Alter von ≥ 60 Jahren. Epid Bull. 2021;1:3–25.
- Grohskopf LA, Blanton LH, Ferdinands JM, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2022–23 influenza season. MMWR Recomm Rep. 2022;71(1):1–28.
- Chang LJ, Meng Y, Janosczyk H, et al. Safety and immunogenicity of high-dose quadrivalent influenza vaccine in adults ≥65 years of age: a phase 3 randomized clinical trial. Vaccine. 2019;37:5825–5834.
- Kapiainen S, et al. Terveydenhuollon yksikkökustannukset Suomessa. THL; 2014. Available from: https://urn.fi/URN:ISBN:978-952-302-079-5
- Szende A, Janssen B, Cabases J. (Eds) Self-reported population health: an international perspective based on EQ-5D. Dordrecht, Heidelberg, New York, London: Springer; 2014.
- Burström K, Johannesson M, Diderichsen F. Swedish population health-related quality of life results using the EQ-5D. Qual Life Res. 2001;10(7):621–635.
- Ferreira LN, Ferreira PL, Pereira LN, et al. The valuation of the EQ-5D in Portugal. Qual Life Res. 2014;23(2):413–423.
- Turner D, Wailoo A, Nicholson K, Cooper N, Sutton A. Systematic review and economic decision modelling for the prevention and treatment of influenza A and B. Health Technol Assess 2003;7(35) doi: 10.3310/hta7350
- Baguelin M, Camacho A, Flasche S, et al. Extending the elderly- and risk-group programme of vaccination against seasonal influenza in England and Wales: a cost-effectiveness study. BMC Med. 2015;13:236.
- Base de dados de medicamentos de uso humano; 2021. Available from: https://extranet.infarmed.pt/INFOMED-fo/index.xhtml
- Beutels P, Vandendijck Y, Willem L et al, Seasonal influenza vaccination: prioritizing children or other target groups? - Part II. Health Technology Assessment (HTA). Brussels. Belgian Health Care Knowledge Centre (KCE). 2013. KCE Reports 204. Aavilable from: https://kce.fgov.be/en/publications/all-reports/seasonal-influenza-vaccination-prioritizing-children-or-other-target-groups-part-ii
- Swartenbroekx N, Obyn C, Guillaume P, et al. Manual for cost-based pricing of hospital interventions. Health Technology Assessment (HTA). Brussels. Belgian Health Care Knowledge Centre (KCE). 2012. KCE Reports 178C. DOI: 10.57598/R178C
- IQVIA. Burden of Acute Respiratory Infections (BARI) study; 2020 (data on file).
- SNS—Monitorização diária do serviço de urgencia; 2021. Available from: https://www.sns.gov.pt/monitorizacao-do-sns/servicos-de-urgencia/
- Sciensano. Belgian Institute for Health—seasonal influenza surveillance. Available from: https://www.sciensano.be/en/health-topics/influenza/role-0
- StatBel. Causes of death by month, sex, age group and region. Available from: https://statbel.fgov.be/en/open-data/causes-death-month-sex-age-group-and-region
- SNS—Portal da transparencia; 2021. Available from: https://transparencia.sns.gov.pt/explore/?sort=title&q=gripe
- Jacks A, Ollgren J, Ziegler T, et al. Influenza-associated hospitalisations in Finland from 1996 to 2010: unexpected age-specific burden during the influenza A(H1N1)pdm09 pandemic from 2009 to 2010. Euro Surveill. 2012;17(38):20276.
- Finnish Institute for Health and Welfare (THL). Vaccine coverage. Available from: https://www.thl.fi/roko/rokotusrekisteri/atlas/atlas.html?show=influenza
- Portal de dados abertos da Administração Pública. Available from: https://dados.gov.pt/pt/datasets/taxa-de-cobertura-da-vacina-antigripal-sazonal-1/
- Finnish Institute for Health and Welfare (THL). National Infectious Diseases Register [cited 2021 Feb 10].
- Govaert TM, Thijs CT, Masurel N, et al. The efficacy of influenza vaccination in elderly individuals. A randomized double-blind placebo-controlled trial. JAMA. 1994;272(21):1661–1665.
- Official Statistics of Finland. Medicinal products database [web publication]. Helsinki: Kela; 2021. Available from: https://www.kela.fi/web/en/medicinal-products-database
- Fleming DM, Andrews NJ, Ellis JS, et al. Estimating influenza vaccine effectiveness using routinely collected laboratory data. J Epidemiol Community Health. 2010;64:1062–1067.
- Rothberg MB, Haessler SD, Brown RB. Complications of viral influenza. Am J Med. 2008;121(4):258–264.
- Becker DL, Chit A, DiazGranados CA, et al. High-dose inactivated influenza vaccine is associated with cost savings and better outcomes compared to standard-dose inactivated influenza vaccine in Canadian seniors. Hum Vaccin Immunother. 2016;12(12):3036–3042.
- Chit A, Roiz J, Briquet B, et al. Expected cost effectiveness of high-dose trivalent influenza vaccine in US seniors. Vaccine. 2015;33(5):734–741.
- Colrat F, Thommes E, Largeron N, et al. Economic evaluation of high-dose inactivated influenza vaccine in adults aged ≥65 years: a systematic literature review. Vaccine. 2021;39(Suppl 1):A42–A50.
- van Aalst R, Russo EM, Neupane N, et al. Economic assessment of a high-dose versus a standard-dose influenza vaccine in the US veteran population: estimating the impact on hospitalization cost for cardio-respiratory disease. Vaccine. 2019;37(32):4499–4503.
- World Health Organization. Influenza; 2019 [cited 2020 May 1]. Available from: https://www.who.int/biologicals/vaccines/influenza/en/
- Bonmarin I, Belchior E, Lé vy-Bruhl D. Impact of influenza vaccination on mortality in the French elderly population during the 2000-2009 period. Vaccine 2015;33:1099–1101.