Abstract
Objectives
Type-2 Diabetes mellitus (T2DM) increases both the patient risk of cardiovascular disease (CVD) and renal outcomes, such as chronic kidney disease (CKD). Recent clinical trials of the glucose-lowering drug-class of sodium-glucose co-transporter-2 inhibitors (SGLT2is) have shown benefits in preventing CVD events and progression of CKD, leading to an update of the Dutch T2DM treatment guideline for patients at risk. The aim of this study is to assess the health and economic impact of the guideline-recommended utilization of SGLT2is in the Netherlands.
Methods
The patient population at risk was determined by multiplying Dutch T2DM prevalence rates with the total numbers of inhabitants of the Netherlands in 2020. Subsequently, two analyses, comparing a treatment setting before and after implementation of the new guideline for SGLT2is, were conducted. Clinical and adverse event rates in both settings as well as direct healthcare costs were sourced from the literature. Total costs were calculated by multiplying disease prevalence, event rates and costs associated to outcomes. One-time disutilities per event were included to estimate the health impact. The potential health and economic impact of implementing the updated guideline was calculated.
Results
Using a 5-year time horizon, the guideline-suggested utilization of SGLT2is resulted in a health impact equal to 4835 quality adjusted life years gained (0.0031 per patient per year) and €461 million cost-savings. The costs of treatment with SGLT2is were €813 million. Hence the net budget impact was €352 million for the total Dutch T2DM population, which translated to €0,57 per patient per day.
Conclusion
SGLT2is offer an option to reduce the number of CVD and CKD related events and associated healthcare costs and health losses in the Netherlands. Further research is needed to include the benefits of improved T2DM management options from a broader societal perspective.
The glucose-lowering drug-class of sodium-glucose co-transporter-2 inhibitors (SGLT2is) has shown benefits in preventing cardiovascular events and progression of kidney disease in patients with type-2 diabetes leading to a revision of the respective Dutch treatment guideline.
The 5-year budget impact of the adoption of SGLT2is in the new treatment guideline was equal to €352 million or €0.57 per patient per day, with a total of 4385 quality adjusted life years gained.
The introduction of SGLT2is for Dutch type-2 diabetes patients has the potential to substantially reduce the number of cardiovascular as well as renal disease events and related healthcare costs while also delivering a health benefit.
Highlights
Introduction
In the Netherlands, the overall prevalence of type-2 diabetes mellitus (T2DM) is pronounced, with roughly 1.03 million people living with this condition in 2019 and with an average age of 61 yearsCitation1. T2DM increases the risk for cardiovascular disease (CVD) by a factor of 2–4 and reduces life expectancy on average by 4 years, with absolute risks being the highest in patients with established chronic kidney disease (CKD)Citation2,Citation3. T2DM also increases the risk for cardiorenal outcomes, in particular heart failure and end stage renal diseaseCitation2.
Cardiovascular risk management is therefore mainstay in all patients with T2DM. At first, conservative treatment options such as smoking cessation and adoption of a healthy lifestyle are recommended for all people with T2DM, but additional risk factor treatment should be considered, especially in patients above the age of 40 yearsCitation4. This approach of risk factor management led to an almost 50% decrease in the prevalence of myocardial infarction, stroke, and retinopathy. Nevertheless, recent studies demonstrate that for end-stage kidney disease, acute myocardial infarction and stroke, the long-term improvements stalled and plateaued after 2010Citation5. More recent cardiovascular outcome studies confirm the high residual cardiorenal risk despite adequate cardiovascular risk managementCitation6,Citation7.
Notably, 25–30% of patients with T2DM have a known (cardio-)vascular condition whereas 28% of patients in the first treatment line live with some form of chronic kidney diseaseCitation8–11. Recent clinical trials of the glucose-lowering drug-class of sodium-glucose co-transporter-2 inhibitors (SGLT2is) have shown benefits in preventing CVD events and progression of CKD, when used as a complementary treatment on top of glycaemic control and risk factor management in T2DM patientsCitation12–14. In the Netherlands, four SGLT2is are currently available: canagliflozin (Invokana), empagliflozin (Jardiance), dapagliflozin (Forxiga), and ertugliflozin (Steglatro), of which the first three are reimbursed and became the preferred choice of therapy in T2DM patients with a very high risk for cardiovascular eventsCitation15. However, for patients without an increased risk, SGLT2is remain as second-line treatment. Dapagliflozin and empagliflozin are the most often used SGLT2isCitation16. Following the outcomes from clinical trials, the Dutch College of General Practitioners (NHG) and the Dutch Society of Internal Medicine (NIV) updated their T2DM related treatment guideline to incorporate SGLT2is as monotherapy or as an add-on to metformin in patients at risk for CVD due to previous disease history or CKDCitation15.
The aim of this study is to assess the health and budget impact of the utilization of SGLT2is based on this new T2DM treatment guideline compared to the previous situation in the Netherlands.
Methods
The budget impact of SGLT2is from a Dutch healthcare system perspective is calculated combining T2DM disease prevalence with the probability of subsequent clinical events and their costs. In this section we further elaborate on how the size of the target patient population and event rates were estimated, the event-specific costs were incorporated in the model to calculate the final health and budget impact.
Population & model
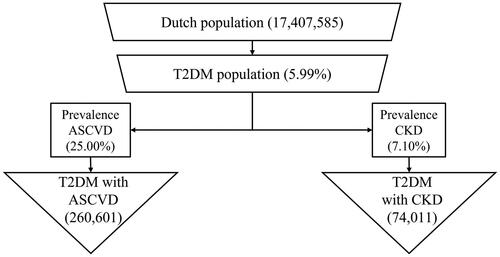
The model population of Dutch T2DM patients was determined by multiplying Dutch T2DM disease prevalence rates with the total numbers of inhabitants of the Netherlands, using the population data from 2020Citation17–19. Subsequently, the target-group-specific prevalence rates of both ASCVD and CKD in T2DM patients were multiplied with the initial population of T2DM patients to estimate the total number of patients with ASCVD and CKD in the NetherlandsCitation8. See for a flow diagram for the estimation of the populations used in this model. Both groups are at risk of developing clinical events.
The number of estimated patients at risk was then used to calculate the absolute number of clinical events in two analyses. The first analysis, labelled “previous setting”, calculated the number of events in the treatment setting prior to the new diabetes mellitus treatment guideline. The second analysis, labelled new setting, considers the broad complementary use of SGLT2is on top of the previous guidelines recommend medication, which is in line with the new diabetes mellitus treatment guideline. Differences in number of clinical events and adverse events were calculated based on the estimated numbers of clinical and adverse events for each the previous and new clinical guideline setting. The events are defined and described in more detail below.
For both analyses, annual clinical and adverse events per year were calculated for different time-horizons of 1, 3, 5 and 10 subsequent years. Both analyses adjusted the prevalent population at the beginning of each year for the respective mortality in the previous year. Lastly, the model was extended with the possibility to test the degree to which SGLT2is are prescribed within the specified target population. These shares are set in quartile steps (25, 50, 75, 100%). The chosen value is determining on what proportions of patients in the new setting were experiencing the SGLT2i treatment effect.
Event rates & treatment effect
The clinical events accounted for in this analysis were major adverse cardiovascular events (MACE), heart failure (HF) related hospitalization, cardiovascular mortality, myocardial infarction (MI), stroke and first events of kidney-related outcomes, which included macroalbuminuria, renal injury, and renal failure.
For the previous setting, annual event rates for all included events were sourced as weighted averages from the EMPA-REG, CANVAS and DECLARE clinical trials as reported in a published meta-analysis, as well as the CREDENCE clinical trialCitation6,Citation7,Citation12,Citation20,Citation21. For the new guideline the annual event rates from the previous setting without the explicit recommendation for additional pharmacological treatment analysis were adjusted for the efficacy of SGLT2is using a hazard ratio (HR), sourced from the same meta-analysis and the CREDENCE clinical trialCitation6,Citation12.
The analysis included the occurrence of specific adverse events, similarly populated by the aforementioned meta-analysis; i.e. amputations, fractures and diabetic ketoacidosisCitation12. For the analysis reflecting the setting prior to the new guideline, annual adverse event rates were sourced and weighted in the same manner as the clinical event rates as described earlier. Subsequently, for the new guideline setting, the annual adverse event rates were adjusted for the utilization of SGLT2is with a HR, sourced from a published meta-analysisCitation12. However, it should be noted that the model did not account for an increased risk for subsequent events after the initial events.
Costs & utilities
The annual number of events avoided was multiplied with the cost per event to estimate the budget impact. Direct costs of clinical events as well as adverse events were obtained by means of a literature search specific to the Netherlands and inflated to 2020 price level in EurosCitation22–30. The value for the particular event of first event kidney related outcomes was calculated as weighted average of the development of macroalbuminuria, renal injury, and renal failure, in which costs due to renal injury were assumed to be equal to value of the development of macroalbuminuria. Weights for that calculation were sourced from the distribution of renal outcomes in the EMPA-REG trialCitation7. All clinical events in this study, except for cardiovascular mortality and adverse events which were treated as one-time costs, created constant follow-up costs in the years after the event, and were obtained from a long-term cost-effectiveness study conducted for T2DM patientsCitation31. The amount of treatment costs of the SGLT2 medication was based on the market shares and acquisition costs, which amounted to €513.94 per year per patientCitation32.
The ISPOR Budget Impact Analysis guidelines were followed, as they provide guidance about the acquisition and use of data for budget-impact analysis and on how to report resultsCitation33. Following the guideline, we adopted a healthcare payers perspective, as the model is made for the budget holder, and a 5-year time horizon was chosen to more accurately outline cost savings associated with SGLT2is. This also involves the way that health outcomes and their related costs in the total target population are reflected in each year, after SGLT2is are introduced into clinical practice. In line with the guidelines, disutilities for all clinical and adverse events were also included for the year in which a particular event occurredCitation33. The disutilities associated with all clinical and adverse events were sourced from a study estimating diabetes-related comorbidities from US and UK EQ-5D questionnaire data on chronic conditionsCitation34. The disutility value of first event kidney related outcomes was obtained in the exact same manner as the related cost value as described above. For the particular case of diabetes ketoacidosis, the disutility value was sourced from a study analyzing the impact of diabetes-related complication in type 1 diabetes-mellitus patientsCitation35. The sum of all disutilities over the simulated time frame were compared for both guidelines and included as a complementary health impact analysis. presents the input values for the different outcomes defined.
Table 1. Costs and disutilities per event type.
To allow for a better comparison between the previous and updated guideline, the budget impact is expressed in cost per patient year. Hence, the patient years simulated in the model were calculated by subtracting the number of subjects with a disease -event in an annual cycle from the starting population in the same annual cycle. In case of mortality, it was assumed that this occurred on average half-way an annual model cycle. Subsequently, the obtained amount of patient years per model year were accumulated over the chosen time-horizon.
Finally, to test the robustness of the model, we performed a one-way sensitivity analysis (OSA) by varying all input parameters between a lower and upper bound value. A complete overview of the parameters can be found in Supplementary Table A1. In addition, scenario analyses were run for different time horizons as well as varying market utilization of SGLT2is.
Results
The total number of patients at risk at the start of the analysis was 334,612, split into 260,601 subjects with ASCVD and 74,011 with CKD. Consistently in all analyses, the total amount of occurring clinical events were lower if the new diabetes mellitus treatment guideline was considered as compared with the previous setting in which that guideline was absent. This resulted in a health gain of 4835 quality adjusted life years based on applying a five-year time horizon. After five years the largest differences were reported for first event of kidney-related outcomes, MACE, total mortality and HF related hospitalization with 65, 46, 46 and 52 averted events per 10,000 patient years, respectively. Occurrence of adverse events increased slightly in the new guidelines analysis when compared to the previous guideline setting. Incremental adverse events per 10,000 patient years in the new setting amounted to 11 amputations, 10 fractures and 4 cases of diabetic ketoacidosis after 5 years.
The total amount of averted events per patient year as well as incremental occurred adverse events for all respective time horizons are reported in . presents the total number of clinical events averted as the difference between the previous and new guideline when 100% implemented. These are all first events. A positive number indicates that the new guidelines analysis results in fewer clinical events compared with the previous guideline. Consequently, a negative number indicates more events in the new setting. For adverse events a positive number indicates additional events relative to the previous setting.
Table 2. The potential total number of clinical events averted following the introduction of the new DM guideline.
The total amount of costs after 5 years in the previous setting would be €1.84 billion, whereas the implementation of the new treatment guideline would result in €1.38 billion for the total population considered. Hence, the introduction of SGLT2is according to the new guideline saved roughly €461 million in total costs, combining averted events and additional adverse events. The costs of treatment with SGLT2is for the population considered were €813 million. This results in a net budget impact of €352 million for the total Dutch T2DM population, which translates into €209 per patient year and a daily amount of €0.57 per patient.
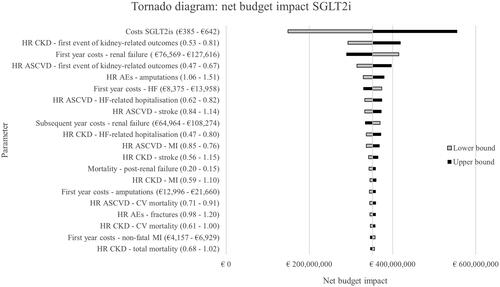
The results from the OSA are presented in the tornado diagram in . The net budget impact is most greatly affected by the SGLT2i costs. Following this, HRs for kidney-related outcomes and costs for renal failure have the greatest impact on the budget impact.
The budget impact as total costs, per patient-day for all time horizons applied are reported in . This table presents the budget impact as the difference between additional medication costs obtained through the utilization of SGLT2is and net savings of clinical events averted.
Table 3. Budget impact results for different time horizons.
The main analysis assumes 100% implementation of the newly introduced T2DM guideline. provides budget-impact results based on different real-world SGLT2i utilization scenarios for a 5-year time-horizon.
Table 4. Budget impact results based on assuming different SGLT2i market share scenarios.
Discussion
The application of SGLT2is based on the new T2DM treatment guideline and the created combination of clinical events averted as well as additional treatment costs as a consequence of SGLT2i use and costs for adverse events, resulted in a net budget impact of SGLT2is of €352 million after 5 years which translates to €0.57 per patient-day.
The health and budget study outcomes highlight the beneficial impact of a complementary treatment option for T2DM patients following the new clinical guideline versus its previous guideline. The preventive effect of SGLT2is in terms of clinical events averted, gains in significance in the light of a large patient group dealing with T2DM as well as an ageing population with longer life-expectancies and thus more frequent and longer periods of to be treated T2DM per patient. SGLT2is offer an immediate form of preventing detrimental and expensive health conditions which often require constant follow-up care. ASCVD and CKD hereby are the most prime examples and both conditions are significantly averted by the utilization of SGLT2is. When comparing the costs per patient per day of €0.57 to other medications used frequently in the same patient group, one can conclude that these are relatively small as – for instance – in 2020 insulin or dabigatran were reimbursed at €0.99 and €2.19 per defined daily dose, respectivelyCitation16.
This study applied comparative data, i.e. hazard ratios, from a recently published meta-analysis on SGLT2isCitation12. However, two alternative studies have conducted similar meta-analysesCitation13,Citation14, but did not report HRs for SGLT2is versus standard of care that are needed for this health and budget impact model analysis. The particular network meta-analysis by Palmer et al. was used to update the Dutch DM guidelineCitation13. The other meta-analysis included two additional trials, but findings were consistent with both other meta-analysesCitation12,Citation14. In the current study, the meta-analysis of Zelniker et al. was finally used as it primarily focused on patients with a history of CVD and quantified the comparative effectiveness for relevant outcome measures, which was considered to be in line with the prospective Dutch patient population to be treated with SGLT2is following the new T2DM treatment guidelineCitation4,Citation12,Citation15.
With respect to preventing expensive clinical events, one should keep in mind that this analysis did not account for the increased risk of subsequent events after the initial event. Previous research has elaborated on the increased risk of follow-up (cardiovascular-) events for type-1 diabetes mellitus after acute coronary syndrome, T2DM patients with prior CVD diagnosis, patients after acute kidney injury and kidney-transplant recipientsCitation36–39. Considering these findings, the estimates in this study can be regarded as conservative, as prevention of subsequent events would potentially drive the budget-impact of SGLT2is closer to budget neutrality or even net cost-savings. A further characteristic to be noted is the non-existent extra costs next to the medication costs, as SGLT2is are easy to administer and hence can be seen as a minor intervention in terms of further healthcare resources used.
Next to SGLT2is, glucagon-like peptide-1 receptor agonists (GLP-1) are also listed in the new treatment guideline for T2DM patients with ASCVD in the Netherlands. Although comparable in terms of efficacy, the annual costs of GLP-1 treatment options are higher at €1,380.28 versus €513.94 for SGLT2isCitation32. Consequently, it can be stated that due to the ability of SGLT2is to be utilized in both CKD and ASCVD patients as well as the more favourable price, SGLT2is should be preferred.
One of the limitations of this study is the lack of Dutch patient level data on the prevalence of ASCVD and CKD within the T2DM patient population, especially those with both ASCVD and CKD. Since this overlap of patients would be present in both arms of the analysis, no overestimation of our findings was expected. Despite the relatively simple intervention and analytical method, we were able to accurately quantify a conservative estimate of the health and budget impact of the introduction of the new treatment guideline for T2DM patients. Finally, an inherent limitation of budget impact analyses is the healthcare payer perspective. This means that we did not include the societal impact (e.g. losses in labour productivity, travel time or other indirect costs) of T2DM and differences in disease events following SGLT2i treatment.
Further research is needed to include the long-term impact of recurring events after a first event and inclusion of indirect benefits of improved T2DM management options from a broader health benefits, budget, and societal perspective, preferably in an incident cohort including all T2DM patients using real-world data.
Conclusion
The introduction of SGLT2is as second-line treatment for Dutch T2DM patients following the new treatment guideline has the potential to substantially reduce the number of cardiovascular and renal disease events, resulting in a net budget impact of €209 per patient year or conversely €0.57 per patient-day when analyzing a 5-year time horizon. SGLT2is offer a beneficial option to reducing the number of clinical events and associated healthcare costs in the Netherlands.
Transparency
Author contributions
Concept and design: MHS, MJP, CB. Analysis and interpretation of data: MHS, AVVS, EHS, PPGS, MJP, CB. Drafting of the manuscript: MHS, AVVS, EHS, PPGS, MJP, CB. Critical revision of the paper for important intellectual content: MHS, AVVS, EHS, PPGS, MJP, CB. Statistical analysis: MHS, AVVS, MJP, CB. Obtaining funding: MJP, CB. Supervision: MJP, CB.
Acknowledgements
None reported.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplemental Material
Download MS Excel (12.9 KB)Declaration of funding
This study was financially supported (unrestricted grant) by collaborative initiative from AstraZeneca, Boehringer Ingelheim and Mundipharma. All data in this study is non-proprietary.
Declaration of financial/other relationships
MHS, AVVS, EHS and PPGS declare that they have no conflict of interest. MJP holds stocks of Health-Ecore B.V. and Pharmaeconomics Advice Groningen. CB reports grants from AstraZeneca, Boehringer Ingelheim and Mundipharma during the conduct of the study, he also received grants and honoraria from various other medical and pharmaceutical companies and he holds stocks in Health-Ecore B.V.
References
- Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment]. Diabetes mellitus | Volksgezondheid en Zorg [Diabetes mellitus | Public Health and Care] [Internet]. [cited 2022 Jun 28]. [Dutch]. Available from: https://www.vzinfo.nl/diabetes-mellitus
- Dal Canto E, Ceriello A, Rydén L, et al. Diabetes as a cardiovascular risk factor: an overview of global trends of macro and micro vascular complications. Eur J Prev Cardiol. 2019;26(2_suppl):25–32.
- Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment]. Levensverwachting mensen met diabetes aanzienlijk lager [Internet]. 2021 [cited 2022 Jun 28]. [Dutch]. Available from: https://www.rivm.nl/nieuws/levensverwachting-mensen-met-diabetes-aanzienlijk-lager
- Barents ESE, Bilo HJG, Bouma M, et al. Diabetes mellitus type 2 | NHG-Richtlijnen [Diabetes mellitus type 2 | Dutch College of General Practitioners Guidelines] [Internet]. 2023 [cited 2022 Jun 26]. [Dutch]. Available from: https://richtlijnen.nhg.org/standaarden/diabetes-mellitus-type-2
- Yang Q, Tong X, Schieb L, et al. Vital signs: recent trends in stroke death rates – United States, 2000–2015. MMWR Morb Mortal Wkly Rep. 2017;66(35):933–939.
- Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295–2306.
- Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–2128.
- Heintjes EM, Houben E, Beekman-Hendriks WL, et al. Trends in mortality, cardiovascular complications, and risk factors in type 2 diabetes. Neth J Med. 2019;77(9):317–329.
- Hart HE, Rutten GE, Bontje KN, et al. Overtreatment of older patients with type 2 diabetes mellitus in primary care. Diabetes Obes Metab. 2018;20(4):1066–1069.
- Hart HE, Ditzel K, Rutten GE, et al. De-Intensification of blood glucose lowering medication in people identified as being over-treated: a mixed methods study. Patient Prefer Adherence. 2019;13:1775–1783.
- Van Der Meer V, Wielders HPM, Grootendorst DC, et al. Chronic kidney disease in patients with diabetes mellitus type 2 or hypertension in general practice. Br J Gen Pract. 2010;60(581):884–890.
- Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393(10166):31–39.
- Palmer SC, Tendal B, Mustafa RA, et al. Sodium-glucose cotransporter protein-2 (SGLT-2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists for type 2 diabetes: systematic review and network meta-analysis of randomised controlled trials. BMJ. 2021;372:1–14.
- McGuire DK, Shih WJ, Cosentino F, et al. Association of SGLT2 inhibitors with cardiovascular and kidney outcomes in patients with type 2 diabetes: a meta-analysis. JAMA Cardiol. 2021;6(2):148–158.
- Federatie Medisch Specialisten [Federation Medical Specialists].Medicamenteuze behandeling zeerhoogrisicopatiënten DM2 - Richtlijn - Richtlijnendatabase [Drug treatment of very-high-risk type 2 diabetes patients - Guideline - Guideline Database] [Internet]. [cited 2022 May 12]. [Dutch]. Available from: https://richtlijnendatabase.nl/richtlijn/medicamenteuze_behandeling_zeerhoogrisico_patienten_dm2/startpagina_-_farmacotherapie_bij_zeerhoogrisicopati_nten_met_diabetes_mellitus_type_2_dm2.html
- Zorginstituut Nederland [National Health Care Institute]. Databank Genees- en hulpmiddelen Informatie Project [Database Medicines and Medical Devices Information Project] [Internet]. [cited 2022 May 12]. [Dutch]. Available from: https://www.gipdatabank.nl
- Vanhommerig, J. Jaarcijfers aandoeningen - Huisartsenregistraties. [Annual figures diseases - General Practitioner Registrations] [cited 2022 Jul 30]. [Dutch]. Available from: https://www.nivel.nl/nl/nivel-zorgregistraties-eerste-lijn/jaarcijfers-aandoeningen-huisartsenregistraties
- Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment]. Diabetes mellitus | Leeftijd en geslacht | Volksgezondheid en Zorg [Diabetes mellitus | Age and gender | Public Health and Care] [Internet]. [cited 2022 Jun 28]. [Dutch]. Available from: https://www.vzinfo.nl/diabetes-mellitus/leeftijd-en-geslacht
- Centraal Bureau voor de Statistiek [Statistics Netherlands]. Population development. [Internet]. [cited 2022 Jul 30]. Available from: https://www.cbs.nl/en-gb/series/population-development
- Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644–657.
- Wiviott SD, Raz I, Bonaca MP, DECLARE–TIMI 58 Investigators, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347–357.
- Postmus D, Abdul Pari AA, Jaarsma T, et al. A trial-based economic evaluation of 2 nurse-led disease management programs in heart failure. Am Heart J. Mosby, Inc. 2011;162(6):1096–1104.
- Alva ML, Gray A, Mihaylova B, et al. The impact of diabetes-related complications on healthcare costs: new results from the UKPDS (UKPDS 84). Diabet Med. 2015;32(4):459–466.
- Soekhlal RR, Burgers LT, Redekop WK, et al. Treatment costs of acute myocardial infarction in The Netherlands. Netherlands Hear J. 2013;21(5):230–235.
- Buisman LR, Tan SS, Koudstaal PJ, et al. Hospital costs of ischemic stroke and transient ischemic attack in The Netherlands. Value Heal. 2014;17(7):A485.
- Boersma C, Gansevoort RT, Pechlivanoglou P, et al. Screen-and-treat strategies for albuminuria to prevent cardiovascular and renal disease: cost-effectiveness of nationwide and targeted interventions based on analysis of cohort data from The Netherlands. Clin Ther Excerpta Medica Inc. 2010;32(6):1103–1121.
- Mazairac AHA, Blankestijn PJ, Grooteman MPC, et al. The cost-utility of haemodiafiltration versus haemodialysis in the convective transport study. Nephrol Dial Transplant. 2013;28(7):1865–1873.
- Niessen LW, Dijkstra R, Hutubessy R, et al. Lifetime health effects and costs of diabetes treatment. Neth J Med. 2003;61(11):355–364.
- Meerding WJ, Mulder S, Van Beeck EF. Incidence and costs of injuries in The Netherlands. Eur J Public Health. 2006;16(3):271–277.
- Dhatariya KK, Parsekar K, Skedgel C, et al. The cost of treating diabetic ketoacidosis in an adolescent population in the UK: a national survey of hospital resource use. Diabet Med. 2019;36(8):982–987.
- Hunt B, Glah D, van der Vliet M. Modeling the Long-Term Cost-Effectiveness of IDegLira in patients with type 2 diabetes who are failing to meet glycemic targets on basal insulin alone in The Netherlands. Diabetes Ther. 2017;8(4):753–765.
- Z-index BV. Z-index database. [cited 2022 Jun 18]. [Dutch]. Available from: https://www.z-index.nl
- Sullivan SD, Mauskopf JA, Augustovski F, et al. Budget impact analysis – principles of good practice: report of the ISPOR 2012 budget impact analysis good practice II task force. Value Heal. 2014;17(1):5–14.
- Sullivan PW, Ghushchyan VH. EQ-5D scores for diabetes-related comorbidities. Value Heal. 2016;19(8):1002–1008.
- Peasgood T, Brennan A, Mansell P, et al. The impact of diabetes-related complications on preference-based measures of health-related quality of life in adults with type I diabetes. Med Decis Mak. 2016;36(8):1020–1033.
- Okkonen M, Havulinna AS, Ukkola O, et al. Risk factors for major adverse cardiovascular events after the first acute coronary syndrome. Ann Med. 2021;53(1):817–823.
- Young JB, Gauthier-Loiselle M, Bailey RA, et al. Development of predictive risk models for major adverse cardiovascular events among patients with type 2 diabetes mellitus using health insurance claims data. Cardiovasc Diabetol. BioMed Central. 2018;17(1):1–13.
- Odutayo A, Wong CX, Farkouh M, et al. AKI and long-term risk for cardiovascular events and mortality. J Am Soc Nephrol. 2017;28(1):377–387.
- Rahamimov R, Van Dijk TY, Molcho M, et al. Acute kidney injury and long-term risk for cardiovascular events in patients after kidney transplantation. Kidney Blood Press Res. 2019;44(5):1149–1157.