Abstract
Introduction
rVIII-SingleChain, a recombinant factor VIII (rFVIII), has demonstrated safety and efficacy in patients with hemophilia A in clinical trials and real-world evidence. This analysis aimed to estimate the potential budget impact of increasing the usage of rVIII-SingleChain for the prophylactic treatment of hemophilia A over 3 years in Italy.
Methods
Patients with moderate and severe hemophilia A receiving prophylaxis were included in the analysis. Epidemiological data were obtained from published literature. Mean product consumption and mean annual bleeding rate for rVIII-SingleChain, rFVIIIFc, octocog alfa and BAY 81-8973 were based on pooled real-world data from Italy, Germany and US. A budget impact model has been developed in order to compare two scenarios: a base-case scenario where current rVIII-SingleChain shares are kept constant over 3 years and an alternative scenario where rVIII-SingleChain shares increase by taking from other rFVIII products. Analysis 1 was based on the current Italian list prices and Analysis 2 considered current regional acquisition prices for both scenarios.
Results
Annually, adult patients treated with rVIII-SingleChain prophylaxis are expected to consume 324,589 units per patient, resulting in annual costs of €240,196 per patient. In Analysis 1, comparing the base case (constant market share of 9% rVIII-SingleChain over time) with the alternative scenario (higher rVIII-SingleChain market share and increasing from 15% in the first year to 25% in the third year), the total expenditure for prophylaxis using rFVIII products is expected to decrease by €1.4 million in Year 1, by €3.1 million in Year 2 and by €5.4 million in Year 3. In Analysis 2 based on regional prices, the results remained consistent.
Discussion/Conclusion
This analysis suggests that increasing utilization of rVIII-SingleChain in hemophilia A patients may lead to cost savings as a result of reduced consumption with uncompromised efficacy in bleed protection.
PLAIN LANGUAGE SUMMARY
Why was the study done?
Hemophilia A is a rare inherited bleeding disorder. People with severe hemophilia are more likely to bleed compared to people without hemophilia and bleeds can occur spontaneously or in response to trauma. Patients are treated with medication to reduce the chance of bleeding. However, the cost of treating patients with hemophilia can be high and place demands on the healthcare system.
What did we do and find?
This study looked at the cost of treating people with hemophilia in Italy and used a type of economic analysis (called budget impact modelling) to estimate the effect of increasing the use of a particular medication (rVIII-SingleChain), compared to other medications that are available. Different variations of the model were tested to compare a range of scenarios.
The results of this analysis suggested that increasing the use of rVIII-SingleChain may lead to cost-savings for the Italian healthcare system, compared to using the other currently available treatments. This analysis suggests that the use of rVIII-SingleChain enables people with hemophilia A to remain protected from bleeds, whilst using less product compared to other available medications.
What is the influence of this study on the wider field?
This type of analysis can be useful to healthcare systems, to guide the decision-making process regarding which medications to use or when making decisions related to healthcare policy.
Introduction
Hemophilia A is an inherited, X-linked recessive disorder characterized by reduced levels or absence of coagulation factor VIII (FVIII)Citation1. It affects approximately 1 in 4000 males, accounting for 80–85% of all hemophilia casesCitation1; in Italy, the prevalence of hemophilia A is between 6.8 and 13.8 in 100,000 inhabitants, with 6.1 in 100,000 inhabitants having severe hemophilia ACitation2. The severity of hemophilia A is determined by the level of FVIII activityCitation3,Citation4.
Factor replacement products represent the current standard of care for patients with severe hemophilia A, for both prevention and treatment of bleedingCitation1. Administration of factor replacement products as prophylaxis is associated with excellent efficacy and improved quality of life compared to episodic (on-demand) treatmentCitation5. However, prophylaxis is associated with a high frequency of injections, meaning that the patient burden and healthcare costs are significant considerationsCitation1,Citation6. Considering the economic impact of hemophilia is importantCitation6. In Italy, the mean total per-patient cost of treating a patient with severe hemophilia A was €220,344; the majority of these costs were related to treatment with factor replacement products (€212,385), while €6,013 was related to indirect treatment costsCitation7.
The range of products available for prophylaxis includes standard-acting or long-acting recombinant FVIII (rFVIII) products, and non-factor replacement therapy with emicizumabCitation1. Recombinant single-chain FVIII (rVIII-SingleChain; AFSTYLA; CSL Behring, Marburg, Germany) is a construct comprising covalently bonded heavy and light chains of recombinant FVIII (rFVIII)Citation8,Citation9. A pivotal study of the AFFINITY clinical trial program demonstrated that rVIII‑SingleChain is well tolerated and effective in preventing and treating bleeding eventsCitation10. Favorable efficacy and tolerability data have been reported in further studies of rVIII‑SingleChainCitation11,Citation12. Real-world evidence has confirmed the potential for reduced dosing frequency and improved quality of life with rVIII-SingleChain versus prior treatment with standard-acting FVIIICitation13–16. The most recent real-world study was conducted on 616 hemophilia A patients across the US, Germany and ItalyCitation16. Prophylaxis with rVIII-SingleChain was associated with improved bleed protection, less frequent dosing, and lower consumption compared with the standard-acting FVIII products octocog alfa (ADVATE, Takeda) and BAY 81-8973 (KOVALTRY, Bayer). This study also showed comparable protection and consumption with rVIII‑SingleChain versus the long-acting FVIII product recombinant factor VIII Fc fusion protein (rFVIIIFc; ELOCTA, Sobi). In a previous real-world study, prophylactic treatment with rVIII-SingleChain was shown to reduce factor consumption while maintaining effective bleeding control, when compared with rFVIIIFc or another long-acting product, polyethylene glycol polymer-conjugated recombinant FVIII (PEG-rFVIII; ADYNOVATE/ADYNOVI, Takeda)Citation14.
The objective of this analysis was to estimate the budget impact for the Health Care System of increasing the use of rVIII-SingleChain for prophylactic treatment in patients with moderate or severe hemophilia A in a real-world setting in Italy. Budget impact analyses are used to estimate the likely change in expenditure to a budget holder following a decision to either reimburse a new healthcare intervention or following a change in policyCitation17.
Methods
Model description and settings
A budget impact analysis was developed to estimate the annual budget impact of the increasing usage of rVIII-SingleChain for prophylactic treatment of moderate and severe hemophilia A patients over 3 years from the Italian National Health Service (NHS) perspective. In addition to rVIII-SingleChain, the currently available products in the Italian setting are rFVIIIFc (ELOCTA, Sobi), BAX 855 (ADYNOVATE/ADYNOVI, Takeda), BAY 94-9027 (JIVI, Bayer), N8-GP (ESPEROCT, NovoNordisk), BAY 81-8973 (KOVALTRY, Bayer), Human cl-rhFVIII (NUWIQ, Octapharma), octocog alfa (ADVATE, Takeda), BDD-rFVIII (REFACTO AF, Pfizer), N8 (NOVOEIGHT, NovoNordisk), or a non-factor replacement product (emicizumab, HEMLIBRA, Roche). The model was developed to account for two distinct patient populations: patients receiving prophylaxis (including regular prophylactic treatment and the treatment of breakthrough bleeds occurring while receiving a prophylactic regimen) and patients without prophylaxis receiving episodic (on demand) treatment of bleeds (hereafter referred to as “episodic treatment”); since the consumption of factor replacement products is lower in episodic treatment compared to prophylaxis in the Italian setting, the current analysis focuses on prophylactic treatment only. See the Appendix for additional methods and results considering episodic treatment.
To perform the budget impact analysis, a base-case and an alternative scenario were considered. In the base-case scenario, the current patient share of rVIII-SingleChain is kept constant over 3 years. In the alternative scenario, rVIII-SingleChain shares increase by taking certain assumed percentages from the standard-acting products (BAY 81-8973, Human cl-rhFVIII, octocog alfa, BDD-rFVIII, N8).
Target population estimation
Patients with moderate and severe hemophilia A receiving prophylaxis in Italy were considered in this analysis. Epidemiological data were obtained from the published literature and presented in . The growth rate of the population of patients with moderate and severe hemophilia A was assumed to remain constant at 13 patients per year; this value was derived from the mean number of children born with severe or moderate hemophilia A between 2012 and 2017Citation2,Citation18–22. Patients with hemophilia A were stratified based on age, average weight and proportion of patients on prophylaxis versus episodic treatment using the published literature.
Table 1. Assumptions around epidemiological data.
Dosing and consumption
Dosing information for regular prophylaxis treatment was obtained from pooled real-world data analysis from Italy, Germany and US for all age groups (octocog alfa, rVIII‑SingleChain, rFVIIIFc and BAY 81-8973)Citation16 and from the European product label for the remaining products as real-world data in the Italian setting are lacking (). When the dosage was provided as a range in the product label, the mean value was used. Dosing for the treatment of breakthrough bleeds was based on the label information and the desired FVIII level for each age group, according to the indication ().
Table 2. Dosing and price information.
Table 3. Efficacy and dosing information for treatment of breakthrough bleeds.
Annual consumption of rFVIII products and emicizumab included in the analyses considered the per-patient consumption of prophylaxis. Annual consumption of regular rFVIII prophylaxis was calculated by multiplying the weekly dose (which takes into account both dose per infusion and infusion frequency per week) for each age group by the average weight of the age group by 52 weeks. The annual consumption of treatment for breakthrough bleeds was calculated by multiplying the annual bleeding rates (ABRs) by the number of infusions needed to stop bleedings and the dose per infusion (). ABRs for each product were derived in the same way as dosing information described above; for octocog alfa, rVIII‑SingleChain, rFVIIIFc and BAY 81-8973 ABRs were obtained from pooled real-world data analysis from Italy, Germany and USCitation14, and for all other products where real-world data in the Italian setting were lacking, ABRs were obtained from the European product label (). The total annual consumption of prophylactic treatment was calculated as the sum of regular prophylaxis and treatment of breakthrough bleeds.
For emicizumab, patients begin treatment with a loading dose for four weeks, and remain on a maintenance dose thereafter; annual consumption of prophylaxis for each age group was calculated in four steps:
First, the weekly dose per injection (for loading dose and maintenance dose separately) was multiplied by the average weight of the age group to obtain the weekly loading dose and the weekly maintenance dose per patient within each age group.
For each year, the annual consumption for new emicizumab patients included 4 weeks at the loading dose and the remaining 48 weeks at the maintenance dose and was calculated as follows: the weekly loading dose per patient multiplied by 4 weeks plus the weekly maintenance dose per patient multiplied by 48 weeks. This was then multiplied by the proportion of patients starting with emicizumab prophylaxis in each age group.
For each year, the annual consumption for patients continuing emicizumab was calculated as the proportion of patients continuing emicizumab prophylaxis multiplied by the weekly maintenance dose per patient, multiplied by 52 weeks.
The total annual consumption for each year was calculated as the sum of steps 2 and 3 for each age group.
Costs
To establish the total cost the model considered costs of treatment (including regular prophylaxis and the treatment of breakthrough bleeds) whereas hospitalization and indirect costs were not included since the biggest proportion of costs associated with hemophilia are related to the drug acquisitionCitation6. The per-patient costs of prophylaxis (Analysis 1) were based on the ex-factory list price per unit (), which was held constant over the 3 years, and consumption information as described above. This analysis assumed that drug prices would remain stable, whereas in practice they can change over time. By adopting a short-time horizon (e.g. 3-years), this study aimed to minimize this limitation. Total cost of each treatment was based on its cost per patient and the patient share. The anticipated market uptake of rVIII-SingleChain and the patient share estimates of all competitor products were assumed ().
Table 4. Patient shares of hemophilia A products in considered scenarios.
Analysis 1
In Analysis 1, the patient-level consumption and cost as well as the budgetary difference between the base-case and alternative scenarios were estimated using list prices. The estimation of the consumption and costs were specified for each product and by age group. Since adults represent 79% of the total populationCitation23, only consumption and costs for adults are reported in the text; data stratified by age group are presented in the corresponding tables.
Analysis 2
Analysis 2 was identical to Analysis 1 except considering current regional acquisition prices. All the other parameters remained the same.
Results
Analysis 1
Target population and patient shares for the base-case and alternative scenario
It was estimated that 1,275 patients would be eligible to receive rFVIII or emicizumab prophylaxis in Year 1 of the analysis, which increased to 1,284 in Year 2 and 1,294 in Year 3 (). Patient shares for rVIII-SingleChain were assumed to be 8.6% over 3 years in the base-case scenario, whereas the figures in the alternative scenario were 15, 20 and 25% in Years 1, 2 and 3, respectively ().
Consumption and annual cost per patient
The analysis suggests that adult patients treated with rVIII-SingleChain prophylaxis are expected to consume 324,589 IU per patient annually. This is lower compared to other rFVIII products, except for BAY 94-9027; N8-GP, octocog alfa, and BAY 81-8973 are expected to show the greatest annual consumptions per patient of 433,312, 426,897, and 409,811 IU, respectively. Consumption for the treatment of breakthrough bleeds accounted for 0% to 2% of total consumption across products. Details of annual consumption for all products, stratified by age group, can be found in for products with pooled real-world data and in for products with data from European product labels.
Table 5. Annual consumption and annual costs of prophylaxis per patient for products with pooled real-world data.
Table 6. Annual consumption and annual costs of prophylaxis per patient for products with data from the European product labels.
The annual costs per patient for rFVIII products and emicizumab were also calculated. For an adult patient receiving prophylaxis, the annual cost of rVIII-SingleChain was €240,196, compared to €454,186 for emicizumab, €282,770 for BAY 81-8973 and €281,653 for N8-GP. A full list of annual costs per patient, stratified by age group is reported in for products with pooled real-world data and in for products with data from European product labels.
Budget impact results with current list prices
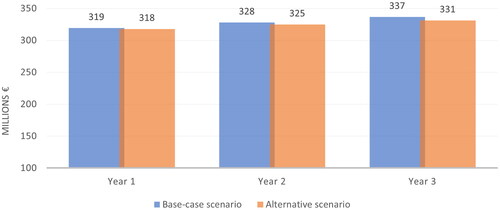
Results for patients receiving prophylaxis treatment were obtained. In the base-case scenario, the total prophylaxis cost was estimated to be €319,275,616, €327,953,865 and €336,700,972 in Year 1, Year 2 and Year 3, respectively ( and ). The corresponding expenditures decreased to €317,925,037, €324,876,039 and €331,280,210, respectively, in the alternative scenario, thereby yielding a savings of €1,350,579 in Year 1, €3,077,826 in Year 2 and €5,420,762 in Year 3. Total savings for the entire 3 years would be €9,849,167 from NHS perspective.
Table 7. Cost by product, total cost, and budget impact for prophylaxis, per year, using current list prices.
Analysis 2
Budget impact results with regional acquisition prices
When the analysis was undertaken using the regional drug acquisition prices, the total prophylaxis cost in the base-case scenario was estimated to be €269,462,830, €272,914,113, and €276,046,871 in Year 1, Year 2 and Year 3, respectively ( and ). The corresponding expenditure decreased to €266,240,884, €266,726,092 and €266,523,134, respectively, in the alternative scenario, thereby yielding a savings of €3,221,946 in Year 1, €6,188,022 in Year 2 and €9,523,736 in Year 3. Total savings for the entire 3 years would be €18,933,704 from an Italian NHS perspective.
Figure 2. Treatment costs of hemophilia A prophylaxis (analysis 2, analysis considering regional drug acquisition prices).
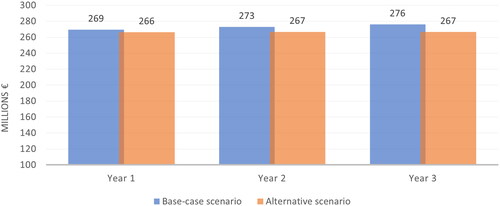
Table 8. Cost by product, total cost, and budget impact for prophylaxis, per year, using regional drug acquisition prices.
Additional analyses considering patients treated only with episodic treatment and the overall patient population (considering those on episodic treatment and those on a prophylactic regimen), are reported in the Appendix.
Discussion
The treatment of hemophilia A is associated with significant costs due to the high number of infusions required to keep FVIII levels above the minimum trough levelCitation1. The budget impact model developed in this study utilized data from multiple sources, including real-world data. The principal findings were that prophylaxis treatment with rVIII-SingleChain reduced factor consumption compared to other commonly used rFVIII products resulting in per patient and overall treatment costs reduction.
This is the first study attempting to evaluate the economic consequences of increasing the use of rVIII-SingleChain for prophylaxis treatment in Italy. Compared with rFVIIIFc or BAX 855, the use of rVIII-SingleChain was shown to reduce mean factor consumption by 11–14% and to reduce the expected annual treatment cost by 34%, consistent with the results of a US study of prophylactic treatment in hemophilia ACitation14.
Budget impact analysis is a valuable tool for payers to understand the economic impact of using a product at the population level. It facilitates the assessment of financial impact over the years ahead and enables product costs to be considered against budget constraintsCitation24–27. Moreover, it enables informed decision-making regarding the affordability of a treatment for the healthcare system. A previous budget impact model in the Italian setting demonstrated a positive financial impact for the introduction of rFVIIIFc as a therapeutic alternative for the treatment of hemophilia A; however, unlike the current study, this analysis did not incorporate real-world data into the model and did not include a comparison with rVIII-SingleChainCitation27. This study suggests that increasing the use of rVIII-SingleChain in place of other established treatments in hemophilia A, including rFVIIIFc, would have a fully positive impact on the Italian healthcare system by reducing the cost of prophylaxis without increasing or potentially reducing when compared with certain products, the number of bleeding events requiring treatment.
Strengths of this study include the use of real-world data (ensuring a large sample size [n = 616] and applicability to routine clinical practice)Citation16 and the inclusion of Analysis 2 (ensuring applicability when acquisition costs of different regions of Italy are applied). Limitations include the use of hypothetical scenarios, meaning uncertainty regarding the actual cost savings that may be achieved. Therefore, future changes to the market shares or pricing of rVIII-SingleChain or comparator treatments could affect the results. This analysis assumed that drug prices would remain stable, whereas in practice they can change over time. The study aimed to address this limitation by adopting a short time horizon (i.e. 3 years). Different data sources were used for different products when calculating the annualized bleeding rate (ABR) and consumption data; pooled real-world data were used for four products (rVIII‑SingleChain, rFVIIIFc, octocog alfa, BAY 81-8973), and the product label was used for the other products. Since real-world consumption might be higher than consumption according to the labels, the results for products based on label data are conservative. Also, the analysis considered pooled ABR and dosing from Italy, Germany and the US which might lead to a slight under or overestimation of the actual drugs’ ABR and dosing rates in the Italian setting. Another assumption of this analysis was that the distributions of patients’ age and hemophilia severity in the real-world dataset were representative of those in Italy.
Given the large number of parameters in the model, future research could consider running sensitivity analyses, if more suitable input data are available, to establish the budget impact due to the variability of different parameters. Future analyses of rVIII-SingleChain in Italy could also be performed using a more homogeneous source of real-world data. Application of the same budget impact model as reported here to other future real-world data could further provide an assessment of the robustness of the study results.
In conclusion, this analysis suggests that increasing the percentage of hemophilia A patients treated with prophylactic rVIII-SingleChain in Italy may lead to cost savings by reducing coagulation factor consumption while maintaining effective protection against bleeding. Thus, it would be desirable for policymakers to maintain and increase the degree of utilization of rVIII-SingleChain. The results were consistent regardless of whether regional drug acquisition costs or list prices were applied.
Transparency
Author contributions
EDB, SY, RT, MP, ED, LS, MB, FR, AC and RM were involved in the study design and data analysis for this paper; EDB, SY, RT, MP, ED, LS, MB, FR, AC and RM were involved in drafting the paper and revision of the paper; EDB, SY, RT, MP, ED, LS, MB, FR, AC and RM approved the final version for publication.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplemental Material
Download Zip (280.1 KB)Acknowledgements
Medical writing support was provided by Meridian HealthComms, Plumley, UK in accordance with good publication practice (GPP3) and funded by CSL Behring.
Declaration of funding
This study was sponsored by CSL Behring. Medical writing support for this report was funded by CSL Behring.
Declaration of financial/other relationships
EDB has no disclosures to declare; SY, RT and MP are employees of CSL Behring; LS and ED are paid consultants of Certara USA Inc.; AC, FR, MB, RM has no disclosures to declare.
References
- Srivastava A, Santagostino E, Dougall A, the WFH Guidelines for the Management of Hemophilia panelists and co-authors, et al. WFH guidelines for the management of hemophilia. Haemophilia. 2020;26(S6):1–158.
- Abbonizio F, Arcieri R, Associazione Italiana Centri Emofilia (AICE), et al. Registro Nazionale delle Coagulopatie Congenite (Rapporto 2018. (Rapporti ISTISAN 20/14). Roma: istituto Superiore di Sanità; 2020. p. 53.
- Berntorp E, Fischer K, Hart DP, et al. Haemophilia. Nat Rev Dis Primers. 2021;7(1):45.
- Franchini M, Mannucci PM. Hemophilia a in the third millennium. Blood Rev. 2013;27(4):179–184.
- Manco-Johnson M, Abshire T, Shapiro A, et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. NJEM. 2007;9(357):535–544.
- Kodra Y, Cavazza M, Schieppati A, et al. The social burden and quality of life of patients with haemophilia in Italy. Blood Transfus. 2014;12(Suppl 3):S567–S75.
- O’Hara J, Hughes D, Camp C, et al. The cost of severe haemophilia in Europe: the CHESS study. Orphanet J Rare Dis. 2017;12(1):106.
- Zollner SB, Raquet E, Muller-Cohrs J, et al. Preclinical efficacy and safety of rVIII-SingleChain (CSL627), a novel recombinant single-chain factor VIII. Thromb Res. 2013;132(2):280–287.
- Raso S, Hermans C. Lonoctocog alfa (rVIII-SingleChain) for the treatment of haemophilia A. Expert Opin Biol Ther. 2018;18(1):87–94.
- Mahlangu J, Kuliczkowski K, Karim FA, AFFINITY Investigators, et al. Efficacy and safety of rVIII-SingleChain: results of a phase 1/3 multicenter clinical trial in severe hemophilia A. Blood. 2016;128(5):630–637.
- Santagostino E, Fischer K, Koenigs C, et al. Interim analysis of the extension study with rVIII-SingleChain in previously untreated patients (PUPs) with severe hemophilia A (CSL627-3001). Blood. 2019;134(Supplement_1):162–162.
- Stasyshyn O, Djambas Khayat C, Iosava G, et al. Safety, efficacy and pharmacokinetics of rVIII-SingleChain in children with severe hemophilia A: results of a multicenter clinical trial. J Thromb Haemost. 2017;15(4):636–644.
- Hassoun AA. Real-World experience of rVIII-SingleChain treatment in a French single center. Res Pract Thromb Haemost. 2020;4(Suppl 1):PB0980.
- Simpson ML, Desai V, Maro GS, et al. Comparing factor use and bleed rates in U.S. Hemophilia a patients receiving prophylaxis with 3 different long-acting recombinant factor VIII products. J Manag Care Spec Pharm. 2020;26(4):504–512.
- Yan S, Maro GS, Desai V, et al. A real-world analysis of commonly prescribed FVIII products based on US medical charts: consumption and bleeding outcomes in hemophilia A patients. J Manag Care Spec Pharm. 2020;26(10):1258–1265.
- Olivieri M, Simpson M, Yan S, et al. Analysis of pooled real-world data from Germany, Italy, and the United States of rVIII-SingleChain compared with standard- and long-acting FVIII products for prophylaxis of hemophilia A. Curr Med Res Opin. 2022;38(7):1133–1139.
- [online] BIA. York; York Health Economics Consortium 2016 [cited 2022 April]. Available from: https://yhec.co.uk/glossary/budget-impact-analysis/.
- Abbonizio F, Giampaolo A, Arcieri R, et al. Registro Nazionale delle Coagulopatie Congenite. Rapporto 2013. (Rapporti ISTISAN 15/14). Roma: Istituto Superiore di Sanità; 2015.
- Abbonizio F, Giampaolo A, Arcieri R, et al. Registro Nazionale delle Coagulopatie Congenite. Rapporto 2014. (Rapporti ISTISAN 16/20). Roma: Istituto Superiore di Sanità; 2016.
- Abbonizio F, Giampaolo A, Arcieri R, et al. Registro Nazionale delle Coagulopatie Congenite. Rapporto 2015. (Rapporti ISTISAN 17/14). Istituto Superiore di Sanità; 2017.
- Abbonizio F, Giampaolo A, Riccioni R, et al. Registro Nazionale delle Coagulopatie Congenite. Rapporto 2016. (Rapporti ISTISAN 17/44). Istituto Superiore di Sanità; 2017.
- Abbonizio F, Hassan H, Riccioni R, et al. Registro Nazionale delle Coagulopatie Congenite. Rapporto 2017. (Rapporti ISTISAN 19/8). Istituto Superiore di Sanità; 2019.
- Berntorp E, Dolan G, Hay C, et al. European retrospective study of real-life haemophilia treatment. Haemophilia. 2017;23(1):105–114.
- McMullen S, Buckley B, Hall E, 2nd, et al. Budget impact analysis of prolonged Half-Life recombinant FVIII therapy for hemophilia in the United States. Value Health. 2017;20(1):93–99. Jan
- Fujii T, Kidoguchi Y, Takahashi N, et al. Budget impact analysis of jivi (Damoctocog alfa pegol, Bay 94-9027) in severe hemophilia a in Japan. J Med Econ. 2021;24(1):218–225.
- Watanabe A, Huey Lee S, Chai-Adisaksopha C, et al. Budget impact of emicizumab for routine prophylaxis of bleeding episodes in patients with hemophilia a with inhibitors. Value Health Reg Issues. 2022;28:7–13.
- Lorenzoni V, Triulzi I, Turchetti G. Budget impact analysis of the use of extended half-life recombinant factor VIII (efmoroctocog alfa) for the treatment of congenital haemophilia a: the Italian National Health System perspective. BMC Health Serv Res. 2018;18(1):596.
- European Medicines Agency. Summary of product characteristics – AFSTYLA; 2019. Available from: https://www.ema.europa.eu/en/documents/product-information/afstyla-epar-product-information_en.pdf.
- European Medicine Agency. Summary of product characteristics – ELOCTA; 2020. Available from: https://www.ema.europa.eu/en/documents/product-information/elocta-epar-product-information_en.pdf.
- European Medicines Agency. Summary of product characteristics – ADYNOVI; 2019. Available from: https://www.ema.europa.eu/documents/product-information/adynovi-epar-product-information_en.pdf.
- European Medicines Agency. Summary of product characteristics – NUWIQ. 2019. Available from: https://www.ema.europa.eu/documents/product-information/nuwiq-epar-product-information_en.pdf.
- European Medicines Agency. Summary of product characteristics – REFACTO AF. 2019. Available from: https://www.ema.europa.eu/documents/product-information/refacto-af-epar-product-information_en.pdf.
- European Medicines Agency. Summary of product characteristics – JIVI; 2020. Available from: https://www.ema.europa.eu/documents/product-information/jivi-epar-product-information_en.pdf.
- European Medicines Agency. Summary of product characteristics – ESPEROCT; 2020. Available from: https://www.ema.europa.eu/documents/product-information/esperoct-epar-product-information_en.pdf.
- European Medicines Agency. Summary of product characteristics – KOVALTRY; 2020. Available from: https://www.ema.europa.eu/en/documents/product-information/kovaltry-epar-product-information_en.pdf.
- European Medicines Agency. Summary of product characteristics – ADVATE; 2020. Available from: https://www.ema.europa.eu/documents/product-information/advate-epar-product-information_en.pdf.
- European Medicines Agency. Summary of product characteristics – NOVOEIGHT; 2020. Available from: https://www.ema.europa.eu/documents/product-information/novoeight-epar-product-information_en.pdf.
- European Medicines Agency. Summary of product characteristics – HEMLIBRA; 2021. Available from: https://www.ema.europa.eu/documents/product-information/hemlibra-epar-product-information_en.pdf.
- Gazzetta Ufficiale Serie Generale. 158(191), 17-08-2017. [cited]. Available from: https://www.gazzettaufficiale.it/eli/gu/2017/08/17/191/sg/pdf.
- Gazzetta Ufficiale Serie Generale. 157(174), 27-07-2016. Available from: https://www.gazzettaufficiale.it/eli/gu/2016/07/27/174/sg/pdf.
- Gazzetta Ufficiale Serie Generale. 161(14), 18-01-2020. Available from: https://www.gazzettaufficiale.it/eli/gu/2020/01/18/14/sg/pdf.
- Gazzetta Ufficiale Serie Generale. 161(21), 27-01-2020. Available from: https://www.gazzettaufficiale.it/eli/gu/2020/01/27/21/sg/pdf.
- Gazzetta Ufficiale Serie Generale. 178(76), 16-07-2020. Available from: https://www.gazzettaufficiale.it/eli/gu/2020/07/16/178/so/24/sg/pdf.
- Gazzetta Ufficiale Serie Generale. 163(52), 03-03-2022. Available from: https://www.gazzettaufficiale.it/eli/gu/2022/03/03/52/sg/pdf.
- Gazzetta Ufficiale Serie Generale. 156(125), 01-06-2015. Available from: https://www.gazzettaufficiale.it/eli/gu/2015/06/01/125/sg/pdf.
- Gazzetta Ufficiale Serie Generale. 156(198), 27-08-2015. Available from: https://www.gazzettaufficiale.it/eli/gu/2015/08/27/198/sg/pdf.
- Gazzetta Ufficiale Serie Generale. 152(298), 23-12-2011. Available from: https://www.gazzettaufficiale.it/eli/gu/2011/12/23/298/sg/pdf.
- Gazzetta Ufficiale Serie Generale. 155(190), 18-08-2014. Available from: https://www.gazzettaufficiale.it/eli/gu/2014/08/18/190/sg/pdf.
- Gazzetta Ufficiale Serie Generale. 161(71), 18-03-2020. Available from: https://www.gazzettaufficiale.it/eli/gu/2020/03/18/71/sg/pdf.
- Biogen Canada Inc. Product monograph – ELOCTATE; 2016. Available from: https://www.biogen.ca/content/dam/corporate/en_CA/pdfs/products/ELOCTATE/ELOCTATE_PM_E.pdf.
- Novo Nordisk Canada Inc. Product monograph – ESPEROCT; 2020. Available from: https://www.novonordisk.ca/content/dam/nncorp/ca/en/products/esperoct-product-monograph.pdf.
- Octapharma Canada Inc. Product monograph – NUWIQ; 2018. Available from: https://pdf.hres.ca/dpd_pm/00044390.pdf.
- Shire Pharma Canada ULC. Product monograph – ADVATE; 2018. Available from: https://pdf.hres.ca/dpd_pm/00046596.pdf.
Appendix
Additional methods
Target population
The number of patients without prophylaxis receiving episodic treatment was obtained from the published literatureCitation23 (Table S1).
Dosing and consumption
Dosing information for the treatment of bleeds for episodic treatment was the same as that for the treatment of breakthrough bleeds ().
The annual consumption of episodic treatment was calculated in the same manner as for the treatment of breakthrough bleeds: the ABR of patients not receiving prophylactic treatment (Table S2) was multiplied by the number of infusions need to stop a bleed () and the dose per infusion (). Published literature was used for ABR for episodic treatmentCitation23.
Costs
The per-patient costs of the treatment of bleeds were based on the ex-factory list price per unit (), which was held constant over a 3-year period, and consumption information as described above.
Analysis 1
In Analysis 1, the patient-level consumption and cost related to the episodic treatment of bleeds as well as the budgetary difference between a base-case and alternative scenario were estimated using list prices. The estimation of the consumption and costs were specified for each product and by age group. Finally, the budget impact was shown both for the population of patients on an episodic regimen as well as for the overall patient population (considering the results of patients on an episodic regimen and those on a prophylactic regimen, already shown in the main text).
Analysis 2
Analysis 2 was conducted with the same parameters but considered the regional drug acquisition prices.
Additional results
Analysis 1
Target population and patient shares for the base-case and alternative scenario
It was estimated that 507 patients with hemophilia A would receive episodic treatment in Year 1 of the analysis, which increased to 510 in Year 2 and 514 in Year 3 (Table S1). Patient shares remain as reported for the prophylaxis analysis ().
Annual cost and consumption per patient
The analysis suggests that annually, adult patients treated episodically with rVIII-SingleChain are expected to consume 11,529 units per patient. For an adult patient, the annual cost of rVIII-SingleChain for episodic treatment only was €8,532. Details of annual consumption and costs by product, stratified by age group, can be found in Table S3.
Budget impact results with current list prices
When considering patients only receiving episodic treatment, total costs were estimated to be €3,725,516 in Year 1 of the base-case scenario, whereas the corresponding expenditure amounted to €3,782,806 in the alternative scenario (Table S4 and Figure S1). Corresponding costs for Year 2 and Year 3 were €3,772,263 and €3,824,647 in the base-case scenario and €3,875,067 and €3,976,195 in the alternative scenario. The alternative scenario resulted in an increase in the budget by €57,290 in Year 1, €102,804 in Year 2 and €151,548 in Year 3. Cumulative budget increases would be equal to €311,642 over 3 years.
Results for the overall patient population (considering those on episodic treatment and those on a prophylactic regimen) were also obtained. In the base-case scenario, the total expenditure for hemophilia A patients was estimated to be €323,001,132 in Year 1 and increased to €331,726,128 in Year 2 and €340,525,620 in Year 3 (Table S5 and Figure S2). In the alternative scenario, the total spending amounted to €321,707,843, €328,751,106 and €335,256,405 in Years 1, 2 and 3, respectively. The analysis suggests that the increasing uptake of rVIII-SingleChain would result in savings up to €1,293,289 in Year 1, €2,975,022 in Year 2 and €5,269,214 in Year 3 while cumulative savings over the 3-year period would amount to €9,537,525.
Analysis 2
Budget impact results with regional acquisition prices
When the analysis considered regional drug acquisition prices, the total expenditure for patients treated only with episodic treatment in the base-case scenario, using drug acquisition prices, was estimated to amount to €3,267,142 in Year 1, €3,283,276 in Year 2 and €3,296,852 in Year 3 (Table S6). In the alternative scenario, these values were €3,283,487, €3,313,031 and €3,343,764, respectively. Increasing the uptake of rVIII-SingleChain for episodic treatment would result in additional expenditures of €16,345 in Year 1, €29,755 in Year 2 and €46,913 in Year 3, for a total cumulative additional expenditure of €93,013 over 3 years.
The estimated total expenditure for the overall patient population (considering those on episodic treatment and those on a prophylactic regimen), in the base-case scenario amounted to €272,729,972 in Year 1, increasing to €276,197,389 in Year 2 and €279,343,722 in Year 3 (Table S7). Corresponding figures for the alternative scenario were €269,524,371, €270,039,122 and €269,866,899, respectively. Increasing the uptake of rVIII-SingleChain would therefore result in savings of €3,205,601, €6,158,266 and €9,476,824 in Years 1, 2 and 3, respectively. Over the 3-year period, the cumulative savings would amount to €18,840,691.