Abstract
Aims
For patients with cartilage defects of the knee, a new biocompatible and in situ cross-linkable albumin-hyaluronan-based hydrogel has been developed for matrix-associated autologous chondrocyte implantation (M-ACI) – NOVOCART Inject plus (Ninject; TETEC AG, Reutlingen, Germany). We aimed to estimate the potential cost-effectiveness of NInject, that is not available on the market, yet compared to spheroids of human autologous matrix-associated chondrocytes (Spherox; CO.DON GmbH, Leipzig, Germany) and microfracture.
Materials and methods
An early Markov model was developed to estimate the cost-effectiveness in the United Kingdom (UK) from the payer perspective. Transition probabilities, response rates, utility values and costs were derived from literature. Since NInject has not yet been launched and no prices are available, its costs were assumed equal to those of Spherox. Cycle length was set at one year and the time horizon chosen was notional patients’ remaining lifetime. Model robustness was evaluated with deterministic and probabilistic sensitivity analyses (DSA; PSA) and value of information analysis (VOIA). The Markov model was built using TreeAge Pro Healthcare.
Results
NInject was cost-effective compared to microfracture (ICER: ₤5,147) while Spherox was extendedly dominated. In sensitivity analyses, the ICER exceeded conventional WTP threshold of ₤20,000 only when the utility value after successful first treatment with NInject was decreased by 20% (ICER: ₤69,620). PSA corroborated the cost-effectiveness findings of NInject, compared to both alternatives, with probabilities of 60% of NInject undercutting the aforementioned WTP threshold and being the most cost-effective alternative. The VOIA revealed that obtaining additional evidence on the new technology will likely not be cost-effective for the UK National Health Service.
Limitations and conclusion
This early Markov model showed that NInject is cost-effective for the treatment of articular cartilage defects in the knee, compared to Spherox and microfracture. However, as the final price of NInject has yet to be determined, the cost-effectiveness analysis performed in this study is provisional, assuming equal prices for NInject and Spherox.
Introduction
Cartilage defects in the knee mostly result from degenerationCitation1 or traumaCitation2 and are associated with decreasing health-related quality of life (HRQoL)Citation3,Citation4, productivityCitation5 and increasing painCitation6,Citation7. Since articular cartilage has only a limited capacity for self-repair, the surgical treatment of defects is important to avoid an increasing severity, aiming to reduce complaints and to prevent the need for knee replacementCitation8. There are a number of treatment options for treating these defects, the choice of which depends on a number of factors, including the size and depth of the defect, the patient’s age and/or activity levelCitation9, and the availability of treatment options. Related to the defect size, for small chondral defects (<2 or 2.5 cm2)Citation9 microfracture is recommended. This procedure is a one-step surgical treatment option for symptomatic localized, full-thickness cartilage lesions in the knee. Small holes are drilled into the surface of the bone in the area of the damaged cartilage, leading to blood and cells from the bone marrow entering the defect area. For lesions greater than 2 cm2 autologous chondrocyte implantation (ACI) is the current gold standard and recommended by NICECitation10. In contrast to microfracture, M-ACI is a two-stage procedure mostly performed by arthroscopy. The patient’s chondrocytes are harvested from cartilage of the knee, cultured and then reimplanted. In 2017, CO.DON received EU marketing authorization for a new advanced therapy medicinal product (ATMP) for M-ACI, called spheroids of human autologous matrix-associated chondrocytes (Spherox; CO.DON GmbH, Leipzig, Germany)Citation11. Spheroids of human autologous matrix-associated chondrocytes are indicated for the treatment of “defect sizes up to 10 cm2 in adults and adolescents with closed epiphyseal growth plate in the affected joint”Citation11 (p.2). Recently, TETEC AG developed a biocompatible and in situ cross-linkable albumin-hyaluronan-based hydrogel as a carrier material for M-ACI proceduresCitation12 called NOVOCART Inject (Ninject; TETEC AG, Reutlingen, Germany). NInject is, similar to Spherox, indicated in adults and adolescents with closed epiphyseal growth plate with defect sizes ≥4 to ≤12cm2 (defect sizes defined as inclusion criteria of study population in the single-arm (phase III) trial for NInjectCitation12) While a few studies exploring the cost-effectiveness of (M-)ACI compared to microfracture existCitation13–15, there is no data comparing the two M-ACI procedures that are available in the EU. Thus, the aim of this study was to estimate the potential cost-effectiveness of NInject compared to (i) spheroids of human autologous matrix-associated chondrocytes and (ii) microfracture for patients suffering from cartilage defects of the knee. All analyses were reflective of the UK setting and performed from the healthcare payer perspective, i.e. the National Health Service (NHS).
Methods
Model overview
A decision model was developed to compare the potential cost-effectiveness of NInject to (i) spheroids of human autologous matrix-associated chondrocytes and (ii) microfracture. Although microfracture is indicated for smaller defects than NInject or spheroids of human autologous matrix-associated chondrocytes, it is still used as a comparator in clinical trials of M-ACI technologies, health technology assessments and in clinical practice, hence it was chosen as an additional comparator in the decision modelCitation16–19.
As NInject is not available on the market, yet, an early Markov model was developedCitation20,Citation21. The structure of the model followed the multiple technology appraisal (MTA) for ACI, published by Mistry et al.Citation15, as well as the single technology appraisal (STA) for spheroids of human autologous matrix-associated chondrocytes in the UKCitation14,Citation22. In line with the above, our model was based on a cohort of 1,000 notional patients, all of whom were 33 years old and 60% of whom were maleCitation14,Citation15,Citation22.
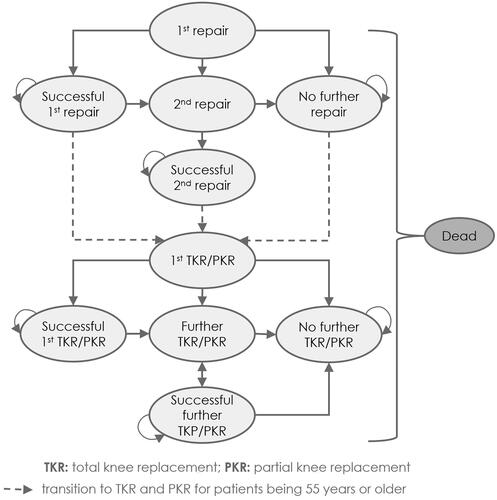
All notional patients (the term “notional patients” is used throughout the manuscript to indicate that the model concerns a hypothetical cohort of patientsCitation23,Citation24) entered the model () with a symptomatic defect in the knee, which was either repaired with NInject, spheroids of human autologous matrix-associated chondrocytes or microfracture in the first state “1st repair”. Based on the STACitation14,Citation22, following the 1st repair, the notional patients could transition to three different states: successful 1st repair, no further repair, or 2nd repair (). Notional patients with a successful 1st repair were symptom-free, either permanently or temporarily, whereby “temporarily symptom-free” means that notional patients had a successful 1st repair but developed pain again in subsequent years. In contrast, notional patients with no further repair were not symptom-free, but “[relied] on analgesics for pain relief rather than have another attempt at repair, although the patients may receive knee replacement later”Citation14 (p.193). In the Markov state “2nd repair”, notional patients who were initially treated with NInject or spheroids of human autologous matrix-associated chondrocytes could be treated a second time for their symptomatic defect in the knee. Therefore, a change in the type of M-ACI was not allowed, i.e. if notional patients were initially treated with NInject, the second treatment had to be performed with NInject again (and analogously for Spherox). This was due to the fact that no evidence has been published yet regarding HRQoL or success rates of such treatment switches that could inform the model. Independently of the type of M-ACI, notional patients who received a second repair could further transition to “successful 2nd repair” or “no further repair”. Notional patients who still, or again, suffered from articular cartilage defect in the knee were eligible to receive partial or total knee replacement (PKR resp. TKR) after their first or second repair if they were aged 55 or older. Transition from the 1st knee replacement (PKR or TKR) to further Markov states followed the same logic as described for the 1st repair state. The transition to death was possible from any state during the entire model.
Figure 1. Overview of the Markov model comparing NInject with (i) spheroids of human autologous matrix-associated chondrocytes or (ii) microfracture as 1st repair. Ovals represent the Markov states within the model. Single-headed arrows indicate the potential for unidirectional transition from a Markov state to another. Double-headed arrows indicate the potential for bidirectional transitions. Recursive arrows indicate that notional patients may remain in the current state at the end of the model cycle.
To include all costs incurred and quality-adjusted life-years (QALYs) reaped over the notional patients’ lifetime, a lifetime horizon was chosen, with a cycle length of one year, with half-cycle correction applied for utilities. Both costs and QALYs were discounted using an annual rate of 3.5%Citation25. Incremental cost-effectiveness ratios (ICERs) were calculated using the incremental lifetime costs divided by the incremental lifetime accumulated QALYs. Further, the cost-effectiveness was calculated based on the healthcare system of the UK and performed from the healthcare payer perspective (NHS). Therefore, a willingness-to-pay (WTP) threshold of costs per QALY was defined at ₤20,000. The Markov model was built using TreeAge Pro Healthcare (Version 2021 R1.2).
Transition probabilities
Treatment-specific transition probabilities of having a successful 1st/2nd repair were derived from published response rates, while the probabilities of requiring a 2nd repair were based on published failure rates ()Citation14,Citation26,Citation27. Response rates for NInject and microfracture were derived from a matched-pairs analysis in which both treatment options were compared to each otherCitation26 (see Supplemental Material I), whereas the response and failure rates for spheroids of human autologous matrix-associated chondrocytes were taken from the “CO.DON Wirksamkeit und Sicherheit” COWISI RCTCitation28. Failure rates after 1 year of NInject from the single-arm phase III study were extracted from an internal database of the sponsor (TETEC AG; 2022Citation27), data on file.
Table 1. Overview of the response and failure rates depending on type of repair.
For the calculation of age- and sex-specific mortality rates, data from the Office of National Statistics were usedCitation29. In line with the STA for spheroids of human autologous matrix-associated chondrocytes, mortality was adjusted for notional patients who received knee replacements. Therefore, in the year of replacement, mortality was assumed to increase by 0.35% for 1st knee replacement and by 1.1% for further knee replacementsCitation14. A complete summary of the transition probabilities used in the model is shown in the supplementary materials.
Utilities
We adopted utility values which were used in the economic evaluation of autologous chondrocyte implantation (ACI) compared to microfracture by Mistry et al.Citation15. Mistry et al. used utility values for ACI and microfracture from a RCT comparing ACI to microfracture in which HRQoL was derived by using the SF-36 health surveyCitation30. Utility values for knee replacements in Mistry et al. were based on two other studies in which HRQoL was elicited using the EQ-5D questionnaireCitation31,Citation32. For successful 1st and 2nd repair, utility values were dependent on the years notional patients remain in “success” (given in ). Time-dependent tunnel state variables were used to count the number of years notional patients stayed in a successful health state. Utility values for Markov health states independent of time are given in .
Table 2. Utility values used in the Markov model (time-dependent), from Mistry et al.Citation15.
Table 3. Utility values used in the Markov model (time-independent), from Mistry et al.Citation15.
Costs
Costs incurred in each Markov state in which procedures (NInject, spheroids of human autologous matrix-associated chondrocytes, microfracture, TKR, or PKR) were performed. The overall costs that were used in the Markov model are given in and were adopted from the MTA for ACICitation15 and STA for spheroids of human autologous matrix-associated chondrocytesCitation14,Citation22 and inflated to 2021 using the Hospital and Community Health Services (HCHS) indexCitation33. The overall costs include procedure costs as well as costs for outpatient and rehabilitation visits. In line with the approach used by Mistry et al.Citation15, we assumed three rehabilitation visits per year for notional patients being treated with M-ACI or microfracture and six, three, and two outpatient visits per year for notional patients being treated with M-ACI, microfracture, and PKR/TKR, respectively. Costs for outpatient and rehabilitation visits were derived from the national reference costsCitation34. In line with the MTA for ACICitation15 and STA for spheroids of human autologous matrix-associated chondrocytesCitation14,Citation22, no costs for notional patients having had a successful procedure or remaining in the Markov health state “no further repair”were included based on clinical expert advice, assuming that costs for analgesics are negligible and patients are not followed up routinelyCitation15. Since NInject has not been launched yet and no reimbursement price has been set, costs for NInject were assumed to be equal to those of spheroids of human autologous matrix-associated chondrocytes.
Table 4. Overall costs used in the Markov model.
Complications
(Dis)utilities and costs associated with adverse events were not included. As outlined, the model structure was adapted from an MTA for ACI published by Mistry et al.Citation15 and the STA for human autologous matrix-associated chondrocyte spheroids in the UKCitation14,Citation22. Both models did not include adverse events as there were no differences between treatment arms. For spheroids of human autologous matrix-associated chondrocytes, the COWISI trial showed no significant difference between treatment groups in the overall incidence of adverse events, the number of patients with any adverse events, or the number of patients with "probably" or "possibly" treatment-related adverse eventsCitation14,Citation28. No differences in adverse events were found for NInject compared to microfractureCitation26,Citation27.
Sensitivity analyses
Model robustness was assessed with deterministic (DSA) and probabilistic sensitivity analyses (PSA). For DSA, intended to assess the volatility of cost-effectiveness estimates, the model parameters for costs, utilities and transition probabilities were varied one after another while holding all other parameters constant. Parameters were either altered by −/+20%Citation35 or within a plausible range, if they had a value of 0. Results were then ranked in a Tornado diagram and ordered from most to least impactful on the base case ICER. For a less static assessment of possible ICER ranges, a Monte Carlo PSA with 100,000 iterations using gamma distributions for costs and beta distribution for utilities and transition probabilities, with variables changing simultaneously at random, was performed. The variables with parameters are provided in the supplementary materials. Results of the DSA are presented as scatter plots showing the results of the iterations in terms of costs and QALYs and as a cost-effectiveness acceptability frontier that show the uncertainty of results and at the same time allows the identification of the optimal interventionCitation36.
Scenario analyses
In the base case scenario no costs were assumed –as these costs are negligible– for notional patients relying on analgesics for pain relief (rather than attempting another repair) in the no further repair state. We additionally used the estimate of ₤220 (equivalent to two outpatient visits per year) in a scenario analysis (scenario analysis I).
In a second scenario analysis, the cost of NInject was altered to determine the maximum product price and the maximum total cost of the intervention (including the cost of cells, harvesting, implementation, outpatient and rehabilitation visits) at which the WTP threshold of ₤20,000/QALY was not exceeded (scenario analysis II).
Value of information analysis
Health technology assessment bodies – but also decentral research funding agencies – decide whether a technology should be reimbursed relying on the available evidence and information or whether further evidence is requiredCitation37,Citation38. In order to investigate the necessity of further evidence gathering and the potential cost-effectiveness of future research, a value of information analysis (VOIA) was performed as recommended for early Markov modelsCitation21.
In the performed VOIA, expected value of perfect information (EVPI) per notional patient and expected value of parameter perfect information (EVPPI) on four parameter groups for all three investigates treatment strategies were analyzed. The parameter groups used in the VOIA were chosen in a way that they could be obtained in a single clinical trial and included short-term effectiveness of M-ACI and microfracture technologies in the 1st repair, effectiveness of the technologies in 2nd repair, effectiveness of knee replacement, and overall costs of the technologies. Assignment of the parameters to the groups is shown in Supplementary material II. EVPI as the PSA was calculated via 100,000 Monte Carlo iterations. EVPPI calculation was performed with 200 outer loop and 100 inner loop iterationsCitation38. The VOIA was contrasted against €0–€40,000 willingness-to-pay threshold values.
To quantify the potential overall amount to be spent by the healthcare system to collect further information if deemed cost-effective, it is recommended to multiply the per-patient EVPI by the total present and (discounted) future population who could benefit from the information. This may comprise the current prevalence, plus the incidence over an “appropriate” time horizonCitation39. As the health technology assessment for NInject for UKNHS is currently pending, and therefore no reliable figures on prevalence, incidence and expected patient numbers exist, it has been decided not to extrapolate the EVPI value per patient to the health system at this stage.
Results
Base case results
In the base case scenario, lifetime costs and QALYs were highest if notional patients were treated with Ninject, followed by spheroids of human autologous matrix-associated chondrocytes, and microfracture (). The usage of spheroids of human autologous matrix-associated chondrocytes was extendedly dominated by Ninject.
Table 5. Total lifetime costs and quality-adjusted life-years (QALYs) for notional patients treated with NInject, spheroids of human autologous matrix-associated chondrocytes, or microfracture, and calculation of the incremental cost-effectiveness ratios (ICERs) comparing NInject to (i) spheroids of human autologous matrix-associated chondrocytes or (ii) microfracture.
It was found that Ninject is a cost-effective treatment compared to microfracture with an ICER of ₤5,147 per QALY gained.
Robustness of results assessed in sensitivity analyses
Results of the deterministic sensitivity analysis (DSA)
Because spheroids of human autologous matrix-associated chondrocytes was extendedly dominated in the base case, the results of the one-way sensitivity analyses are presented as a Tornado diagram showing the effect the variables had on the ICER for 1st repair treatment with Ninject vs microfracture.
When comparing Ninject to microfracture, the ICER exceeded the WTP threshold only when the utility of 0.817 – which notional patients with a successful 1st treatment with Ninject have from the 5th year onwards – was reduced by 20% to 0.654. In this case, ICER increased to ₤69,620 per QALY. However, the model was robust in DSA for the change of all other variables ().
Figure 2. Tornado diagram for sensitivity analyses for NInject compared to microfracture. The first five variables having the most impact on the base case ICER [₤5,147] are ranked top to bottom in the order of decreasing impact.
Note. Values to the right indicate ICER when changing base case values by +20%. Values to the left indicate ICER when changing base case values by ‒20%.
![Figure 2. Tornado diagram for sensitivity analyses for NInject compared to microfracture. The first five variables having the most impact on the base case ICER [₤5,147] are ranked top to bottom in the order of decreasing impact.Note. Values to the right indicate ICER when changing base case values by +20%. Values to the left indicate ICER when changing base case values by ‒20%.](/cms/asset/fd46a21f-8ac1-4181-9df4-54c924c2adba/ijme_a_2194805_f0002_c.jpg)
Results of the probabilistic sensitivity analysis (PSA)
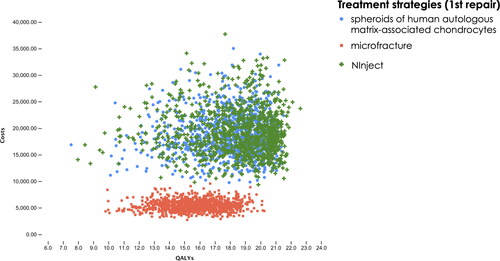
The PSA confirmed the results of the deterministic base case: over 100,000 iterations, Ninject extendedly dominated spheroids of human autologous matrix-associated chondrocytes ().
Figure 3. Scatter Plot of Monte Carlo simulation of total costs and QALYs. Red squares present results for microfracture, blue circles for spheroids of human autologous matrix-associated chondrocytes and green crosses for NInject. Due to clear representation, the scatter plot shows only 1,000 iterations of the Monte Carlo simulation.
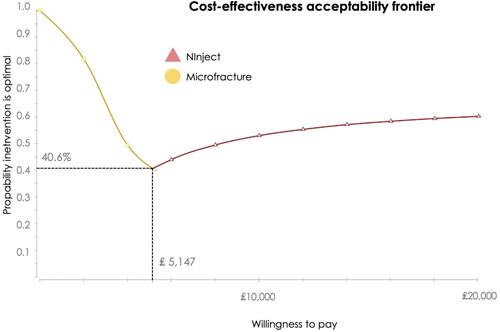
The PSA also demonstrated that Ninject was the cost-effective option at a WTP threshold per QALY of ₤20,000 in ∼60% of 100,000 random samples, 22% of the times microfracture was the most cost-effective alternative, and in 18% of samples spheroids of human autologous matrix-associated chondrocytes showed as cost-effective. The cost-effectiveness acceptability showed that Ninject was the optimal alternative from a threshold value of £5,147 onwards, as it is the healthcare technology with the highest expected average Net Monetary Benefit vs. Spherox and microfractureCitation40 ().
Results when assuming costs for notional patients treated with analgesics (scenario sensitivity analysis I)
When costs for patients relying on analgesics were considered in the model, NInject was less costly and more effective, and therefore strongly dominated spheroids of human autologous matrix-associated chondrocytes. Comparing NInject to microfracture, the base case ICER of ₤5,147 decreased by ∼₤574 to ₤4,573.
Results for different costs of NInject (scenario sensitivity analysis II)
Cost-effectiveness at a WTP threshold of ₤20,000/QALY gained of NInject was lost when assuming overall costs (including costs of cells, for harvesting, implementation, outpatient and rehabilitation visits) for NInject greater than ₤23,200 instead of ₤16,659 () – resulting in an ICER of ₤20,103/QALY gained. This corresponds to cells costs of approximate ₤15,500.
Value of information analysis
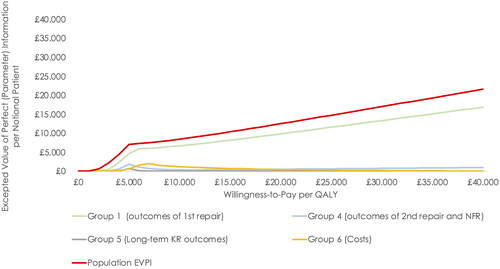
The EVPI, indicating whether collecting further evidence to support healthcare decision-making is potentially cost-effective or not, ranged from £12,516 to £17,011 per notional patient above the range usually considered cost-effective in England (£20,000–£30,000 per QALY) (). The EVPI provides an excepted upper bound to spend on further research for this population group that might be cost-effective to perform. The EVPPI revealed that potential value in collecting further information was greatest for parameters about the effectiveness of NInject and spheroids of human autologous matrix-associated chondrocytes (group I parameters): at a willingness-to-pay threshold of £20,000, EVPPI was £9,840 for reducing uncertainty in this parameter group.
Figure 5. Value of information analysis - Expected value of perfect information and expected value of parameter perfect information per notional patient. EVPI was based on 100,000 Monte Carlo iterations. EVPPI was based on 100 and 200 Monte Carlo iterations for the inner and outer loop, respectively. Abbreviations. Expected value of perfect information; EVPPI, expected value of parameter perfect information; NFR, no further repair; KR, knee replacement.
Discussion
Summary of results
Our study found that NInject was cost-effective compared to spheroids of human autologous matrix-associated chondrocytes (extendedly dominated) and microfracture (ICER: ₤5,147 per QALY gained) at a WTP threshold of ₤20,000 per QALY. Applying a DSA, cost-effectiveness of NInject compared to microfracture was robust, except that cost-effectiveness was lost when one variable, namely the utility after successful first treatment with NInject from the 5th year onward, was decreased by −20%. Results of the PSA suggest for NInject to be a cost-effective treatment option compared to spheroids of human autologous matrix-associated chondrocytes and microfracture. When costs for notional patients being treated with analgesics were considered, NInject would appear even more cost-effective.
Discussion of results
The presented potential cost-effectiveness results for NInject compared to microfracture are well in line with the ICERs given in the final appraisal determination for spheroids of human autologous matrix-associated chondrocytes by NICE, which reported an ICER of ₤4,360 to ₤5,294 for notional patients treated with spheroids of human autologous matrix-associated chondrocytes compared to microfractureCitation39,Citation41. Therefore, in our study using an early Markov model, the potential cost-effectiveness of NInject was driven by the high proportion of notional patients who had a successful first repair and consequently entered the model with high utility values. It should be mentioned, that success rates of all three treatment options highly depend on the choice of proper indications for the treatment options. As success rates were used from clinical trials, deviations in real-world setting are possible – depending on where patients were treated. Lower success rates could worsen cost-effectiveness. In contrast, the costs per QALY gained if notional patients were treated with ACI in general compared to microfracture reported in the MTA ranged from £14,395 to £15,598Citation15. The three times higher ICERs in the MTA have resulted, among others, from higher procedure costs for ACI. However, as NInject and microfracture are intended for the treatment of different defect sizes (NInject: ≥4 to ≤12cm2 as defined in the single-arm phase III trialCitation12 and ACI in general for defects greater than 2 cm2 as recommended by NICECitation10; microfracture: <∼2 cm2, respectively), spheroids of human autologous matrix-associated chondrocytes seem to be the more appropriate comparator due to the approval for defect sizes of up to 10 cm2. Thus, by comparing NInject to spheroids of human autologous matrix-associated chondrocytes, the costs per QALY gained are ₤608, assuming equal costs for both treatments. Even assuming an increase of 20% of the costs for NInject, total costs per QALY gained increased to ₤10,243.
The EVPPI functions highlight that, for most of the parameters under consideration, further evidence is not expected to change decision makers’ opinion about the cost-effectiveness of NInject made on the grounds of current information.
For 5 out of the 6 parameter groups included in VOIA, EVPPI is well below ₤1,000 at a WTP threshold of ₤20,000. EVPPI for additional information on the short-term effectiveness of NInject and spheroids of human autologous matrix-associated chondrocytes were ₤9,800 per notional patient, so that further research in the future should focus on these parameters. However, given the fact that the repair itself is around ₤16,162 (), it seems highly unlikely that additional evidence can be acquired to these costs per patient and obtaining additional evidence on the new technology will likely not be cost-effective for the UK health care system. Therefore, all in all, the decision of funding Ninject could well be made in the light of the existing evidence, if deemed totally reliable by the UKNHS decision makers.
Limitations
For our early Markov model, data from different studies were used for the quantification of intervention effects (utility values). Thus, it cannot be excluded that patients considered in these studies differed in their baseline characteristics (e.g. defect sizes or prior surgeries). In particular, it should be noted, that the indications and contraindications for the three treatment options are different and a randomized study comparing NInject and Spherox does not exist that ensure equal baseline characteristics of patients. Future research is encouraged to inform economic decision models with patient individual data that may allow risk adjustment of patients at baseline. Furthermore, intervention effects based on ACIs that are no longer approved for the treatment of articular cartilage defects of the knee in the UK were included. As intervention effects were separately entered into the model for ACI in general (equal for NInject and spheroids of human autologous matrix-associated chondrocytes) and microfracture, this limitation is unlikely to bias the comparative cost-effectiveness of NInject vs. spheroids of human autologous matrix-associated chondrocytes in favor of one. Moreover, we assumed that all notional patients entered the model with a treatment of either NInject, spheroids of human autologous matrix-associated chondrocytes, or microfracture, and did not adjust for the possibility of notional patients entering the model in other Markov health states. Further, for NInject, the clinical effectiveness and safety were evaluated in a prospective single-arm trial, as the comparison of NInject to another M-ACI or microfracture was assessed to be non-justifiable on ethical grounds, due to different defect sizes the interventions were approved for. Thus, to compare NInject and microfracture head-to-head, a matched-pairs analysis was performed, of which the transition probability (of having a successful treatment with NInject or microfracture) was used in our study. The implications of this model should be reassessed once the final trial results of NInject were published in a peer-reviewed journal. The defect size was not part of the matching and remains a potential confounder. Furthermore, since this early Markov model followed an already accepted model structure, neither health care resources, nor cost were included into the model for potential adverse events related to the three procedures under investigation. The same comments holds for potential adverse event-related disutilitiesCitation15.
In the context of study designs and data availability, Pinho-Gomes and CairnsCitation42 discussed the challenges in the evaluation of ATMPs – such as M-ACI – by screening 14 health technology assessments conducted by NICE. They found several concerns in cost-effectiveness evaluations caused by limited study data and lack of evidence especially in long-term follow-up periods. They described that committees used information from observational studies and clinical experts to deal with these shortcomingsCitation42. Finally, as NInject has not been approved by EMA yet, we assumed equal costs for NInject and spheroids of human autologous matrix-associated chondrocytes. Thus, the final results of the cost-effectiveness analysis will be mainly driven by the price that will ultimately be determined for NInject. Since the author group does not have any information on the price, we assumed equal prices with the existing similar technology in base-line. In addition, it would be conceivable that costs between the two M-ACI treatment options differ, e.g. depending on the success rate of biopsy growth since not successful harvesting of cells requires additional surgery and/or outpatient visits.
Conclusion
Overall, NInject could be shown to be potentially cost-effective compared to spheroids of human autologous matrix-associated chondrocytes and microfracture for the treatment of articular cartilage defects in the knee from a UK healthcare payer perspective. However, as the final price of NInject has not been determined yet, the presented potential cost-effectiveness findings hinge on the assumption of equal prices for NInject and spheroids of human autologous matrix-associated chondrocytes.
Transparency
Author contributions
Study concept and design: TS, YZ, and SW. Analysis: TS, YZ, and SW. Interpretation: MS, VM, RC, PJE, EK, PN, RV, CG, and AR. Manuscript drafting: TS, YZ, SW, and AR. Manuscript editing: MS, VM, RC, PJE, EK, PN, RV, CG, and AR. All authors were responsible for revising and critically appraising the manuscript for intellectual content and approved the final submission.
Acknowledgements
The authors would like to thank Tobias Vogelmann (LinkCare GmbH, Ludwigsburg, Germany) for his support with statistical analyses and modelling in TreeAge.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose. The Editors in Chief helped with adjudicating the final decision on this paper.
Supplemental Material
Download MS Word (52.3 KB)Declaration of funding
This study has received funding from TETEC AG. TETEC AG is responsible for initiation of the study and decision to publish.
Declaration of financial/other relationships
AR and CG are employees of TETEC AG. TS is owner and employee and SW is an employee of LinkCare GmbH; which received consulting honoraria from TETEC AG, CO.DON and B.BRAUN. Martyn Snow is a consultant for Smith & Nephew. PJE has shares in Chondropeptix and has received consulting fees from Episurf and KioMed. EK reports consulting for Cartiheal ldt, Green Bone, Geistlich, and Bioveex, and speaking for Zimmer Biomet and Fidia Farmaceutici SPA. PN has received grants for educational purposes, including CO.DON AG and is a consultant for TETEC AG and B.BRAUN. RV is consultant at Orteq Sports Medecine. The other authors declare nor conflicts of interest.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
References
- Niemeyer P, Feucht MJ, Fritz J, et al. Cartilage repair surgery for full-thickness defects of the knee in Germany: indications and epidemiological data from the german cartilage registry (KnorpelRegister DGOU). Arch Orthop Trauma Surg. 2016;136:891–897.
- Arøen A, Løken S, Heir S, et al. Articular cartilage lesions in 993 consecutive knee arthroscopies. Am J Sports Med. 2004;32(1):211–215.
- Clavé A, Potel J-F, Servien E, et al. Third-generation autologous chondrocyte implantation versus mosaicplasty for knee cartilage injury: 2-year randomized trial. J Orthop Res. 2016;34:658–665.
- Vanlauwe J, Saris DBF, Victor J, et al. Five-year outcome of characterized chondrocyte implantation versus microfracture for symptomatic cartilage defects of the knee: early treatment matters. Am J Sports Med. 2011;39:2566–2574.
- Jackson DW, Simon TM, Aberman HM. Symptomatic articular cartilage degeneration. Clin Orthop Relat Res. 2001;391:S14–S25.
- Johnson-Nurse C, Dandy DJ. Fracture-separation of articular cartilage in the adult knee. J Bone Joint Surg Br. 1985;67-B:42–43.
- Fossum V, Hansen A, Wilsgaard T, et al. Collagen-covered autologous chondrocyte implantation versus autologous matrix-induced chondrogenesis: a randomized trial comparing 2 methods for repair of cartilage defects of the knee. Orthop J Sports Med. 2019;7:2325967119868212.
- Hoburg A, Niemeyer P, Laute V, et al. Matrix-Associated autologous chondrocyte implantation with spheroid technology Is superior to arthroscopic microfracture at 36 months Regarding activities of daily living and sporting activities after treatment. Cartilage. 2021;13:437S–448.
- Niemeyer P, Albrecht D, Andereya S, et al. Autologous chondrocyte implantation (ACI) for cartilage defects of the knee: a guideline by the working group ‘clinical tissue regeneration’ of the german society of orthopaedics and trauma (DGOU). Knee. 2016;23:426–35.
- National Institute for Health and Care Excellence (NICE). Autologous chondrocyte implantation for treating symptomatic articular cartilage defects of the knee: technology appraisal guidance [TA477]; 2017. Available from: https://www.nice.org.uk/guidance/TA477/chapter/1-Recommendations.
- European Medicines Agency. Spherox – spheroids of human autologous matrix-associated chondrocytes: product information – Annex I – Summary of product characteristics; 2021. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/spherox#product-information-section.
- Niemeyer P, Hanus M, Belickas J, et al. Treatment of large cartilage defects in the knee by hydrogel-based autologous chondrocyte implantation: two-year results of a prospective, multicenter, single-arm phase III trial. Cartilage. 2022;13:19476035221085144.
- Vogelmann T, Roessler PP, Buhs M, et al. Long-term cost-effectiveness of matrix-associated chondrocyte implantation in the german health care system: a discrete event simulation. Arch Orthop Trauma Surg. 2023;143(3):1417–1427.
- National Institute for Health and Care Excellence (NICE). Autologous chondrocyte implantation using chondrosphere for treating symptomatic articular cartilage defects of the knee: technology appraisal guidance [TA508]; 2018. Available from: https://www.nice.org.uk/guidance/ta508/documents/html-content-2.
- Mistry H, Connock M, Pink J, et al. Autologous chondrocyte implantation in the knee: systematic review and economic evaluation. Health Technol Assess. 2017;21:1–294.
- Saris D, Price A, Drogset JO, et al. SUMMIT prospective, randomized, controlled trial: response rates to matrix-induced autologous chondrocyte implant (MACI) versus microfracture (MFX) by lesion characteristics. Orthop J Sports Med. 2013;1(4 Suppl):2325967113S00029.
- Saris DBF, Vanlauwe J, Victor J, et al. Treatment of symptomatic cartilage defects of the knee. Am J Sports Med. 2009;37:10–9.
- Niemeyer P, Laute V, Zinser W, et al. A prospective, randomized, Open-Label, multicenter, phase III noninferiority trial to compare the clinical efficacy of Matrix-Associated autologous chondrocyte implantation With spheroid technology Versus arthroscopic microfracture for cartilage defects of the knee. Orthop J Sports Med. 2019;7:232596711985444.
- Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen. N19-02 – ACI am Kniegelenk – Abschlussbericht – Version 1.1. Published Online First; 2020. Available from: www.iqwig.de.
- Annemans L, Genesté B, Jolain B. Early modelling for assessing health and economic outcomes of drug therapy. Value in Health. 2000;3:427–34.
- IJzerman MJ, Koffijberg H, Fenwick E, et al. Emerging use of early health technology assessment in medical product development: a scoping review of the literature. Pharmacoeconomics. 2017;35:727–40.
- Armoiry X, Cummins E, Connock M, et al. Autologous chondrocyte implantation with chondrosphere for treating articular cartilage defects in the knee: an evidence review group perspective of a NICE single technology appraisal. Pharmacoeconomics. 2019;37:879–86.
- Svendsen VG, Lokkerbol J, Danner UN, et al. Design and testing of a health economic markov model for treatment of anorexia nervosa. Expert Rev Pharmacoecon Outcomes Res. 2022;22:1243–51.
- Sonnenberg FA, Beck JR. Markov models in medical decision making. Med Dec Mak. 1993;13:322–38.
- National Institute for Health and Care Excellence, editor. NICE health technology evaluations: the manual. Process and methods; 2022. Available from: www.nice.org.uk/process/pmg36.
- Niemeyer P, Angele P, Juras V, et al. Minimally invasive matrix-coupled autologous chondrocyte transplantation (MACT) with a regeneration-promoting hydrogel –0. 40 AGA Congress Abstract Book | 14–16 September 2023 | Berlin; 2022. p. 48. Available from: https://kongressarchiv.aga-kongress.info/wp-content/uploads/2022/10/AGA22_Abstractband.pdf., Abstract AGA22-202
- TETEC AG. Data on file: treatment of large cartilage defects in the knee by hydrogel-based autologous chondrocyte implantation: two-year results of a prospective, multicenter, single-arm phase III trial: number of reoperations after one year; 2022.
- National Institute for Health and Care Excellence (NICE). Single technology appraisal: autologous chondrocyte implantation with chondrosphere for treating articular cartilage defects [ID851]: committee Papers; 2018. Available from: https://www.nice.org.uk/guidance/ta508/documents/committee-papers.
- Office for National Statistics. Death registered in England and Wales: 2020; 2021. Available from: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/bulletins/deathsregistrationsummarytables/2020.
- Gerlier L, Lamotte M, Wille M, et al. The cost utility of autologous chondrocytes implantation using ChondroCelect in symptomatic knee cartilage lesions in Belgium. Pharmacoeconomics. 2010;28:1129–46.
- Dong H, Buxton M. Early assessment of the likely cost-effectiveness of a new technology: a markov model with probabilistic sensitivity analysis of computer-assisted total knee replacement. Int J Technol Assess Health Care. 2006;22:191–202.
- Jansson K-Å, Granath F. Health-related quality of life (EQ-5D) before and after orthopedic surgery. Acta Orthop. 2011;82:82–89.
- PSSRU. Unit costs of health & social care 2016; 2016. Available from: https://www.pssru.ac.uk/pub/uc/uc2016/full.pdf?uc=2016-full.
- National Health Service. National reference costs 2015–16; 2015. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/577083/Reference_Costs_2015-16.pdf.
- Gray AM, Clarke PM, Wolstenholme JL, et al. Applied methods of cost-effectiveness analysis in health care. New York: Oxford University Press; 2011.
- Fenwick E, Claxton K, Sculpher M. Representing uncertainty: the role of cost-effectiveness acceptability curves. Health Econ. 2001;10:779–787.
- Chilcott J, Brennan A, Booth A, et al. The role of modelling in prioritising and planning clinical trials. Health Technol Assess. 2003;7(23):iii, 1–125.
- Briggs AH, Karl C, Sculpher MJ. Decision modelling for health economic evaluation. Oxford: Oxford University Press; 2006;237.
- Wilson ECF. A practical guide to value of information analysis. Pharmacoeconomics. 2015;33(2):105–121.
- Stinnett AA, Mullahy J. Net health benefits: a new framework for the analysis of uncertainty in cost-effectiveness analysis. Med Decis Making. 1998;18(2 Suppl):S68–S80.
- National Institute for Health and Care Excellence (NICE). Final appraisal determination: autologous chondrocyte implantation using chondrosphere for treating symptomatic articular cartilage defects of the knee; 2017. Available from: https://www.nice.org.uk/guidance/ta508/documents/final-appraisal-determination-document.
- Pinho-Gomes A-C, Cairns J. Evaluation of advanced therapy medicinal products by the national institute for health and care excellence (NICE): an updated review. Pharmacoecon Open. 2022;6(2):147–167.
- Saris D, Price A, Widuchowski W, SUMMIT Study Group, et al. Matrix-applied characterized autologous cultured chondrocytes versus microfracture. Am J Sports Med. 2014;42(6):1384–1394.
- Knutsen G, Drogset J, Engebretsen L, et al. A randomized trial comparing autologous chondrocyte implantation with microfracture. J Bone Joint Surg Am. 2007;89:2105–2112.