Abstract
Aims
Gastro-esophageal reflux disease (GERD) is a common, chronic gastrointestinal condition characterized by heartburn, chest pain, regurgitation, and bloating. The current standard of care includes chronic treatment with proton pump inhibitors (PPIs) or, in selected patients, laparoscopic anti-reflux surgery. RefluxStop is a novel implantable device indicated for GERD patients eligible for laparoscopic surgical treatment. The aim of this analysis was to assess the cost-effectiveness of RefluxStop against available treatment options for GERD.
Material and methods
A Markov model was developed to assess the cost-effectiveness of RefluxStop compared with PPI-based medical management (MM) and two surgical management options, LNF and magnetic sphincter augmentation (MSA, LINX system), in people with GERD. Clinical outcomes and costs were estimated over a lifetime horizon from the UK National Health Service perspective and an annual discount rate of 3.5% was applied.
Results
RefluxStop showed favorable surgical outcomes compared with both LNF and MSA. The base case incremental cost-effectiveness ratios compared with MM, LNF, and MSA were £4,156, £6,517, and £249 per QALY gained, respectively. At the UK cost-effectiveness threshold of £20,000 per QALY gained, the probability that RefluxStop was cost-effective against MM, LNF, and MSA was 100%, 93%, and 100%, respectively.
Limitations
The model presented the results of a comparison, with evidence for RefluxStop derived from its single-arm CE mark trial and that for comparators from the literature. The varied clinical care pathway of individual GERD patients was necessarily simplified for modeling purposes, and necessary assumptions were made; however, the model results proved robust to sensitivity analyses.
Conclusions
Introduction of RefluxStop was estimated to extend life expectancy and improve quality-of-life of GERD patients when compared with MM, LNF, and MSA. The results of the cost-effectiveness analysis demonstrated that RefluxStop is highly likely to be a cost-effective treatment option within NHS England.
Introduction
Gastro-esophageal reflux disease (GERD) is a chronic gastrointestinal condition characterized by retrograde flow of gastric contents into the esophagusCitation1. Common symptoms include heartburn, chest pain, regurgitation, bloating, excessive salivation, and impaired sleepCitation1. GERD is a common disorder; its age-standardized prevalence was recently estimated at 8,819 (95% uncertainty interval [UI]: 7,781–9,863) cases per 100,000 population globally and 9,920 (95% UI: 8,721–11,140) per 100,000 population in the UKCitation2. Patients with GERD are at an increased risk of developing esophageal adenocarcinoma and its precursor lesion, Barrett’s esophagus, defined by a change in the lining of the esophagus from squamous to columnar epitheliumCitation3. In a recent meta-analysis, the prevalence of histologically confirmed Barrett’s esophagus in patients with GERD was 7.2%, while esophageal adenocarcinoma was rare, affecting 1.2% of patients with histologically confirmed Barrett’s esophagus and 0.1% of the broader population of patients with GERDCitation4.
Current guideline from the UK National Institute of Health and Care Excellence (NICE) recommends proton pump inhibitors (PPI) as first-line treatment for GERDCitation5. However, more than half of the patients on daily PPI therapy have been reported to experience persistent symptoms of GERDCitation6. NICE recommends that patients who do not respond adequately to PPI treatment may be offered H2 receptor antagonistsCitation5 and those who do not wish to continue with long-term PPI therapy, or are intolerant to it, should be considered for laparoscopic Nissen fundoplicationCitation5. However, surgical experts have suggested that the group of patients who would benefit from surgical treatment is broader than that described in the NICE guideline, and includes patients with predominantly volume reflux, ongoing esophagitis despite maximal medical therapy, or a large hiatus herniaCitation7. Recently, magnetic sphincter augmentation (MSA) using the LINX system has been employed in patient’s refractory to PPI therapyCitation8,Citation9. In January 2023, NICE considered the evidence on the safety and efficacy of MSA sufficient to support using the procedure subject to standard arrangements for clinical governance, consent, and auditCitation10.
RefluxStop (Implantica, Zug, Switzerland) is an implantable, non-active, single-use device indicated for the treatment of patients with GERDCitation11. RefluxStop restores normal anatomy by blocking the movement of the lower esophageal sphincter into the thorax and maintaining the anatomically correct position of the angle of esophagogastric angle without affecting the passage of foodCitation11. The efficacy and safety of RefluxStop were evaluated in a prospective, single-arm, multicenter CE mark trial, in which 50 patients with chronic GERD requiring daily PPI medication were implanted the device using a standardized surgical techniqueCitation11. The primary efficacy endpoint was the percent reduction of GERD symptoms from baseline measured using the GERD-Health-Related Quality-of-Life (GERD-HRQL) total score, and the primary safety objective was the assessment of serious adverse device effects (SADEs) and procedure-related serious adverse events (SAEs). Average GERD-HRQL total score improved from baseline by 89% at 6 months and by 86% at 1 yearCitation11; the trial therefore met its primary endpoint. At 6 months from surgery, 24-hour pH monitoring showed a mean reduction in the percentage of overall time with pH <4 from 16.35% at baseline to 0.80%; 98% of patients had normal 24-hour pHCitation11. No patients experienced new-onset dysphagia after the surgery, despite this being a common adverse effect of other available anti-reflux operationsCitation11. In terms of safety, no SADEs were reported in the trialCitation11. Four patients experienced procedure-related SAEs within 6 months from surgery, and one patient experienced a release of fundoplication sutures (classified as an SAE) between 6 months and 1 year from surgeryCitation11. RefluxStop received its CE mark approval in August 2018Citation12.
The aim of this study was to assess the cost-effectiveness of RefluxStop in comparison with PPI-based medical management, laparoscopic Nissen fundoplication, and MSA in people with GERD and, ultimately, support decision-makers in maximizing the health benefits of the funded interventions for GERD while carefully considering limited resources of the healthcare system.
Methods
A cost-effectiveness analysis was conducted comparing RefluxStop with PPI-based medical management and two surgical management options, Nissen fundoplication and MSA, in people with GERD. In line with the NICE requirements for health technology assessmentsCitation13, clinical outcomes and costs were assessed over a lifetime horizon and the perspective of the analysis was that of the National Health Service (NHS) in England and Wales, i.e. only direct medical costs were included, without accounting for societal costs (e.g. those related to lost productivity due to absence from work). Clinical outcomes were quantified using standard measures, i.e. quality-adjusted life-years (QALYs). In line with NICE requirementsCitation13, all costs and QALYs were discounted at 3.5% per year. The model cycle length was 1 month, and half cycle correction was applied; therefore, any events in the model were assumed to occur in the middle of each month.
Model structure
The analytical framework used to assess cost-effectiveness of RefluxStop was a state transition (Markov) model, in which a cohort of patients progressed through a series of mutually exclusive health states reflecting all possible outcomes of the four GERD treatment options assessed. The Markov structure is consistent with previously published studies assessing the cost-effectiveness of treatments for GERD from the UK perspectiveCitation14–17. The structure of the model differed between medical () and surgical () treatment options to reflect the different nature of treatments; however, in both cases the health states considered standard and high-dose medical management, refractoriness to PPIs, initial surgery and reoperations, development of Barrett’s esophagus and esophageal carcinoma, and death. The model structure also included adverse events (AEs) associated with medical and surgical management options.
Figure 1. Model structure applied to medical management (panel A) and surgical treatment options (panel B). Abbreviations. C. Diff, Clostridium difficile; CKD, chronic kidney disease; PPIs, proton pump inhibitors; reop, reoperation.
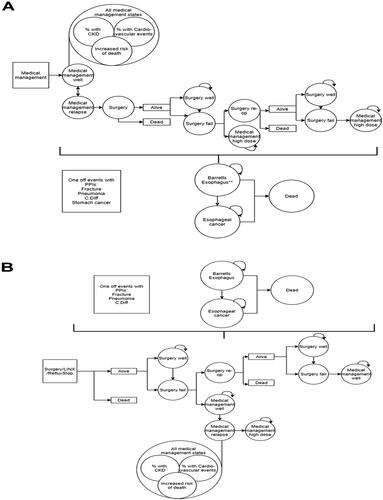
The medical management arm of the model was designed to reflect the NICE-recommended standard of care for GERDCitation5. In this arm (), all patients were assumed to receive standard-dose medical management with PPI as a first-line treatment (i.e. entered the model in the ‘Medical management well’ state), with a risk of GERD relapse applied each month. When relapse occurred, patients received a double dose of PPIs (described as the ‘Medical management relapse’ state) and could subsequently return to a standard PPI dose (the ‘Medical management well’ state) or have surgery, which in this model arm was restricted to Nissen fundoplication, recommended by NICE as part of standard care for GERDCitation5. Post-surgical health states of the model describe successful (‘Surgery well’) or unsuccessful (‘Surgery fail’) outcomes of the procedure. Patients who had an unsuccessful surgery could require a reoperation (‘Surgery re-op’) or receive treatment with a double dose of PPI (‘Medical management high dose’), which was assumed to continue for the rest of the patient’s lifetime. Patients whose reoperation also failed went on to receive lifelong double-dose PPI treatment.
In the surgical treatment arms of the model (Nissen fundoplication, MSA, and RefluxStop) (structure presented in ), all patients entered the model at the point of initial surgery. This initial surgery could be successful (captured in the ‘Surgery well’ state) or unsuccessful (‘Surgery fail’). Options for patients whose initial surgery failed included reoperation (‘Surgery re-op’) or treatment with standard PPI dose (‘Medical management well’). Once on medical management, patients experienced a monthly probability of GERD relapse at which point they were assumed to switch to a double dose of PPIs and remain on it for the rest of their lifetime (‘Medical management high dose’). With regards to reoperations, in the RefluxStop and Nissen fundoplication treatment arms, patients undergoing reoperation were assumed to receive the same surgery type as their initial procedure; however, in the MSA treatment arm, reoperation was assumed to be a Nissen fundoplication. Patients whose reoperation was unsuccessful were assumed to receive treatment with a standard dose of PPI (‘Medical management well’).
All patients in the model were at risk of developing Barrett’s esophagus, from which a small proportion (0.06% per month) would progress to esophageal cancer. In addition, both general population mortality and mortality associated specific health states were considered as described below.
Model inputs
Clinical and quality-of-life inputs
The model followed a hypothetical cohort of 1,000 patients whose starting age was 52 years and 56% were male, in line with the RefluxStop CE mark trialCitation11. These demographic characteristics are also broadly similar to other large trials of GERD treatments, including the UK REFLUX trialCitation18 and the recent trial comparing medical and surgical treatment in US patients with refractory GERDCitation19. Clinical and quality-of-life inputs informing the model are summarized in .
Table 1. Clinical and quality-of-life inputs used in the model.
RefluxStop’s efficacy and safety data informing the model was sourced from the CE mark trialCitation11, utilizing unpublished 3-year follow-up dataCitation20. Clinical efficacy data for medical management, Nissen fundoplication, and MSA were sourced from recent available publications. For surgical interventions, the monthly probability of surgical failure was split into two different periods (up to 1 year post-surgery and >1 year from surgery), reflecting the reporting in studies of each intervention.
Intra-operative complications and post-surgical AEs were included for all surgical procedures modeled (whether initial or reoperations); the events considered were sourced from a recent review on the outcomes of Nissen fundoplicationCitation21. Patients in the medical management states were at risk of AEs associated with long-term PPI use, including clostridium difficile (C.Diff) infectionCitation22, fractures related to osteoporosisCitation23, community-acquired pneumoniaCitation24, gastric cancerCitation25, chronic kidney disease (CKD)Citation26, and cardiovascular (CV) eventsCitation27. Of these, fractures, pneumonia, stomach cancer, and C.Diff infection were modeled as one-off events, while CKD and CV events were modeled using the proportion in state method, i.e. assuming that at any given time, a proportion of the patients in medical management states had CKD or CV events. This method assumed that the rate of death from CKD and CV events was the same as the rate at which PPI users developed these complications.
As noted previously, Barrett’s esophagus and esophageal cancer are potential long-term sequelae of GERDCitation3. The monthly probability of developing Barrett’s esophagus was 0.083%, based on the model developed to inform the NICE guideline on dyspepsia and GERDCitation16, and was assumed to apply to all treatment arms. The monthly risk of developing esophageal cancer in patients with Barrett’s esophagus was 0.06%, based on evidence from a systematic review and meta-analysisCitation28.
In addition to general population mortality, the model considered excess mortality associated with surgery, esophageal cancer, and AEs related to long-term PPI use, which determined mortality in different model arms. With regards to surgical mortality, a very small risk of intra-operative mortality (0.05%) was applied to all initial surgeries across all model arms, and this risk was assumed to be doubled for reoperations. Patients who developed esophageal cancer had a monthly risk of death of 3.4%, in line with survival data reported by the UK Office of National Statistics (ONS)Citation29. A relative mortality risk of 1.57 was applied to all patients in the medical management states (regardless of model arm), reflecting the increased risk of death associated with adverse effects of long-term PPI use, as described in a recent systematic reviewCitation30. As such, mortality estimation in the model did not favor any of the arms.
Health Related Quality-of-Life (HRQoL) was described using utility decrements. The utility decrements for those who have had a successful surgery was based on the proportion reporting persistent dysphagia, a potential ongoing adverse effect of surgery. A utility decrement of 0.24 was applied for 1 month following all initial surgical procedures and reoperations (in all model arms), and was based on the quality-of-life impact of undergoing a laparoscopic cholecystectomyCitation15 which represents a conservative approach, since laparoscopic cholecystectomy is a more complex procedure compared with GERD surgery. Utility decrements in patients receiving PPIs were based on a cost-effectiveness analysis of PPIs and laparoscopic Nissen fundoplication in the treatment of GERD based on the REFLUX trial, a large UK-based trial funded by the NHSCitation15,Citation18. Another analysis based on this trial informed the utility decrement associated with unsuccessful Nielsen fundoplication surgery, which was assumed to also apply to MSA and RefluxStopCitation18. Patients with Barrett’s esophagus and esophageal cancer also experienced utility decrements associated with these conditions. All HRQoL model inputs are listed in .
Cost inputs
Costs were sourced from NHS schedule costs 2019/20Citation31 whenever possible. Where data are used from older sources in the literature, the values were inflated using the Personal Social Services Research Unit (PSSRU) inflation indicesCitation32. Key cost inputs applied in the model are summarized in .
Table 2. Key cost inputs applied in the model.
The model considered the costs of PPI medications (omeprazole, lansoprazole, pantoprazole, rabeprazole, and esomeprazole) and surgical procedures. The cost of surgical treatments included procedure costs and, for MSA and RefluxStop only, device and training costs. The procedure cost of Nissen fundoplication was assumed to apply also to MSA and RefluxStop insertion procedures as all the three procedures use the same standard laparoscopic approach. However, the MSA procedure is likely to be of shorter duration, therefore we have estimated 25% less procedure cost in a scenario analysis and the change of results was insignificant.
Patients who developed Barrett’s esophagus incurred an initial diagnostic and treatment cost, and a monthly management cost, in line with the management pathway described in the cost-effectiveness analysis of endoscopic management therapy for Barrett’s esophagus patientsCitation33. All patients were assumed to undergo diagnostic endoscopy; and those with dysplasia (16.6%) were assumed to receive treatment with endoscopic mucosal ablation and radiofrequency ablation, followed by monitoring with endoscopy every 2 years and treatment with PPIsCitation33. Patients without dysplasia incurred the costs of PPIs and monitoring with endoscopy onlyCitation33. Patients who progressed from Barrett’s esophagus to esophageal cancer were assumed to follow the care pathway outlined in the model developed to support NICE clinical guidance development in dyspepsia and GERDCitation16. The costs associated with esophageal cancer included initial diagnostics and treatment costs (comprising diagnostic endoscopy, a GP appointment, esophagectomy, and chemotherapy), a monthly cost of treatment with PPI and, for any patients dying from cancer, a terminal care cost.
With regards to adverse events associated with PPIs, monthly costs were applied to patients who developed CKD or experienced a CV event. The costs of one-off events included those of fractures, pneumonia, and C. diff. infection. A lifetime cost associated with supportive care and chemotherapy was applied to patients who developed stomach cancer.
The costs of adverse events associated with surgery included those of conversion to open surgery, esophageal dilation, major surgical complications, and device removal. Intraoperative splenic, liver, or gastro-esophageal injuries were assumed to already be captured in the costs of the procedure itself.
Economic analysis
For each arm, lifetime estimates of per patient QALYs, life-years (LYs), life expectancy, and total costs were generated, as well as the incremental differences in these outcomes between treatment arms. Three incremental measures comparing the treatment arms were generated: (1) incremental cost-effectiveness ratio (ICER), which represents the cost per QALY gained; (2) incremental net health benefit (NHB), which assesses the impact of the intervention on population health; and (3) incremental net monetary benefit (NMB), representing the value of an intervention in monetary terms. Both NMB and NHB were calculated using the standard NICE cost-effectiveness threshold of £20,000 per QALY gained. A positive incremental NMB or NHB value indicates that the treatment would benefit the healthcare system at the cost-effectiveness threshold value, with larger NHB/NMB values indicating a greater benefit.
In addition to the base case analysis described above, deterministic sensitivity analyses were conducted to assess the inherent uncertainty associated with model results. In deterministic sensitivity analyses, one model input was varied at a time, and the effect on model results recorded to identify the most influential inputs. The ranges used to vary the parameters were based on reported confidence intervals (CIs), or assumptions were made where these were not available. In probabilistic sensitivity analyses, all model inputs associated with uncertainty were simultaneously sampled from plausible distributions and the model run over 1,000 iterations, with each iteration using a different set of inputs. The standard errors used to generate probabilistic values were derived from reported CIs wherever possible. Where this information was not available, the standard error was assumed to be equal to 10% of the mean value.
Results
Base case results
Life expectancy estimated by the model was higher in the RefluxStop arm than all comparator arms, with differences of 0.73–2.18 years in favor of RefluxStop (). Patients in the RefluxStop arm also accrued between 0.63 and 1.83 more QALYs than patients in the comparator arms (). Against both Nissen fundoplication and MSA, RefluxStop also compared favorably in terms of surgical outcomes, including the number of surgeries, surgical failures, endoscopic dilation and, against MSA only, device removals ().
Table 3. Clinical outcome estimates in the base-case analysis (per 1,000 patients unless otherwise stated).
Table 4. Cost-effectiveness outcomes estimated in the base case analysis, per patient.
RefluxStop was cost-effective at the NICE cost-effectiveness threshold of £20,000 per QALY against all comparators assessed (). The ICERs against medical management, Nissen fundoplication, and MSA were £4,156 per QALY, £6,517 per QALY, and £249 per QALY, respectively. The NHB (1.41 against PPI-based medical management, 0.43 against Nissen fundoplication, and 1.59 against MSA) and NMB (£28,225 against PPI-based medical management, £8,554 against Nissen fundoplication, and £31,797 against MSA) against all of the assessed comparators were positive.
The costs of RefluxStop were somewhat offset by the lower costs of PPI treatment (savings of £945, £296, and £917 vs medical management, Nissen fundoplication, and MSA, respectively) and the management of PPI-associated events (savings of £204, £53, and £165 vs medical management, Nissen fundoplication and MSA, respectively). Compared with the two surgical treatment options, Reflux stop also provided per-patient lifetime savings in terms of surgical outcomes and complications (respectively vs Nissen fundoplication and MSA: savings of £76 and £11 for intraoperative events and complications requiring surgery, £324 and £145 for endoscopic dilation, and, against MSA only, a saving of £44 related to device removal).
Sensitivity analyses
In deterministic sensitivity analyses (Supplementary Figure S1) the most influential model inputs were the probability of surgical failure with RefluxStop and the probability of surgery after medical relapse. The results of the model appeared to be robust to changes in individual input parameters. When varied individually, none of the parameters, except for the monthly surgical failure rate with RefluxStop when compared with Nissen fundoplication, changed the direction of the results. Long-term cost-effectiveness was largely unaffected when different cost estimates and risk of adverse events were explored.
Reducing the cost of the LINX device by 20% (£4,126 instead of £5,502) increased the ICER from £249 to £1,104 per QALY gained.
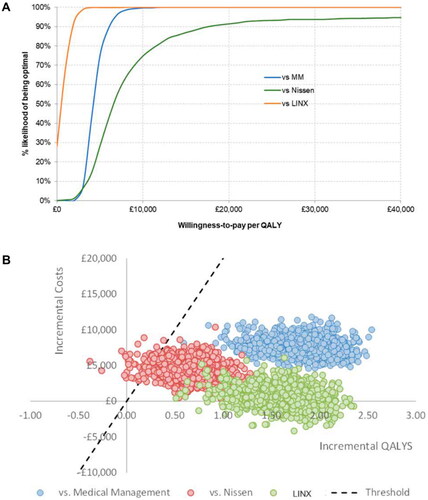
In probabilistic sensitivity analysis, the probabilities of RefluxStop being cost-effective against medical management, Nissen fundoplication, and MSA were 100%, 93%, and 100%, respectively () at the NICE threshold of £20,000 per QALY. Most of the probabilistic iterations were below the cost-effectiveness threshold (). The average ICERs obtained from 1,000 iterations of the probabilistic analyses were £4,336 per QALY when compared with medical management, £6,789 per QALY when compared with Nissen fundoplication, and £502 per QALY when compared with MSA, and were therefore similar to the base case ICERs against each of the comparators.
Figure 2. Results of the probabilistic sensitivity analyses against all three comparators presented as CEACS (panel A) and as a CE plane (panel B). The dashed black line in panel B indicates the NICE cost-effectiveness threshold of £20,000 per QALY. The points lying to the right of this line indicate iterations in which RefluxStop was cost-effective vs the assessed comparator (marked by the color of the individual points) and the points lying to the left of the dashed line indicate those iterations in which RefluxStop was not cost-effective.
Discussion
RefluxStop was predicted to have a positive effect on patients’ life expectancy, that also persisted when quality-of-life was taken into account, as evidenced by incremental gains in life-years and QALYs compared with the other treatment options assessed. Furthermore, the estimated number of surgical complications was lower with RefluxStop compared with both laparoscopic Nissen fundoplication and MSA. While the model did estimate a slight increase in the number of cases of Barrett’s esophagus and esophageal cancer with RefluxStop, this is attributable to longer life expectancy in this model arm relative to the comparator arms, as successful surgery does not necessarily stop the development of Barrett’s esophagus or esophageal cancer.
The results of the cost-effectiveness analysis show that RefluxStop is highly likely to be a cost-effective treatment option for GERD patients when compared with the management options currently available in the United Kingdom. The base case ICER estimates were all well below the NICE £20,000 per QALY threshold, and the sensitivity and scenario analyses suggested these estimates were robust to changes in model inputs, particularly to a reduction in the cost of the LINX device. Therefore, the health benefits of RefluxStop balanced its additional cost in this analysis. Positive NHB and NMB of RefluxStop should be interpreted as the intervention providing both health and monetary benefits to the NHS, relative to its comparators.
To date, the clinical effectiveness and safety of RefluxStopCitation11 and, with this analysis, its cost-effectiveness from the NHS England perspective have been demonstrated, providing important evidence supporting wider implementation of RefluxStop. However, spreading innovation in surgery may be difficult in practice and will require additional steps beyond presenting the evidence supporting the use of this technology. The Royal College of Surgeons has developed a framework for adoption of surgical innovation that combines actions from multiple stakeholders into six key steps, from early identification of promising technologies and championing their adoption, through establishing the capacity and organizational structure that supports innovation, formal evaluation by NICE (where appropriate), to financial incentives for adopting the technology and supporting informed patient choiceCitation34. Surgeon training is also an important consideration for the adoption of a new surgical interventionsCitation34 and specific training considerations apply to implantation of the RefluxStop device, including correct placement of the device above the esophageal sphincter and adherence of the stomach fundus wall to the esophagus, building a platform for the deviceCitation11.
The results of this analysis suggest that appropriate measures to drive the wider adoption of RefluxStop are likely to benefit both patients and the NHS in the future. In terms of strengths and limitations of the analysis, a notable strength lies in the fact that many of the model clinical parameters of RefluxStop were derived from an analysis of recently collected 3-year data from the CE mark trialCitation20, from which the 1-year results have previously been publishedCitation11. While this data may be viewed critically, being derived from a single-arm trial, employment of randomized controlled trials (RCTs) in the field of surgery has long been recognized as a challenge, with substantially fewer surgical than medical treatments being supported by RCT-derived evidenceCitation35. The data on the effectiveness and safety of comparator treatments were derived from the literature and therefore were based on somewhat dissimilar populations (with some slight differences noted, e.g. in baseline age, male-to-female ratio, and proportion of patients with esophagitis at baseline) and outcome measures to those applied in the RefluxStop CE mark trial (e.g. definitions of surgical failure varied between the studies informing efficacy in the model). As such, the model presents the results of a naïve comparison, which should be taken into account when interpreting the data. No model can adequately reflect the varied clinical care pathway of individual patients and, as such, the results of health economic modeling should be seen as presenting the results of an ‘average’ patient care scenario. Another common issue in cost-effectiveness modeling is that necessary assumptions have to be made in the absence of informing data. Finally, regional- and individual-level health and/or socioeconomic disparities were not taken into account in this analysis, despite established methodology to account for this important issueCitation36,Citation37. All these factors are sources of uncertainty associated with the model’s results, which were, however, in the case of our analysis largely balanced by the robustness of model estimates demonstrated when the inputs were varied in sensitivity analyses. An RCT of RefluxStop against the surgical standard of laparoscopic Nissen fundoplication is also planned and will provide additional data supporting RefluxStop as a treatment for patients with GERD.
Conclusions
The results of the cost-effectiveness analysis demonstrated that RefluxStop is highly likely to be a cost-effective treatment option for GERD patients when compared with treatment options currently available within NHS England and Wales.
Transparency
Declaration of financial/other relationships
SH, LG, and SM work for a consultancy company that was commissioned by Implantica to develop the cost-effectiveness model. None have any personal conflicts to declare. LG, PG, and AA have no personal conflicts to declare.
Author contributions
SH, LG, and SM designed and developed the model. LG, PG, and AA contributed to the design of the economic model and validated the results from a clinical perspective. All authors contributed to the manuscript.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Previous presentations
The data described in this manuscript was partially presented at ISPOR Europe 2022.
Supplemental Material
Download MS Word (785.1 KB)Acknowledgements
Medical writing and editorial assistance with the preparation of this manuscript was provided by Karolina Badora and funded by Implantica.
Additional information
Funding
References
- Boulton KHA, Dettmar PW. A narrative review of the prevalence of gastroesophageal reflux disease (GERD). Ann Esophagus. 2022;5:7–7.
- Dirac MA, Safiri S, Tsoi D, et al. The global, regional, and national burden of gastro-oesophageal reflux disease in 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet Gastroenterol Hepatol. 2020;5(6):561–581.
- Shaheen N, Ransohoff DF. Gastroesophageal reflux, barrett esophagus, and esophageal cancer. JAMA. 2002;287(15):1972–1981.
- Eusebi LH, Cirota GG, Zagari RM, et al. Global prevalence of barrett’s oesophagus and oesophageal cancer in individuals with gastro-oesophageal reflux: a systematic review and meta-analysis. Gut. 2021;70(3):456–463.
- National Institute for Health and Care Excellence. Clinical guideline [CG184]: gastro-oesophageal reflux disease and dyspepsia in adults: investigation and management. 2019. https://www.nice.org.uk/guidance/cg184
- Delshad SD, Almario CV, Chey WD, et al. Prevalence of gastroesophageal reflux disease and proton pump Inhibitor-Refractory symptoms. Gastroenterology. 2020;158(5):1250–1261.e2.
- Thompson SK, Watson DI. We asked the experts: “when is a laparoscopic fundoplication warranted for gastroesophageal reflux disease?” World J Surg. 2022;46(7):1711–1712.
- Bonavina L, Horbach T, Schoppmann SF, et al. Three-year clinical experience with magnetic sphincter augmentation and laparoscopic fundoplication. Surg Endosc. 2021;35(7):3449–3458.
- Skubleny D, Switzer NJ, Dang J, et al. LINX® magnetic esophageal sphincter augmentation versus nissen fundoplication for gastroesophageal reflux disease: a systematic review and meta-analysis. Surg Endosc. 2017;31(8):3078–3084.
- National Institute for Health and Care Excellence. Interventional procedures guidance [IPG749]: laparoscopic insertion of a magnetic ring for gastrooesophageal reflux disease. 2023. https://www.nice.org.uk/guidance/ipg749
- Bjelović M, Harsányi L, Altorjay Á, et al. Non-active implantable device treating acid reflux with a new dynamic treatment approach: 1-year results: refluxStop™ device; a new method in acid reflux surgery obtaining CE mark. BMC Surg. 2020;20(1):159.
- Implantica. Implantica receives CE Mark Approval for RefluxStop™, a potential Paradigm shift in the Treatment for Acid reflux. 2018. https://www.implantica.com/media/press-releases/2018/implantica-receives-ce-mark-approval-for-refluxstop-a-potential-paradigm-shift-in-the-treatment-for-acid-reflux/
- National Institute for Health and Care Excellence. Process and methods [PMG36]: NICE health technology evaluations: the manual. 2022. https://www.nice.org.uk/process/pmg36/chapter/introduction-to-health-technology-evaluation
- Epstein D, Bojke L, Sculpher MJ, REFLUX trial group. Laparoscopic fundoplication compared with medical management for gastro-oesophageal reflux disease: cost effectiveness study. BMJ. 2009;339:b2576.
- Bojke L, Hornby E, Sculpher M, REFLUX Trial Team A comparison of the cost effectiveness of pharmacotherapy or surgery (laparoscopic fundoplication) in the treatment of GORD. Pharmacoeconomics. 2007;25(10):829–841.
- National Institute for Health and Care Excellence (NICE). Dyspepsia and gastro-oesophageal reflux disease. Appendix H: full Health Economics Report. 2014. https://www.nice.org.uk/guidance/cg184/evidence/appendix-h-full-health-economics-report-pdf-6955335252
- Wahlqvist P, Junghard O, Higgins A, et al. Cost effectiveness of proton pump inhibitors in gastro-oesophageal reflux disease without oesophagitis: comparison of on-demand esomeprazole with conventional omeprazole strategies. Pharmacoeconomics. 2002;20(4):267–277.
- Grant A, Wileman S, Ramsay C, et al. The effectiveness and cost-effectiveness of minimal access surgery amongst people with gastro-oesophageal reflux disease - a UK collaborative study. The REFLUX trial. Health Technol Assess. 2008;12(31):1–181.
- Spechler SJ, Hunter JG, Jones KM, et al. Randomized trial of medical versus surgical treatment for refractory heartburn. N Engl J Med. 2019;381(16):1513–1523.
- Implantica. RefluxStop CE mark trial - 3 year data. Data on File. 2022.
- Karolinska Institute. Literature review and analysis: laparoscopic Nissen Fundoplication. [unpublished]. (Version KILNF001). 2017.
- Trifan A, Stanciu C, Girleanu I, et al. Proton pump inhibitors therapy and risk of Clostridium difficile infection: systematic review and meta-analysis. World J Gastroenterol. 2017;23(35):6500–6515.
- Nassar Y, Richter S. Proton-pump inhibitor use and fracture risk: an updated systematic review and meta-analysis. J Bone Metab. 2018;25(3):141–151.
- Nguyen PA, Islam M, Galvin CJ, et al. Meta-analysis of proton pump inhibitors induced risk of community-acquired pneumonia. Int J Qual Health Care. 2020;32(5):292–299.
- Poly TN, Lin MC, Syed-Abdul S, et al. Proton pump inhibitor use and risk of gastric cancer: current evidence from epidemiological studies and critical appraisal. Cancers. 2022;14(13):3052.
- Nochaiwong S, Ruengorn C, Awiphan R, et al. The association between proton pump inhibitor use and the risk of adverse kidney outcomes: a systematic review and meta-analysis. Nephrol Dial Transplant. 2018;33(2):331–342.
- Batchelor R, Kumar R, Gilmartin-Thomas JFM, et al. Systematic review with meta-analysis: risk of adverse cardiovascular events with proton pump inhibitors independent of clopidogrel. Aliment Pharmacol Ther. 2018;48(8):780–796.
- Yousef F, Cardwell C, Cantwell MM, et al. The incidence of esophageal cancer and high-grade dysplasia in barrett’s esophagus: a systematic review and meta-analysis. Am J Epidemiol. 2008;168(3):237–249.
- Office for National Statistics (ONS). Cancer survival by NHS England Area Team- Adults diagnosed: 1997-2012, followed up to 2013. 2014. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/cancersurvivalbynhsenglandareateamadultsdiagnosed/2014-12-16#oesophageal-cancer
- Shiraev TP, Bullen A. Proton pump inhibitors and cardiovascular events: a systematic review. Heart Lung Circ. 2018;27(4):443–450.
- NHS England. 2019/20 National Cost Collection Data Publication. 2022. https://www.england.nhs.uk/publication/2019-20-national-cost-collection-data-publication/
- Jones K, Burns A. Unit costs of health and social care 2021. Kent: personal Social Services Research Unit (PSSRU); 2021.
- Pollit V, Graham D, Leonard C, et al. A cost-effectiveness analysis of endoscopic eradication therapy for management of dysplasia arising in patients with barrett’s oesophagus in the United Kingdom. Curr Med Res Opin. 2019;35(5):805–815.
- The Royal College of Surgeons of England. From innovation to adoption. Successfully spreading surgical innovation. 2014. https://www.rcseng.ac.uk/-/media/files/rcs/library-and-publications/non-journal-publications/rcs_innovation_to_adoption_2014_web-(1).pdf
- McCulloch P, Taylor I, Sasako M, et al. Randomised trials in surgery: problems and possible solutions. BMJ. 2002;324(7351):1448–1451.
- Asaria M, Griffin S, Cookson R, et al. Distributional Cost-Effectiveness analysis of health care Programmes - A methodological case study of the UK bowel cancer screening programme. Health Econ. 2015;24(6):742–754.
- Lee K-S, Park E-C. Cost effectiveness of colorectal cancer screening interventions with their effects on health disparity being considered. Cancer Res Treat. 2016;48(3):1010–1019.
- Food and Drug Administration (FDA). Summary of Safety and Effectiveness Data (SSED) for LINX Reflux Management System. 2012.
- WHO CVD Risk Chart Working Group World health organization cardiovascular disease risk charts: revised models to estimate risk in 21 global regions. Lancet Glob Health. 2019;7(10):e1332–e1345.
- Collins G, Altman D. Predicting the risk of chronic kidney disease in the UK: an evaluation of QKidney® scores using a primary care database. Br J Gen Pract. 2012;62(597):e243–e250.
- Hussain S, Sing A, Habib A, et al. Proton pump inhibitors use and risk of chronic kidney disease: evidence-based meta-analysis of observational studies. Clin Epidemiol Glob. 2019;7(1):46–52. Mar
- Kanis JA, Johnell O, Oden A, et al. FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int. 2008;19(4):385–397.
- Zhou B, Huang Y, Li H, et al. Proton-pump inhibitors and risk of fractures: an update meta-analysis. Osteoporos Int. 2016;27(1):339–347.
- Sun X, Douiri A, Gulliford M. Pneumonia incidence trends in UK primary care from 2002 to 2017: population-based cohort study. Epidemiol Infect. 2019;147:e263.
- Lambert AA, Lam JO, Paik JJ, et al. Risk of community-acquired pneumonia with outpatient proton-pump inhibitor therapy: a systematic review and meta-analysis. PLoS One. 2015;10(6):e0128004.
- Public Health England. Clostridium difficile infection: mandatory surveillance 2017/18. 2018. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/724368/CDI_summary_2018.pdf
- Janarthanan S, Ditah I, Adler DG, et al. Clostridium difficile-associated diarrhea and proton pump inhibitor therapy: a meta-analysis. Am J Gastroenterol. 2012;107(7):1001–1010.
- Brusselaers N, Wahlin K, Engstrand L, et al. Maintenance therapy with proton pump inhibitors and risk of gastric cancer: a nationwide population-based cohort study in Sweden. BMJ Open. 2017;7(10):e017739.
- National Institute for Health and Care Excellence. Interventional procedures guidance [IPG585]: laparoscopic insertion of a magnetic titanium ring for gastro-oesophageal reflux disease. 2017. https://www.nice.org.uk/guidance/ipg585
- Alicuben ET, Bell RCW, Jobe BA, et al. Worldwide experience with erosion of the magnetic sphincter augmentation device. J Gastrointest Surg. 2018;22(8):1442–1447.
- Kind P, Hardman G, Macran S. UK population norms for EQ-5D: university of York. Centre for Health Economics. 1999. https://www.york.ac.uk/che/pdf/DP172.pdf
- Ainslie WG, Catton JA, Davides D, et al. Micropuncture cholecystectomy vs conventional laparoscopic cholecystectomy: a randomized controlled trial. Surg Endosc. 2003;17(5):766–772.
- Bouvy JC, Ebbers HC, Schellekens H, et al. The cost-effectiveness of periodic safety update reports for biologicals in Europe. Clin Pharmacol Ther. 2013;93(5):433–442.
- Ayazi S, Zheng P, Zaidi AH, et al. Magnetic sphincter augmentation and postoperative dysphagia: characterization, clinical risk factors, and management. J Gastrointest Surg. 2020;24(1):39–49.
- Tsai C, Steffen R, Kessler U, et al. Postoperative dysphagia following magnetic sphincter augmentation for gastroesophageal reflux disease. Surg Laparosc Endosc Percutan Tech. 2020;30(4):322–326.
- National Institute for Health and Care Excellence. British National Formulary (BNF). 2020. https://bnf.nice.org.uk/
- Palser TR, Ceney A, Navarro A, et al. Variation in laparoscopic anti-reflux surgery across England: a 5-year review. Surg Endosc. 2018;32(7):3208–3214.
- Kerr M, Bray B, Medcalf J, et al. Estimating the financial cost of chronic kidney disease to the NHS in England. Nephrol Dial Transplant. 2012;27 (Suppl 3):iii73–80.
- Kent S, Schlackow I, Lozano-Kühne J, et al. What is the impact of chronic kidney disease stage and cardiovascular disease on the annual cost of hospital care in moderate-to-severe kidney disease? BMC Nephrol. 2015;16:65.
- Kidneyfailurerisk. The kidney failure risk equation. 2022. https://kidneyfailurerisk.com/
- Danese MD, Gleeson M, Kutikova L, et al. Estimating the economic burden of cardiovascular events in patients receiving lipid-modifying therapy in the UK. BMJ Open. 2016;6(8):e011805.
- National Institute for Health and Care Excellence (NICE). Technology appraisal guidance [TA464]: bisphosphonates for treating osteoporosis. 2017. https://www.nice.org.uk/guidance/ta464
- Svedbom A, Hernlund E, Ivergård M, et al. Osteoporosis in the european union: a compendium of country-specific reports. Arch Osteoporos. 2013;8(1):137.
- National Institute for Health and Care Excellence. Quality standard [QS110]: Pneumonia in adults. 2016. https://www.nice.org.uk/guidance/qs110
- National Institute for Health and Care Excellence (NICE). Technology appraisal guidance [TA378]: ramucirumab for treating advanced gastric cancer or gastro–oesophageal junction adenocarcinoma previously treated with chemotherapy. 2016. https://www.nice.org.uk/guidance/ta378
- Laudicella M, Walsh B, Munasinghe A, et al. Impact of laparoscopic versus open surgery on hospital costs for Colon cancer: a population-based retrospective cohort study. BMJ Open. 2016;6(11):e012977.
