Abstract
Aims
In Japan, the use of comprehensive genomic profiling (CGP) is only available for cancer patients who have no standard of care (SoC), or those who have completed SoC. This may lead to missed treatment opportunities for patients with druggable alterations. In this study, we evaluated the potential impact of CGP testing before SoC on medical costs and clinical outcome in untreated patients with advanced or recurrent biliary tract cancer (BTC), non-squamous non-small cell lung cancer (NSQ-NSCLC), or colorectal cancer (CRC) in Japan between 2022 and 2026.
Materials and methods
We constructed a decision-tree model reflecting the healthcare environment of Japan, to estimate the clinical outcome and medical costs impact of CGP testing by comparing two groups (with vs without CGP testing before SoC). The epidemiological parameters, detection rates of druggable alterations, and overall survival were collected from literature and claims databases in Japan. Treatment options selected based on druggable alterations were set in the model based on clinical experts’ opinions.
Results
In 2026, the number of untreated patients with advanced or recurrent BTC, NSQ-NSCLC, and CRC was estimated to be 8600, 32,103, and 24,896, respectively. Compared with the group without CGP testing before SoC, CGP testing before SoC increased druggable alteration detection and treatment rate with matched therapies in all three cancer types. The medical costs per patient per month were estimated to increase with CGP testing before SoC in the three cancer types by 19,600, 2900, and 2200 JPY (145, 21, and 16 USD), respectively.
Limitations
Only those druggable alterations with matched therapies were considered in the analysis model, while the potential impact of other genomic alterations provided by CGP testing was not considered.
Conclusions
The present study suggested that CGP testing before SoC may improve patient outcomes in various cancer types with a limited and controllable increase in medical costs.
Introduction
Next-generation sequencing (NGS) based genomic tests have provided physicians with a powerful tool to identify genomic alterations for improving the diagnosis and personalized healthcare of cancer patients. In Japan, the positioning of genomic tests, including multi-gene panel tests, in the healthcare system is currently being discussed to realize personalized cancer genome medicine described in the Basic Plan to Promote Cancer Control Programs (2018) by the Ministry of Health, Labour and Welfare (MHLW)Citation1.
In December 2018, comprehensive genomic profiling (CGP) with NGS for solid cancers was approved in Japan. CGP testing can help patients with druggable genomic alterations select more effective genomically matched therapies instead of traditional chemotherapies, which may contribute to better clinical outcomes. Currently, most conventional genomic tests are essentially single-gene tests or small-panel tests focused on a limited number of genes. These tests require sequential testing which can be time consuming and exhaust patients’ tissue samples, while CGP testing can comprehensively detect hundreds of genomic alterations at once. Therefore, introducing CGP testing into clinical practice is expected to reduce the workload of medical staff, the overall turnaround time needed for sequential testing, and save the tissue samples requiredCitation2–4.
However, as of September 2022, CGP testing in Japan is only covered by National Health Insurance (NHI) in patients with advanced or recurrent solid cancers who have no standard of care (SoC) and those who have already completed SoC. Therefore, CGP testing cannot be performed at an optimal timing based on physicians’ evaluationCitation5 and patients and physicians may miss the opportunity of the most appropriate treatment in an early line, which raises a critical issue for healthcare in the oncology field. In the guidance published by cancer-associated societies in Japan, it is recommended that the optimal timing of CGP testing should take into consideration subsequent treatment plans, and not be limited to rigid constrictions within specific treatment linesCitation6.
On the other hand, the current cost of CGP in Japan is 560,000 JPY (4148 USD) (1USD = 135JPY; as of September 2022), comparatively higher than single-gene tests (25,000 JPY [185 USD] to 51,000 JPY [378 USD]) and Oncomine Dx Target Test Multi-CDx System (140,000 JPY [1037 USD]). Therefore, assessments from not only the clinical perspective, but also the economic perspective are necessary when reviewing and considering the future conditions for CGP use in Japan. Previous studies on patients with advanced or recurrent non-squamous non-small cell lung cancer (NSQ-NSCLC) in the United States and CanadaCitation7,Citation8 evaluated the clinical and economic impacts of expanded CGP use, including CGP testing from early-line treatment, and reported that the incremental costs due to expanded CGP use on health insurance were limited. However, no such study has been conducted or published so far in Japan.
Therefore, the aims of this study are to evaluate the impact of CGP testing before SoC on both medical costs and the clinical outcomes in Japan from 2022 to 2026, by comparing the two scenarios in which CGP testing can or cannot be used before SoC.
Methods
Target population
The target population for analysis in this study was defined as untreated patients who have been newly diagnosed with advanced or recurrent cancer of any of the following three types: biliary tract cancer (BTC), NSQ-NSCLC, and colorectal cancer (CRC). SoC for with and without each type of druggable alterations of these three cancer types has been defined in the clinical practice guidelines, and the medical need for CGP testing before SoC in these cancer types is considered growing in Japan.
BTC is one of the cancer types with poorest prognosis, and the treatment options as SoC recommended in the clinical practice guidelines for BTC are very limitedCitation9–11. On the other hand, multiple new matched therapies based on druggable alterations for BTC, such as tyrosine kinase inhibitors (TKIs) and immune checkpoint inhibitors (ICIs) are currently under development worldwide and expected to become new options for BTC in the future. Therefore, the medical need for CGP testing is expected to increase in the future. Regarding NSQ-NSCLC and CRC, the concept of cancer genomic medicine has been developed and incorporated into the current clinical practice guidelinesCitation12,Citation13, with multiple matched therapies indicated for different druggable alterations already approved or under development in Japan. For this reason, they are also considered as cancer types with high and increasing need for CGP testing before SoC.
Model structure
To evaluate the impact of CGP testing before SoC on both the clinical outcome and medical costs, a decision-tree model considering different treatment options based on various druggable alterations detected from genomic tests for each cancer type was constructed in this study (). The analysis model was constructed using Microsoft Excel.
Figure 1. Economic analysis modeling approach. BTC, biliary tract cancer; CGP, comprehensive genomic profiling; CRC, colorectal cancer; NSQ-NSCLC, non-squamous non-small cell lung cancer; OS, overall survival; SoC, standard of care.
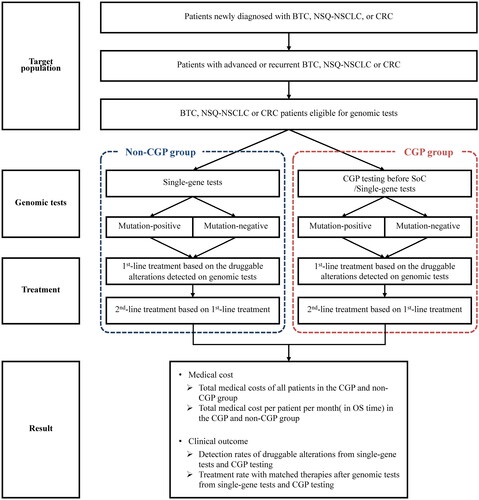
We compared the clinical outcomes and medical costs between two alternative scenarios as two groups; Group 1: either conventional single-gene tests or CGP testing can be performed before SoC (CGP group); Group 2: only conventional single-gene tests can be performed before SoC while CGP testing is not available (non-CGP group). Each kind of genomic test was set to be conducted a maximum of once only before SoC (re-test for any reason was not considered in the model). All druggable alterations clinically meaningful for treatment selection, and all treatment options (including best supportive care) considered based on clinical practice guidelines for each cancer type in this analysis are shown in . Concerning NSQ-NSCLC, genomic test with the Oncomine Dx Target Test Multi-CDx System for four druggable alterations (EGFR, ALK, ROS1, and BRAF) was also considered in the same category as conventional single-gene tests. The approval status of each drug therapy under the NHI in Japan was based on the time of analysis execution (December 2020).
Table 1. Druggable alterations and treatment options.
In both the CGP and non-CGP group, the first-line treatments were selected based on the druggable alterations detected from genomic tests. Patients with no druggable alteration detected from the genomic tests were regarded as druggable-alteration negative. For each treatment option selected as first-line treatment, the progression-free survival (PFS) reported from relevant clinical trials was set as the treatment period in the model. After the completion of first-line treatments, second-line treatments were selected based on different cancer types and previous treatments. Second-line treatments were set to be continued until death in the model. Based on the results of the SHIVA studyCitation14, which found no improvement in prognosis with matched therapy after second-line treatment, the analysis assumed that patients would survive according to the OS reported in clinical trials of the treatment option selected as first-line treatment.
Since CGP testing are not performed on patients with poor condition after SoC, it is not recommended that CGP testing should be performed after SoC in the clinical practice guidelines for each cancer type in Japan. However, it is possible for some patients to take the CGP test after SoC completion. To reflect the current clinical practice in Japan, the use of CGP testing after SoC completion was set as possible for the non-CGP group’s patients in the model.
Parameters
Patient population
The predicted incidence numbers of untreated and newly diagnosed patients with BTC, NSQ-NSCLC, or CRC between 2022 and 2026 were extracted from the results of MHLW-granted research conducted by the National Cancer Center in JapanCitation15. Among all patients, the number with advanced or recurrent BTC, NSQ-NSCLC, or CRC who can undergo genomic tests before SoC was estimated based on market research data from Chugai Pharmaceutical Co., Ltd. (). The rate of undergoing CGP testing before SoC was also estimated from the market research data. The testing rate of each single-gene test in patients with various cancer types was extracted from a claims database (Medical Data Vision Co., Ltd, Tokyo, Japan) between April 2020 and March 2021 (). The number of patients who can undergo genomic tests before SoC was estimated by multiplying “Estimated number of patients”, “Rate of diagnosis of advanced or recurrent” and “Rate of patients to be treated by drug therapy”. Of these, the number of patients who performed CGP testing before SoC was estimated by multiplying “The number of patients who can undergo genomic tests before SoC” and “Rate of undergoing CGP testing before SoC”. The number of patients undergoing CGP testing after SoC completion in the non-CGP group was estimated based on the registration records from June 2019 to June 2021 published by the Center for Cancer Genomics and Advanced Therapeutics (C-CAT) in JapanCitation16.
Table 2. Population of each cancer type.
Table 3. Parameters associated with the genomic tests.
Clinical parameters
The following clinical parameters of various cancers were set in the model: the success probability of genomic tests without any test failure, detection rates of druggable alterations, OS/PFS of each treatment option, and the selection rate of each different treatment option based on the results of genomic tests.
In this analysis, the success probabilities of all conventional single-gene tests and CGP testing were set with a same value reported from Gandara et al.Citation18. The detection rate of each druggable alteration from conventional single-gene tests and CGP testing was set based on the available literature (). The OS and PFS data for each treatment were cited from the relevant clinical trial report (Supplementary Table 1). The selection rates of various first- and second-line treatment options based on druggable alterations were set in the model based on the interview responses from two clinical experts in each cancer type who had substantial clinical experience in the field of oncology in Japan (Supplementary Tables 2–4).
Table 4. Parameters associated with druggable alterations.
Resources/treatments costs
The analysis was conducted from the public healthcare payer’s perspective, and only direct medical costs were considered. The direct medical costs included the costs of genomic tests, the costs of first- and second-line drug treatments, and other inpatient and outpatient treatment-related costs associated with medical procedures.
The fees of all the genomic tests and medical procedures were set based on the latest price revision in 2020, while the prices of all the drugs were set based on the latest price revision in the NHI in Japan in 2021 (). The inpatient and outpatient treatment-related costs were calculated based on the definitions of the standard clinical process (established from the interview responses of clinical experts), and the latest fee for each medical procedure (Supplementary Tables 5).
Table 5. Cost of genomic tests.
Outcomes
The economic analysis, we first estimated the total medical costs of all patients in the CGP and non-CGP group, respectively, and then calculated the medical costs per patient per month (in OS time) in each group. For the clinical outcomes of this study included: (1) detection rates of druggable alterations from single-gene tests and CGP testing before SoC; (2) treatment rate with matched therapies after genomic tests. Matched therapy was defined as a drug therapy using TKIs or ICIs for BTC and CRC, and a drug therapy using TKIs for NSQ-NSCLC.
The change in the medical costs per patient per month in the CGP group versus the non-CGP group was further evaluated for its uncertainty in the sensitivity analysis described below.
Sensitivity analysis
To confirm the impact of each parameter on the results of economic analysis (the change in the medical costs per patient per month between the CGP and non-CGP group), one-way sensitivity analysis was conducted using the 2026 data and represented by tornado diagrams. The range of each parameter in one-way sensitivity analysis was set based on the 95% confidence interval from the original reference source if there is one, or ±20% of the basic value.
Scenario analysis
In the scenario analysis, we expanded our analysis to include the possibility of clinical trial participation in the model. Some patients may be detected with specific druggable alterations which are not covered by the existing conventional single-gene tests but identifiable with CGP testing. Generally, there is no approved matched therapy for patients with such druggable alterations under the NIH in Japan; however, we considered a possibility provided by CGP testing to participate in clinical trials if there are some innovative matched therapies currently under development in Japan or overseas.
The rate of clinical trial participation in such cases was estimated through interviews with clinical experts in each cancer type. The medical costs of participation in a clinical trial were assumed to be paid entirely by the manufacturer (Supplementary Table 6).
Results
This model analysis was conducted for each year, respectively, among the 5 years from 2022 to 2026. No major differences in the results among these 5 years was found, except for minor changes due to cancer incidence growth every year. The following results show the analysis of 2026 as a representative year with the highest predicted cancer incidence.
Advanced or recurrent BTC
Among the 27,570 untreated patients who will be newly diagnosed with BTC in 2026, 8600 advanced or recurrent BTC patients were estimated to take genomic tests (). In the non-CGP group, the only approved conventional single-gene test for BTC was microsatellite instability (MSI) test and the detection rate of MSI-High was estimated to be 0.08% in all patients (7/8600). On the other hand, CGP testing was estimated to be conducted in a total of 2062 patients instead of single-gene tests in the CGP group, detecting multiple druggable alterations at the same time, with the detection rates as follows: 1.75% (36/2062) NTRK-positive, 3.22% (66/2062) FGFR2-positive, and 1.04% (21/2062) as MSI-High. The total detection rate of any druggable alterations from the CGP-testing population was 6.01%, which was 5.93% higher than that of the single-gene test ().
Figure 2. Detection rates of druggable alterations. (A) Advanced or recurrent BTC in 2026. (B) Advanced or recurrent NSQ-NSCLC in 2026. (C) Advanced or recurrent CRC in 2026. . BTC: biliary tract cancer; CGP: comprehensive genomic profiling; CRC: colorectal cancer; MSI: microsatellite instability; MSS: microsatellite stable; NSQ-NSCLC: non-squamous non-small cell lung cancer.
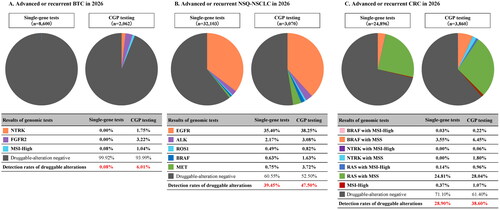
Table 6. Population to be analyzed and number of patients in whom genomic tests was performed.
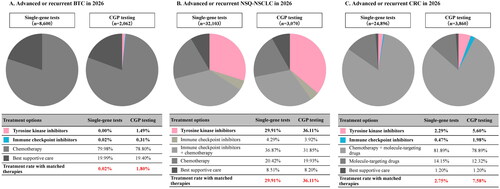
Regarding the first-line treatment rate with matched therapy, no TKI was used, while ICIs were used in 0.02% (2/8600) of patients taking the single-gene test. On the other hand, TKIs and ICIs were used in 1.49% (31/2062) and 0.31% (6/2062) of patients undergoing CGP testing, respectively. The total treatment rate with matched therapy in the CGP-testing population was 1.80%, which was 1.78% higher than that of the single-gene test ().
Figure 3. Treatment rate with matched therapies after genomic tests. (A) Advanced or recurrent BTC in 2026. (B) Advanced or recurrent NSQ-NSCLC in 2026. (C) Advanced or recurrent CRC in 2026. BTC: biliary tract cancer; CGP: comprehensive genomic profiling; CRC: colorectal cancer; NSQ-NSCLC: non-squamous non-small cell lung cancer.
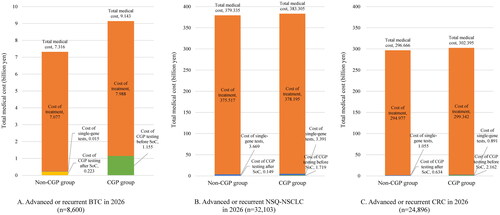
As a yearly estimation of 2026, the cost of CGP testing before SoC in the CGP group was 1.155 billion JPY (8.556 million USD). The total medical costs in the CGP group were 9.143 billion JPY (67.726 million USD) (total cost of genomic test: 1.155 billion JPY [8.556 million USD]; cost of treatments: 7.988 billion JPY [59.170 million USD]), while those in the non-CGP group were 7.316 billion JPY (54.193 million USD) (total cost of genomic tests: 0.239 billion JPY [1.770 million USD]; cost of treatments: 7.077 billion JPY [52.422 million USD]; ). The total cost of genomic tests increased by 0.916 billion JPY (6.785 million USD), while the cost of treatments increased by 0.911 billion JPY (6.748 million USD) in the CGP group compared with the non-CGP group.
Figure 4. Total medical cost. (A) Advanced or recurrent BTC in 2026. (B) Advanced or recurrent NSQ-NSCLC in 2026. (C) Advanced or recurrent CRC in 2026. BTC, biliary tract cancer; CGP, comprehensive genomic profiling; CRC, colorectal cancer; NSQ-NSCLC, non-squamous non-small cell lung cancer; SoC, standard of care.
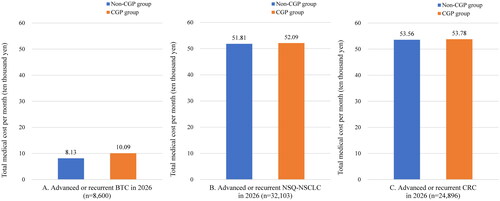
The medical costs per patient per month in the CGP and non-CGP group were 100,900 and 81,300 JPY (747 and 602 USD), respectively, with a change of 19,600 JPY (145 USD) (). Results for the years before 2026 can be found in the Supplementary data.
Figure 5. Total medical cost per patient per month (in OS time). (A) Advanced or recurrent BTC in 2026. (B) Advanced or recurrent NSQ-NSCLC in 2026. (C) Advanced or recurrent CRC in 2026. . BTC, biliary tract cancer; CGP, comprehensive genomic profiling; CRC, colorectal cancer; NSQ-NSCLC, non-squamous non-small cell lung cancer; OS, overall survival.
Advanced or recurrent NSQ-NSCLC
Among the 153,686 untreated patients who will be newly diagnosed with NSQ-NSCLC in 2026, 32,103 advanced or recurrent NSQ-NSCLC patients were estimated to take genomic tests (). In the non-CGP group, the detection rates of druggable alterations from conventional single-gene tests were estimated to be 35.40% (11,365/32,103) EGFR-positive, 2.17% (698/32,103) ALK-positive, 0.49% (158/32,103) ROS1-positive, 0.63% (201/32,103) BRAF-positive, and 0.75% (242/32,103) MET-positive. The total detection rate of druggable alterations was 39.45%. On the other hand, CGP testing was estimated to be conducted in a total of 3070 patients instead of single-gene tests in the CGP group, with the detection rates as follows: 38.25% (1174/3070) EGFR-positive, 3.08% (95/3070) ALK-positive, 0.82% (25/3070) ROS1-positive, 1.63% (50/3070) BRAF-positive, and 3.72% (114/3070) MET-positive. The total detection rate of any druggable alterations from the CGP-testing population was 47.50%, which was 8.05% higher than that of the single-gene test ().
Regarding the first-line treatment rate with matched therapy, TKIs were used in 29.91% (9602/32,103) of patients taking the single-gene test compared with 36.11% (1108/3070) of patients undergoing CGP testing (6.20% higher than that of the single-gene test; ).
As a yearly estimation of 2026, the cost of CGP testing before SoC in the CGP group was 1.719 billion JPY (12.733 million USD). The total medical costs in the CGP group were 383.305 billion JPY (2.839 billion USD) (total cost of genomic tests: 5.110 billion JPY [37.852 million USD]; cost of treatments: 378.195 billion JPY [2.801 billion USD]), while those in the non-CGP group were 379.335 billion JPY (2.810 billion USD) (total cost of genomic tests: 3.818 billion JPY [28.281 million USD]; cost of treatments: 375.517 billion JPY [2.782 billion USD]; ). The total cost of genomic tests increased by 1.292 billion JPY (9.570 million USD), while the cost of treatments increased by 2.678 billion JPY (19.837 million USD) in the CGP group compared with non-CGP group.
The medical costs per patient per month in the CGP and non-CGP group were 520,900 and 518,100 JPY (3859 and 3838 USD), respectively, with a change of 2900 JPY (21 USD) (. Results for the years before 2026 can be found in the Supplementary data.
Advanced or recurrent CRC
Among the 158,498 untreated patients who will be newly diagnosed with CRC in 2026, 24,896 advanced or recurrent CRC patients were estimated to take genomic tests (). In the non-CGP group, the detection rate of druggable alterations from conventional single-gene tests were estimated to be 3.57% (890/24,896) BRAF-positive, 24.95% (6212/24,896) RAS-positive, and 0.37% (93/24,896) MSI-High. The total detection rate of druggable alterations was 28.90%. On the other hand, CGP testing was estimated to be conducted in a total of 3860 patients instead of single-gene tests, with the detection rates as follows: 6.67% (257/3860) BRAF-positive, 1.86% (72/3860) NTRK-positive, 29.00% (1119/3,860) RAS-positive, and 1.07% (41/3860) MSI-High. The total detection rate of any druggable alterations from the CGP-testing population was 38.60%, which was 9.70% higher than that of the single-gene test ().
Regarding the first-line treatment rate with matched therapy, TKIs and ICIs were used in 2.29% (569/24,896) and 0.47% (117/24,896) of all patients taking single-gene tests, respectively. For patients undergoing CGP testing, TKIs and ICIs were used in 5.60% (216/3,860) and 1.98% (77/3,860) of all patients, respectively. The total treatment rate with matched therapy of the CGP-testing population was 7.58%, which was 4.83% higher than that of the single-gene test ().
As a yearly estimation of 2026, the cost of CGP testing before SoC in the CGP group was 2.162 billion JPY (16.015 million USD). The total medical costs in the CGP group were 302.395 billion JPY (2.240 billion USD) (total cost of genomic tests: 3.053 billion JPY [22.615 million USD]; cost of treatments: 299.342 billion JPY [2.217 billion USD]), while those in the non-CGP group were 296.666 billion JPY (2.198 billion USD) (total cost of genomic tests: 1.689 billion JPY [12.511 million USD]; cost of treatments: 294.977 billion JPY [2.185 billion USD]; ). The total cost of genomic tests increased by 1.364 billion JPY (10.104 million USD), while the cost of treatments increased by 4.365 billion JPY (32.333 million USD) in the CGP group compared with non-CGP group.
The medical costs per patient per month in the CGP and non-CGP group were 537,800 and 535,600 JPY (3,984 and 3,967 USD), respectively, with a change of 2,200 JPY (16 USD) (). Results for the years before 2026 can be found in the Supplementary data.
Sensitivity analysis
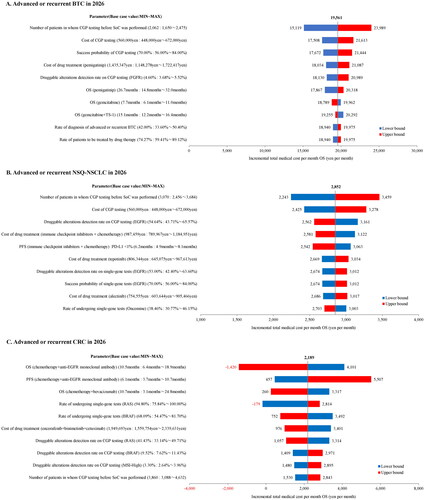
The results of the one-way sensitivity analyses for BTC and NSQ-NSCLC showed that the most impactful parameter on the change of medical costs per patient per month between the CGP and non-CGP group was the CGP-testing rate before SoC. On the other hand, the most impactful parameter in CRC was the OS of chemotherapy plus anti-EGFR monoclonal antibody combination therapy ().
Figure 6. Results of one-way sensitivity analysis. (A) Advanced or recurrent BTC in 2026. (B) Advanced or recurrent NSQ-NSCLC in 2026. (C) Advanced or recurrent CRC in 2026. . BTC: biliary tract cancer; CGP: comprehensive genomic profiling; CRC: colorectal cancer; NSQ-NSCLC: non-squamous non-small cell lung cancer; OS: overall survival; PFS: progression free survival; SoC: standard of care.
Scenario analysis
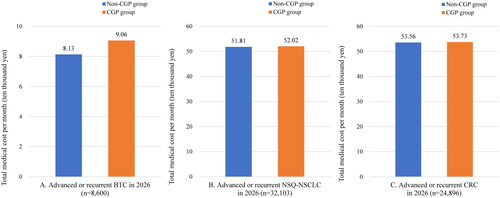
When considering clinical trial participation additionally in the scenario analysis, the total detection rate of druggable alterations from CGP testing was estimated to increase to 39.21% in BTC, 61.31% in NSQ-NSCLC, and 50.11% in CRC. Furthermore, the treatment rates with matched therapy including clinical trial participation increased to 13.80% in BTC, 37.49% in NSQ-NSCLC, and 8.74% in CRC. The difference in medical costs per patient per month between the CGP and non-CGP group for all cancer types were reduced to 9300 JPY (69 USD) per month in BTC, 2200 JPY (16 USD) per month in NSQ-NSCLC, and 1700 JPY (13 USD) per month in CRC ().
Figure 7. Total medical cost per patient per month (in OS time) (Results of scenario analysis). (A) Advanced or recurrent BTC in 2026. (B) Advanced or recurrent NSQ-NSCLC in 2026. (C) Advanced or recurrent CRC in 2026. BTC, biliary tract cancer; CGP, comprehensive genomic profiling; CRC, colorectal cancer; NSQ-NSCLC, non-squamous non-small cell lung cancer; OS, overall survival.
Discussion
Our study evaluated the impacts of CGP testing before SoC, versus non-CGP testing, on clinical outcomes and medical costs in BTC, NSQ-NSCLC and CRC patients in Japan.
For all the three cancer types included in this study, the CGP group has been estimated as having higher detection rates of druggable alterations and higher treatment rates with matched therapies, compared with the non-CGP group. This will contribute to greater survival benefits for patients as the incremental OS benefit per patient initiated on matched therapy or detected with druggable alterations. These results suggest that the appropriate use of CGP testing before SoC, and genomically matched therapies based on these test results may help more physicians to make better clinical decisions and bring more health benefits for cancer patients.
Nowadays, more and more research and development are being conducted to explore new matched therapies with molecule-targeting drugs. In many cases, matched therapies for patients with specific druggable alterations were reported to improve OS significantly compared with conventional chemotherapies. As recent examples, the median OS of crizotinib for ROS1 fusion-positive NSQ-NSCLC was 51.4 monthsCitation26, and the median OS of alectinib for ALK fusion-positive NSQ-NSCLC was not reached even at 81.0 months of follow-upCitation27. For MSI-High CRC, the median OS of pembrolizumab had not been reached at 60.0 months of follow-upCitation28,Citation29. Such new matched therapies have appeared to revolutionize the conventional treatments with median overall survivals greater than 50 months. Witnessing further development and introduction of matched therapies for cancer treatment in future, the medical need for CGP testing from an early line is expected to continue growing in Japan.
Regarding the economic impacts of CGP testing, the increases in medical costs per patient per month due to CGP introduction before SoC were shown to be limited to only 19,600, 2900, and 2200 JPY (145, 21 and 16 USD) per month for BTC, NSQ-NSCLC and CRC, respectively. The total annual costs of CGP testing before SoC in 2026 for BTC, NSQ-NSCLC, and CRC were estimated to be 1.155, 1.719, and 2.162 billion JPY (8.556, 12.733 and 16.015 million USD), respectively, while the actual increase in the total costs of genomic tests were shown to be even smaller at 0.916, 1.292, 1.364 billion JPY (6.785, 9.570, 10.104 million USD), respectively, attributed to the replacement of conventional single-gene tests with CGP. Compared with other medical devices in the oncology field approved in the past 5 years in Japan and their predicted peak annual costs published by the MHLW, such as Archer MET Companion Diagnostic System for NSCLC (peak annual cost: 2.62 billion JPY [19.41 million USD]), Boron Neutron Capture Therapy (BNCT) systems for head and neck cancer (peak annual cost: 5.2 billion JPY [38.5 million USD]), and Oncomine Dx Target Test Multi-CDx System for NGS in NSCLC (peak annual cost: 1.77 billion JPY [13.11 million USD]), the predicted economic impact of CGP testing before SoC was considered at a similar level or lower.
When considering the possibility of clinical trial participation in the scenario analysis, the CGP group has shown the potential to identify more druggable alterations and provide more opportunities of access to matched therapies in an early line, compared with the base-case analysis. The increase in medical costs for the public healthcare payer in the CGP group was also reduced due to the savings of medical costs in clinical trials. The extent of such improvement in clinical outcomes and cost reduction is based on how many eligible patients with druggable alterations can participate in the corresponding clinical trials and will fluctuate with complex factors. However, with the future development in systems like advanced medical care, and patient-proposed healthcare services in Japan, CGP testing is expected to make more contributions in this field.
We also compared our study results with the reports from previous published studies. Signorovitch et al. and Johnston et al. evaluated the impact of expanded CGP testing on clinical outcomes and medical costs for NSCLC patients in the United States and Canada, respectively. Both reported an improvement in clinical outcomes such as OS with a limited cost increase (<0.1 USD per insuree per month)Citation7,Citation8. Proudman et al. reported the study results in CRC patients in the United States that showed replacing 20% of usual testing with CGP was associated with up to a 0.003 USD testing cost increase per insuree per month, and 15.5 additional patients with an opportunity for genomically informed care (12.7 patients for treatment and 2.8 for clinical trials)Citation30. Although the healthcare environment and NHI system in Japan differs from other countries, which makes the direct comparison of results difficult, the results from our study have shown some similar trends with these previous studies. Furthermore, this study is the first study to evaluate the impacts of CGP testing in BTC, which has limited prior research but considered to have growing needs for genomic tests and innovative treatments to further improve its unsatisfying prognosis.
Regarding future prospects for cancer genomic medicine in Japan, the appropriate use of CGP testing is expected to reduce the workload, test time and tissue exhaustion for physicians and patients, to help improve the overall quality of healthcareCitation2–4. Clinical decision-making well informed by CGP testing before SoC will also help physicians find the best possible treatment option in an early line, reducing the use of ineffective treatments that may lead to harm and wasted medical costs. Furthermore, the large amount of genomic information generated from CGP testing can be stored in databases and utilized for the future development of innovative therapies and personalized cancer medicine, which is currently being discussed and undertaken in Japan.
Limitations
There are some limitations of this study including: (1) Since the rate of undergoing CGP testing before SoC, which affects the analysis results was estimated based on market research data, there are uncertainties. Therefore, the results of the analysis may differ depending on the market and policy in Japan at the time of CGP testing before SoC is introduced into clinical practice; (2) clinical parameters extracted from the results of clinical trials and epidemiological studies considered to be the best were set in the analysis model, although there were no studies directly compared the clinical benefit of CGP testing before SoC and after SoC; (3) Based on the results of the SHIVA studyCitation14, the analysis assumed that patients would survive according to the OS of first-line treatment. Because limited targeted therapies were available at the time of the SHIVA study, outcomes may differ from currently approved targeted therapies. In that case, it may underestimate the effectiveness of targeted therapies selected as second-line treatment in either group, especially for some patients in the non-CGP group who performed CGP testing after SoC; (4) the impacts of CGP testing and various matched therapies on patient’s quality of life (QoL) were not considered in the analysis due to data gaps and study feasibility, which is expected to be studied in future; (5) only those druggable alterations with existing matched therapies at the time of analysis were considered in the analysis. While the information of other genomic alterations from CGP testing is also provided in clinical practice, the impact and possible value of such information was not reflected in this study due to model complexity; (6) improvement in clinical outcomes including OS and QoL may help more cancer patients come back to society, increasing the labor force while reducing the care service burden in Japan, which would have additional benefits from a socioeconomic perspective.
If future studies reveal data related to the limitation, it is expected that the usefulness of CGP testing before SoC will be evaluated in terms of cost effectiveness as well as budget impact in Japan.
Conclusion
The present study suggested that CGP testing before SoC may contribute to better clinical outcomes with the increase of druggable alteration detections and higher treatment rate with matched therapies in various solid cancer types. The estimated medical costs increase with CGP testing before SoC, but such increase is considered limited and controllable.
It is expected that CGP testing from an early-line treatment without the limitation of SoC completion as a precondition, and the selection of genomically matched therapies based on the test results will be realized in future, to improve overall healthcare quality for cancer patients in Japan.
Transparency
Declaration of financial/other relationships
WT, TO, KF, HA, and SI are employed by Chugai Pharmaceutical Co., Ltd. KH, an employee of CRECON Medical Assessment Inc., performed analyses for this study and received consulting fee for it from Chugai Pharmaceutical Co., Ltd. YM received consulting fee from Chugai Pharmaceutical Co., Ltd., and speaker honoraria from Tsumura & Co. HS and KT received consulting fee from Chugai Pharmaceutical Co., Ltd. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose. The Editor in Chief and an Editorial Board Member helped with adjudicating the final decision on this paper.
Author contributions
All authors gave a substantial contribution to this work. WT, TO, KF, HA, and SI conceived the concept of this study. KH constructed the analysis model. WT, KH, TO, KF, HA, and SI drafted the manuscript. YM, HS, and KT provided an interpretation of this research and revised the manuscript. All authors have reviewed and approved the manuscript.
Supplemental Material
Download Zip (477.4 KB)Acknowledgement
The authors thank all clinical experts who participated in the interviews on cancer treatments during the model analysis in Japan. This study result was accepted for the 60th Annual Meeting of the Japanese Society of Clinical Oncology (JSCO2022) as an oral presentation.
Additional information
Funding
References
- Ministry of Health, Labour and Welfare. Basic plan to promote cancer control programs. https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/0000183313.html.
- Johnston KM, Sheffield BS, Yip S, et al. Costs of in-house genomic profiling and implications for economic evaluation: a case example of non-small cell lung cancer (NSCLC). J Med Econ. 2020;23(10):1123–1129.
- Yu TM, Morrison C, Gold EJ, et al. Budget impact of next-generation sequencing for molecular assessment of advanced non-small cell lung cancer. Value Health. 2018;21(11):1278–1285.
- Dong L, Wang W, Li A, et al. Clinical next generation sequencing for precision medicine in cancer. Curr Genomics. 2015;16(4):253–263.
- Sunami K. The challenges of drug provision in cancer genome medicine. Journal of Hereditary Tumors. 2022;21(4):101–104.
- Japanese Society of Medical Oncology, Japan Society of Clinical Oncology, Japanese Cancer Association. Clinical practice guidance for next-generation sequencing in cancer diagnosis and treatment; Edition 2.1; [cited 2020 May 15]. Available from: https://www.jca.gr.jp/researcher/topics/2020/files/20200518.pdf.
- Signorovitch J, Zhou Z, Ryan J, et al. Budget impact analysis of comprehensive genomic profiling in patients with advanced non-small cell lung cancer. J Med Econ. 2019;22(2):140–150.
- Johnston KM, Sheffield BS, Yip S, et al. Comprehensive genomic profiling for non-small-cell lung cancer: health and budget impact. Curr Oncol. 2020;27(6):e569–e577.
- Nagino M, Hirano S, Yoshitomi H, et al. Clinical practice guidelines for the management of biliary tract cancers 2019: the 3rd English edition. J Hepatobiliary Pancreat Sci. 2021; Jan28(1):26–54.
- Okusaka T, Nakachi K, Fukutomi A, et al. Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: a comparative multicentre study in Japan. Br J Cancer. 2010;103(4):469–474.
- Morizane C, Okusaka T, Mizusawa J, et al. Combination gemcitabine plus S-1 versus gemcitabine plus cisplatin for advanced/recurrent biliary tract cancer: the FUGA-BT (JCOG1113) randomized phase III clinical trial. Ann Oncol. 2019;30(12):1950–1958.
- Akamatsu H, Ninomiya K, Kenmotsu H, et al. The Japanese lung cancer society guideline for non-small cell lung cancer, stage IV. Int J Clin Oncol. 2019; Jul24(7):731–770.
- Hashiguchi Y, Muro K, Saito Y, et al. Japanese society for cancer of the colon and rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol. 2020; Jan25(1):1–42.
- Tourneau CL, Delord JP, Gonçalves A, et al. Molecularly targeted therapy based on tumour molecular profiling versus conventional therapy for advanced cancer (SHIVA): a multicentre, open-label, proof-of-concept, randomised, controlled phase 2 trial. Lancet Oncol. 2015; Oct16(13):1324–1334.
- Cancer Registry and Statistics. Cancer Prediction. 2015–2039. Cancer information service, National Cancer Center, Japan. https://ganjoho.jp/reg_stat/statistics/dl/index.html.
- Center for Cancer Genomics and Advanced Therapeutics. Status of cancer multi-gene panel Tests as of June 2021. https://for-patients.c-cat.ncc.go.jp/registration_status/.
- Nikkei Medical Oncology survey. Tests of EGFR, ALK, ROS1, and BRAF gene mutations in 60% before primary treatment for lung cancer. December 3, 2020. https://medical.nikkeibp.co.jp/leaf/mem/pub/search/cancer/report/202012/568090.html.
- Gandara DR, Paul SM, Kowanetz M, et al. Blood-based tumor mutational burden as a predictor of clinical benefit in non-small-cell lung cancer patients treated with atezolizumab. Nat Med. 2018;24(9):1441–1448.
- Nakamura H, Arai Y, Totoki Y, et al. Genomic spectra of biliary tract cancer. Nat Genet. 2015;47(9):1003–1010.
- Akagi K, Oki E, Taniguchi H, et al. Nationwide large-scale investigation of microsatellite instability status in more than 18,000 patients with various advanced solid cancers. J Clin Oncol. 2020;38(4_suppl):803–803.
- Saito M, Shiraishi K, Kunitoh H, et al. Gene aberrations for precision medicine against lung adenocarcinoma. Cancer Sci. 2016;107(6):713–720.
- Shimizu J, Masago K, Saito H, et al. Biomarker tests for personalized, first-line therapy in advanced nonsquamous non-small cell lung cancer patients in the real world setting in Japan: a retrospective, multicenter, observational study (the BRAVE study). Ther Adv Med Oncol. 2020;12:1758835920904522.
- Matsushita K. BRAF genetic tests in colorectal cancer treatment from the viewpoint of clinical laboratory. Modern Media. 2019;65(5):9–15.
- Guidelines for tumor-agnostic genome medicine: Japan society of clinical oncology/Japanese society of medical oncology. Guidelines for tumor-agnostic genome medicine in adult/childhood advanced solid cancer. 2nd version. Kanehara-Shuppan. 2019.
- Ishiguro M, Watanabe T, Yoshino T, et al. Examination of the KRAS gene mutation rate in colorectal cancer patients in Japan. 65th annual meeting of The Japan Society of Coloproctology; 2010.
- Shaw AT, Riely GJ, Bang YJ, et al. Crizotinib in ROS1-rearranged advanced non-small-cell lung cancer (NSCLC): updated results, including overall survival, from PROFILE 1001. Ann Oncol. 2019;30(7):1121–1126.
- Yoshioka H, Hida T, Nokihara H, et al. Final OS analysis from the phase III j-alex study of alectinib (ALC) versus crizotinib (CRZ) in Japanese ALK-inhibitor naïve ALK-positive non-small cell lung cancer (ALK + NSCLC). J Clin Oncol. 2021;39(15_suppl):9022–9022.
- André T, Shiu KK, Kim TW, et al. Pembrolizumab in microsatellite-instability-high advanced colorectal cancer. N Engl J Med. 2020;383(23):2207–2218.
- André T, Shiu KK, Kim TW, et al. Final overall survival for the phase III KN177 study: pembrolizumab versus chemotherapy in microsatellite instability-high/mismatch repair deficient (MSI-H/dMMR) metastatic colorectal cancer (mCRC). J Clin Oncol. 2021;39(15_suppl):3500–3500.
- Proudman D, DeVito NC, Belinson S, et al. Comprehensive genomic profiling in advanced/metastatic colorectal cancer: number needed to test and budget impact of expanded first line use. J Med Econ. 2022;25(1):817–825.
