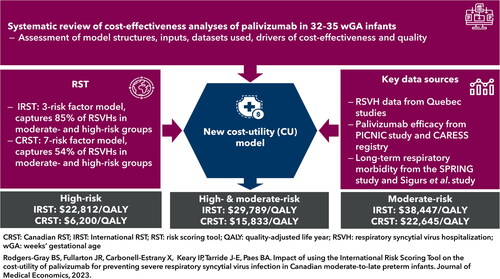
Abstract
Background and objective
To assess the cost-utility of palivizumab versus no prophylaxis in preventing severe respiratory syncytial virus (RSV) infection in Canadian moderate-to-late preterm (32–35 weeks’ gestational age) infants using an (i) International Risk Scoring Tool (IRST) and (ii) Canadian RST (CRST).
Methods
A decision tree was developed to assess cost-utility. Infants assessed at moderate- and high-risk of RSV-related hospitalization (RSVH) by the IRST or CRST received palivizumab or no prophylaxis and then progressed to either (i) RSVH; (ii) emergency room/outpatient medically attended RSV-infection (MARI) or (iii) were uninfected/non-medically attended. Infants admitted to intensive care could incur mortality (0.43%). Respiratory morbidity was accounted in all uninfected surviving infants for 6 years or 18 years (RSVH/MARI). Palivizumab efficacy (72.2% RSVH reduction) and hospital outcomes were from the Canadian CARESS, PICNIC and RSV-Quebec studies. Palivizumab costs (50 mg: CAN$752; 100 mg: $1,505) were calculated from Canadian birth statistics combined with a growth algorithm. Healthcare/payer and societal costs (May 2022; 1.5% discounting) were included.
Results
Cost per quality-adjusted life year (QALY) was $29,789 with the IRST (0.79 probability of being <$50,000) and $15,833 with the CRST (0.96 probability). The model was most sensitive to utility scores, long-term sequelae and palivizumab cost. Vial sharing improved the incremental cost-utility ratio (IRST: $22,319; CRST: $9,231).
Conclusions
Palivizumab was highly cost-effective (vs no prophylaxis) in Canadian moderate-to-late preterm infants using either the IRST or CRST. The IRST has fewer risk factors than the CRST (3 vs 7, respectively), captures more potential RSVHs (85% vs 54%) and provides another option to guide cost-effective RSV prophylaxis in Canada.
Introduction
Respiratory syncytial virus (RSV) is a major childhood pathogen estimated to cause approximately 12.9 million lower respiratory tract infections (LRTI) resulting in 2.2 million hospitalizations (RSVH) and >66,000 deaths annually in children <1 year worldwideCitation1. RSV-LRTI in premature infants and young children has a temporal association with long-term wheezing and asthma, which can persist throughout childhood and late adolescenceCitation2–4, and may result in obstructive lung function in adulthoodCitation5,Citation6. It is well-established that infants born moderate-to-late preterm (32–35 weeks’ gestational age; wGA) are among those at the greatest risk of RSV-LRTI, with approximately 4% of these infants being hospitalizedCitation7,Citation8.
In Canadian infants born moderate-to-late preterm, recently published studies have estimated that the average direct healthcare cost of an RSVH to be CAD$16–28,500Citation9–11. In Quebec alone, the direct cost of RSVHs across two RSV seasons was estimated at $3.7 millionCitation10. Importantly, the true financial burden of RSV-LRTI is likely to be considerably greater, as these estimates do not account fully for medically-attended RSV infections (MARI) – those that require medical attention, but not hospital admission – and the costs of managing the long-term sequelae. RSV-LRTI has also been associated with a considerable financial (indirect costs) and quality of life (QoL) impact on the families of affected infantsCitation12–14.
At present, immunoprophylaxis with the monoclonal antibody palivizumab (Synagis; AstraZeneca Canada) remains the only widely available intervention to prevent RSV-LRTI in high-risk infants, including those born at ≤35wGA. Prior to 2015, guidelines from the Canadian Paediatric Society (CPS) recommended palivizumab prophylaxis for those otherwise healthy infants born 32–35wGA assessed at moderate- (7.1%) or high- (18.7%) risk of RSVH by the Canadian Risk Scoring Tool (CRST)Citation15,Citation16. This was based on a cost-utility analysis published in 2010 that reported an incremental cost-utility ratio (ICUR) versus no prophylaxis for high-risk infants to be $5,274/quality-adjusted life year (QALY) and $34,438/QALY for moderate-risk infantsCitation17. Since 2015, however, the CPS has recommended that palivizumab use be largely restricted to those otherwise healthy infants born ≤30wGA and <6 months old (reaffirmed in 2021)Citation18. Guidelines from the Canadian National Advisory Committee on Immunization (NACI) are similar to those from the CPS although they state that palivizumab may also be considered for premature infants of 30–32wGA and age <3 months who are at high-risk for RSV infection from day care attendance or presence of another preschool child or children in the homeCitation19. A recent review has since reported marked differences in prophylaxis policies for moderate-to-late preterm infants across Canada, resulting in inequities in careCitation20. This variability in policies may reflect limitations in funding and human resources to deliver prophylaxis programs, differing interpretation of the evidence for palivizumab efficacy, variation in regional RSV epidemiology, and/or local pressures or advocacyCitation20.
Since the publication of the last Canadian cost-utility analysis of palivizumab, a new International RST (IRST) that can identify 32–35wGA infants at moderate- (3.3%) or high- (9.5%) risk of RSVH has been developed and validatedCitation21. There has also been a wealth of new data published on the epidemiology and burden of RSV-LRTI for inclusion in cost-analyses. The primary objective of this study was to undertake a new cost-utility analysis of palivizumab prophylaxis guided by the IRST in Canadian moderate-to-late preterm infants using the latest clinical data. A secondary objective was to provide an updated analysis of the cost-utility of palivizumab using the CRST.
Methods
This cost-utility analysis assessed whether palivizumab use in moderate-to-late preterm infants, defined as birth at 32–35wGA, who are at moderate- and high-risk of RSV-LRTI, as determined by use of the IRSTCitation21 at birth, is a cost-effective strategy versus no prophylaxis in Canada. Costs were considered from the societal perspective in the base-case and within all scenario analyses unless specifically stated otherwise. The cost-utility analysis is reported commensurate with the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) 2022Citation22.
Systematic review
Prior to building our updated model, a systematic literature review was undertaken. The research question addressed was “How has the health economics of palivizumab in moderate-to-late preterm infants been modelled and assessed?”. The objective of the review was to inform our model design by identifying the key characteristics of previous cost-effectiveness and cost-utility models, including the varied modelling methodologies, model structures and parameters used, the most influential variables, and the use of novel approaches to handle important calculations such as infant weight at the time of palivizumab dosing. The review identified 20 cost-analyses of palivizumab use in infants born at 32–35wGA or a subset thereof (Supplementary Materials 1).
Model structure
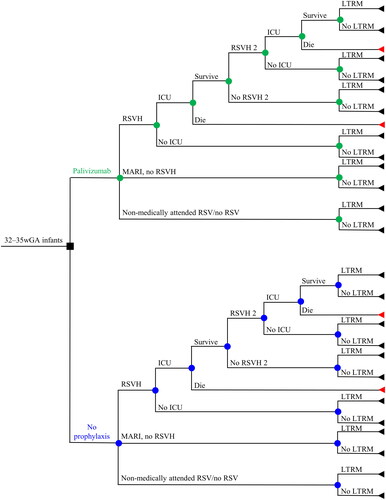
A decision tree cost-utility model was employed wherein prophylaxed/non-prophylaxed infants have an RSV-LRTI resulting in either hospitalization, emergency room/outpatient attendance (MARI), or were uninfected/non-medically attended (). Hospitalized infants could be subsequently admitted to the intensive care unit (ICU) or not, and those admitted to the ICU were assumed to either die or survive. For surviving infants, the possibility of a second identical cycle of RSVH was included. Any surviving infant, regardless of RSV infection status, could potentially experience some degree of childhood wheezing and/or asthma (up to 18 years in the base case for those with RSVH/MARI). A life-time horizon was used to adequately capture the effects of mortality and long-term respiratory sequelae associated with RSV. The model was built using the Canadian Agency for Drugs and Technologies in Health (CADTH) and International Society for Pharmacoeconomics and Outcomes Research (ISPOR) guidelinesCitation23,Citation24.
Figure 1. Decision tree describing the clinical pathway used in the model. Nodes represent points where more than one event is possible; the square node represents the decision addressed by the model. Branches represent the possible events that patients may experience. Triangles represent the decision tree endpoints. Abbreviations. ICU, intensive care unit; LTRM, long-term respiratory morbidity; MARI, medically-attended RSV infection; RSV, respiratory syncytial virus; RSVH, RSV-related hospitalization.
Model assumptions and inputs
IRST
The IRST was developed to predict the risk of RSVH in infants born 32–35wGA and thereby improve the targeting of RSV preventionCitation21. The IRST was derived using logistic regression from a pooled dataset of six observational studies (including the Pediatric Investigators Collaborative Network on Infections in Canada [PICNIC] studyCitation7) which included risk factor data on 13,475 infants born 32–35wGA, 484 of whom had RSVH at ≤1 year (Table S1 in Supplementary Materials 2)Citation21. The IRST included three risk factors: birth between three months before to two months after the start of the RSV season; household smoking and/or smoking while pregnant; and siblings (excluding those as part of a multiple birth with the subject) and/or day care attendance (Figure S1 in Supplementary Materials 2). Cut-off scores for RSVH were ≤19 for low- (RSVH rate: 1.0%), 20–45 for moderate- (3.3%), and 50–56 (9.5%) for high-risk infants (Table S2 in Supplementary Materials 2). The IRST had an area under the receiver operating characteristic curve (AUROC) of 0.773 (sensitivity 68.9%; specificity 73.0%)Citation21, with a score of 1 representing perfect predictive accuracy.
Palivizumab efficacy
The overall efficacy of palivizumab was derived from two Canadian studies: CARESS (Canadian Registry of SynagisCitation25,Citation26) and PICNICCitation7. CARESS collected data on 25,003 children receiving palivizumab between 2005 and 2017 and reported an RSVH rate of 1.0% (70/6,894) in 32–35wGA infantsCitation26,Citation27. The RSVH rate in 33–35wGA infants not receiving prophylaxis in the PICNIC study was 3.6% (66/1,832)Citation7, equating to an overall efficacy rate for palivizumab of 72.2%. The impact of palivizumab on the incidence of respiratory morbidity was calculated using data from Simoes et al.Citation28, Blanken et al.Citation29 and Yoshihara et al.Citation30
RSVH
To reflect differences in the risk of hospitalization across the scoring tools and risk groups, the RSVH rates for infants not receiving palivizumab were drawn directly from the published data for each RST, since these populations excluded infants that received prophylaxis ()Citation16,Citation21. The RSVH rates in those infants receiving prophylaxis was calculated by applying the efficacy of palivizumab (72.2% risk reduction) to the non-prophylaxed rates. In the base case, the RSVH rates for prophylaxed versus non-prophylaxed infants scored at moderate- or high-risk by the IRST were 1.8% and 6.3%, respectively.
Table 1. RSV-related hospitalization (RSVH) rates associated with risk scoring tools.
For infants not receiving palivizumab the incidence of RSVH, ward length of stay (LOS) and ICU admission rate and LOS were not available in the PICNIC study and thus data were drawn from the studies by Papenburg et al.Citation10,Citation31, which examined healthcare utilization and costs of RSV among 33–35wGA infants from Quebec (). For infants that received palivizumab and had RSVH, the equivalent information from CARESSCitation27 was used, except for ward LOS, which was again based on data from Papenburg et al.Citation31. For any infants admitted to ICU, a mortality rate of 0.43% was appliedCitation32,Citation33.
Table 2. Input parameters associated with RSVH and MARI.
Medically-attended RSV infection (MARI)
It was assumed that 2.95% of infants receiving prophylaxis would experience MARI, which was the rate among infants >32–35wGA in the palivizumab arm of the motavizumab trialCitation34. The proportion of these infants presenting to outpatients only, emergency department (ED) only, or both was calculated by applying the rates for these outcomes from the US REPORT (Respiratory Events Among Preterm Infants Outcomes and Risk Tracking) studyCitation35 in infants born 32–35wGA who did not receive prophylaxis (). For non-prophylaxed infants, the rates of MARI observed in REPORTCitation35 were adjusted according to the relative difference in the RSVH rate in this study versus that observed in PICNICCitation7 (3.47% vs 3.6%, respectively).
Long-term respiratory morbidity
The algorithm by Sanchez-Luna et al.Citation36 was used to calculate the effect of palivizumab on long-term respiratory sequelae. Sanchez-Luna et al.Citation36 estimated the risk reduction associated with prophylaxis versus no prophylaxis for 32–35wGA infants up to six years old, using data from the Spanish SPRINGCitation37 study combined with three palivizumab studiesCitation28–30. The same approach was adopted for the current model for years 0–6, and extended to 18 years of age using prospectively collected data on rates of respiratory morbidity in Swedish infants published by Sigurs et al.Citation2,Citation38–40. To adjust for differences in the baseline rates of respiratory morbidity, the Sigurs et al. dataCitation38–40 was fitted to the lower rates observed in SPRINGCitation37. This approach was chosen because SPRINGCitation37 was specific to infants born 32–35wGA, whereas Sigurs et al.Citation2 considered all infants aged <1 year with a RSVH and therefore provided a less gestational age-specific estimate.
For infants with RSVH or MARI, long-term respiratory morbidity was assumed to persist until 18 years of age, albeit with lower rates for the latter (Table S3 in Supplementary Materials 2). Infants without an RSV infection or an RSV infection not requiring any medical management were assumed to have some background respiratory morbidity for up to six years, using the same rates as for MARI.
Utilities
To estimate the impact of RSVH on infants’ QoL, the utility value (0.60) used in the US analysis by Weiner et al.Citation41 was adopted and applied only for the duration of the hospital stay (). This value was derived from a prospective study of 46 children born at ≤35wGA with RSVH and 45 chronologic age-matched controls from the same geographic area with a history of prematurity. The study used various measures to assess the QoL impact of hospitalization on the infant and parentCitation13. Following discharge, a utility value of 0.88 was applied, which was taken from the UK study by Greenough et al.Citation42, which followed premature infants (median 27wGA) with chronic lung disease and RSVH (and a control group without RSVH) until five years of age. For infants with MARI without long-term respiratory morbidity and those without an RSV-LRTI or an RSV infection not requiring medical management, the control group utility value (0.95) from Greenough et al.Citation42 was used. The values from Greenough et al.Citation42 were applied until five years of life, after which perfect health was assumed.
For the impact of long-term respiratory morbidity on QoL, no RSV-specific utility data were identified, so a value of 0.79, as reported in children with mild-and-moderate asthma symptoms, was utilizedCitation43.
Costs
Costs were calculated on an individual infant level and reflected the acquisition and administration costs associated with palivizumab as well as the costs of RSVH, MARI, ICU admission, the ongoing costs associated with respiratory morbidity, and societal costs (). All costs were taken from publicly available Canadian sourcesCitation10,Citation44–49 and were adjusted using the Consumer Price Index for Canada (CPI)Citation50 as of May 2022, with a 1.5% discount applied commensurate with the CADTH guidelinesCitation23.
Table 3. Direct and indirect costs.
The cost of palivizumab was calculated using the Canadian list price (50 mg: $752; 100 mg: $1,505) and the lowest combination of vials per infant weight, assuming no vial sharing, and 100% compliance. Using the average birthweight of a Canadian 32–35wGA infant (2,308 g)Citation51 and assuming an even spread of births across the year, infant weight at palivizumab administration was predicted using the growth algorithm described by Narayan et al.Citation52. Predicated on this approach, the average cost of palivizumab in the base case was estimated to be $6,215/infant, when nurse administration was included (average number of injections required: 4.09).
Model outputs
Outcomes were expressed as the cost per QALY (incremental cost-utility ratio [ICUR]) for palivizumab versus no intervention.
Sensitivity analyses
As per best practiceCitation53, uncertainty was assessed by way of probabilistic (PSA) and deterministic (DSA) sensitivity analysis. In the absence of measures of dispersion in the source materials, analyses employed limits of plus or minus 10% (PSA) and 20% (DSA) on the values of the tested variables. DSA results were used for purely qualitative purposes to rank and thereby identify the most influential variables. Within the PSA (10,000 Monte Carlo simulations), gamma distribution was used as the default for costs, beta distribution for utilities and hospitalization-associated rates, and normal distribution for discount rates and mortality (Table S4 in Supplementary Materials 2). In addition, probabilistic DSA (PDSA) was undertaken based upon the methodology of Vreman et al.Citation54 For the PDSA, the default number of probabilistic repetitions per plotted point was 1,000.
Scenario analyses
The main alternate scenario was to use the seven-variable CRSTCitation16 (AUROC: 0.762; sensitivity: 68.2%, sensitivity: 71.9%) to guide prophylaxis (Figure S2 in Supplementary Materials 2). The CRST has cut-off scores of 0–48, 49–64 and 65–100 for low- (RSVH rate: 1.7%), moderate- (7.1%) and high-risk (18.7%) infants, respectively (Table S2 in Supplementary Materials 2). The single most important risk factor in the CRST for determining an infant’s risk of RSVH is birth during the RSV season, which accounts for 25% of the overall risk score (this compares to 11% with the IRST)Citation16,Citation21. This implies that almost all infants classified at moderate- or high-risk by the CRST are born during the RSV season (and therefore weigh less than low-risk infants); this has implications for the cost of palivizumab as it is dosed by weight. For the analyses using the CRST, infant weight was adjusted to reflect the low-, moderate- and high-risk categories (Figure S3 in Supplementary Materials 2). This resulted in an average palivizumab cost of $5,370 (average 4.01 injections) per moderate/high-risk infant. To further explore the relative benefits of the two RSTs, an updated version of the CRST was created in which the RSVH rate in the low-risk group was brought into line (standardized) with that of the IRST (changing from 1.7% to 1.0%), with the rates for the moderate- and high-risk groups being adjusted accordingly ().
Other scenario analyses included (Table S5 in Supplementary Materials 2): (i) assessment of high- and moderate-risk groups separately; (ii) no discounting; (iii) 3% discounting; (iv) exclusion of societal costs; (v) inclusion of vial sharing (assuming 5% wastage); (vi) alternative value for overall palivizumab efficacy (RSVH rate 1.8% vs 10.1% for placebo; risk reduction: 82.2%) taken from the IMpactCitation55,Citation56 (registrational) study; (vii) alternative value for loss of productivity due to RSV mortality ($8,805,732Citation57); (viii) MARI rate from the REPORTCitation35 study utilized directly without adjustment for baseline RSVH rate in non-prophylaxed infants; (ix) MARI rate in non-prophylaxed infants calculated according to the rate in prophylaxed infants with the efficacy of palivizumab removed; (x) limiting the duration of respiratory sequelae to 13 or 6 years; (xi) reducing mortality rate to 0%; (xii) discounting the acquisition cost of palivizumab by 10%, 20% or 30%.
Model validation
The model structure, data and assumptions were first validated through consultation with globally recognized clinical experts in RSV (XCE and BP) and compared with other cost-analyses of palivizumab identified during the systematic review (Supplementary Materials 1). Model coding was validated using an alternative model built independently from the main model but based on the same structure, assumptions, and data. Finally, the calculation from the non-prophylaxed arm of the model was applied to the Spanish FLIP-2Citation58 (Risk Factors Linked to RSV Infection Requiring Hospitalization in Premature Infants) population of 5,441 infants born at 32–35wGA, and the resulting outcomes (predicted vs actual) compared: number of RSVHs; ICU admittance; and mortality rate.
Software
The model was developed using Microsoft Excel 365.
Results
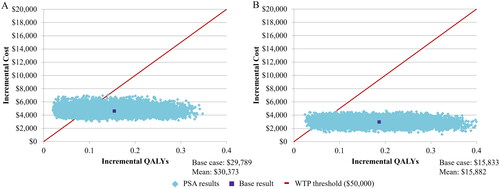
Palivizumab prophylaxis increased treatment costs and indirect costs but led to a reduction in direct healthcare costs and improved QoL compared to no prophylaxis (). In the base case, prophylaxis in moderate- and high-risk infants, as guided by the IRST, resulted in an ICUR of $29,789. When considering high- and moderate-risk infants separately, the corresponding ICURs were $22,812 and $38,447, respectively.
Table 4. Costs and utilities for palivizumab versus no prophylaxis using IRST and CRSTa.
Sensitivity analyses
In the base case, a mean ICUR of $30,373 was predicted in the PSA (vs no-prophylaxis), with a probability of 0.79 to be below a $50,000 willingness to pay (WTP) threshold and 0.91 probability at a $75,000 WTP threshold ( and ). The DSA found the model to be most sensitive to utility scores, long-term morbidity rate, palivizumab cost, and the RSVH rate in non-prophylaxed infants (Figure S4 in Supplementary Materials 2).
Figure 2. Acceptability curve for palivizumab versus no prophylaxis in moderate- and high-risk infants born at 32–35wGA as guided by the IRST and CRST. Abbreviations. IRST, International Risk Scoring Tool; QALY, quality-adjusted life year; wGA, weeks’ gestational age.
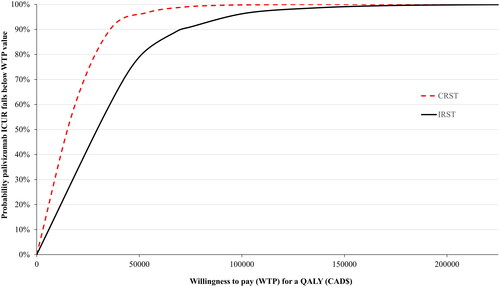
Figure 3. Incremental cost-effectiveness plane for palivizumab prophylaxis in moderate- and high-risk 32–35wGA infants identified by (A) the IRST and (B) the CRST. Results are based on the probabilistic sensitivity analysis after 10,000 Monte Carlo simulations. The solid line represents an acceptability threshold of $50,000/QALY. Abbreviations. CRST, Canadian Risk Scoring Tool; IRST, International Risk Scoring Tool; PSA, probabilistic sensitivity analysis; QALY, quality-adjusted life year; wGA, weeks’ gestational age; WTP, willingness to pay.
The PDSA results indicated that the 10 most influential variables individually identified by the DSA broadly responded linearly, except for the variable “utility with significant respiratory sequelae”, which produced an exponential effect on the ICUR when increased ever closer to parity with long-term normal health (Figure S5 in Supplementary Materials 2). The results strongly suggest that the qualitative DSA observations on the relative impact of these variables are valid.
Scenario analyses
Using the CRST to identify moderate- and high-risk infants provided an ICUR of $15,833 (). The PSA resulted in incremental costs of $15,882/QALY with a 0.96 probability of palivizumab being cost-effective at the $50,000 WTP threshold and 0.99 at $75,000 ( and Citation3). The point estimate for moderate- and high-risk infants using the standardized CRST was $23,841/QALY.
Vial sharing (N.B. palivizumab vials are single use) and using the IMpact trialCitation56 to derive palivizumab efficacy resulted in improved ICURs of $22,319 and $17,883, respectively, for moderate- and high-risk infants using the IRST (). Unsurprisingly, discounting the cost of palivizumab reduced the ICUR, with a 10% discount resulting in a value of $25,837. Conversely, reducing the period of long-term respiratory morbidity in the base case to 13 and 6 years increased the ICUR to $38,391 and $53,728, respectively. The remaining scenarios all had a relatively minor impact on the ICUR.
Table 5. Results of scenario analyses.
Model validation
No marked differences between the model predictions and actual outcomes of FLIP-2Citation58 were found for number of RSVHs (196 vs 202, respectively), ICU admittance (19.0% vs 17.9%), and mortality rate (0.003% vs 0%).
Discussion
Moderate-to-late preterm infants (born 32–35wGA) comprise a substantial population at increased risk of severe RSV-LRTICitation7,Citation8,Citation59. Identification of those 32–35wGA infants with additional risk factors and thus at greatest risk of severe RSV-LRTI can help guide the cost-effective use of palivizumabCitation17,Citation60. In this first cost-utility analysis incorporating the IRST, palivizumab was found to be highly cost-effective when targeted to Canadian infants assessed at moderate- and high-risk of RSVH, with an ICUR of $29,789 (mean $30,373/QALY) versus no prophylaxis. While there is no strict per health outcome WTP threshold in Canada, a value of approximately $50,000/QALY is commonly citedCitation61, with the PSA of the base case generating a 79% probability of being below this level. The ICUR also improved significantly when vial sharing (5% wastage) was considered in a scenario analysis ($22,319), which is a practice recommended in various provincial prophylaxis policiesCitation62,Citation63. The fact that palivizumab remained below the $50,000 threshold ($38,447) in moderate-risk infants alone (without vial sharing) further highlights the robustness of the base case result.
An updated assessment of CRST-guided prophylaxis also found this to be a highly cost-effective strategy, with an ICUR of $15,833 (mean $15,882; 96% probability of being below a $50,000 WTP threshold) in moderate- and high-risk infants. At face value, the CRST appears to be the more favourable of the two RSTs to use, with the ICUR approaching half that of the IRST ($15,833 vs $29,789, respectively). While the two RSTs have a similar level of good predictive accuracy (both AUROCs >0.75) and are well validated, a salient difference, aside from the number of included risk factors (IRST: 3 vs CRST: 7), is that the CRST ascribes a higher baseline RSVH rate (which was found to be a key driver of the ICUR in the DSA) to high- and moderate-risk infants than the IRST (10.9% vs 6.3%, respectively; )Citation16,Citation21. This results in the IRST assigning approximately twice as many infants as being at moderate-risk compared to the CRST (19.9% vs 9.8%, respectively)Citation16,Citation21. In practice, and taking into consideration the relative sensitivity and specificity of the tools, this means that approximately 30% more RSVHs would potentially be prevented by using the IRST than the CRST (85% vs 54%, respectively), but at a cost of a similar proportion of infants receiving prophylaxis unnecessarily (Table S6 in Supplementary Materials 2)Citation64. The impact of the difference in RSVH rates underlying the two RSTs was explored with the standardized CRST (where the RSVH rate in the low-risk group was set at 1.0% to match that of the IRST and the high and moderate rates adjusted accordingly), which produced an ICUR closer to that of the IRST ($23,841 in moderate- and high-risk infants). It should also be recognized that the way infant weight was adjusted to reflect risk category for the CRST (as birth in season was the preeminent risk factor) favours this tool over the IRST (where such an adjustment was not appropriate), as this ultimately led to a proportionally higher number of smaller infants being prophylaxed and, therefore, lower palivizumab cost. Subtle variation in the epidemiological characteristics of the data underpinning the two RSTs might also have influenced the results, such as the IRST encompassing infants born 32wGA while the CRST did not, as well as differences in follow-up duration (). Ultimately, the decision about which RST to use should take into consideration familiarity and ease of use (CRST used in Canada for over a decade vs fewer risk factors to score in the IRST) as well as available budget versus maximising outcomes (CRST more cost-effective vs potential for more RSVHs prevented with the IRST but with a proportion of infants receiving unnecessary prophylaxis). Maximizing the prevention of RSVHs is perhaps particularly relevant, when considering the stress on healthcare resources if there was a contemporaneous upsurge in COVID hospitalizations.
Table 6. Comparison of key attributes of the IRSTCitation21 and the CRSTCitation16.
When compared to the previous cost-utility analysis of palivizumab using the CRSTCitation17, the ICURs in the high-risk group were broadly similar, although approximately 15% higher in the current analysis ($5,274 vs $6,200, respectively). The higher ICUR in the current analysis probably relates to the updated assumptions behind RSV-associated mortality and the palivizumab efficacy rate. In the previous analysis,Citation17,Citation60 a mortality rate of 8.1% (from Sampalis 2003Citation65) was applied to all infants with RSVH, and the efficacy of palivizumab at preventing RSVH was 82% (from the IMpact StudyCitation55,Citation56). The current analysis used more conservative and arguably more representative values of 0.43%Citation32,Citation33 for mortality, and applied this to infants in the ICU only, and a palivizumab efficacy rate of 72.2%Citation7,Citation27. When the efficacy rate from IMpactCitation56 was used in a scenario analysis, the ICUR in the current model fell to $2,476 using the CRST, demonstrating the influence of this input on the ICUR. Similarly, the influence of the higher mortality rate used in the Canadian study is most evident in the high-risk infants. This results from the higher mortality rate and higher hospitalization rate combining to produce a large relative increase in the number of infants dying, and therefore incurring a loss of productivity, in the no palivizumab arm versus the palivizumab arm.
For moderate-risk infants, however, it was the current analysis that provided the more favourable ICUR ($22,645 vs $34,438 reported previouslyCitation17) This almost certainly relates to how long-term respiratory morbidity was modelled in the two analyses. In the current analysis, long-term sequelae was modelled until 18 years (using data from SPRINGCitation37 and Sigurs et al.Citation2,Citation39,Citation40) with palivizumab efficacy appliedCitation28–30, an annual cost attached to its management, and all surviving infants assumed to be at some level of risk according to palivizumab use and RSVH status (Table S3 in Supplementary Material 2). By contrast, the earlier analysisCitation60 included respiratory morbidity for up to 16 years but extrapolated this from the study by Greenough et al.Citation42 that measured a utility difference retrospectively and at one timepoint (5 years) between infants with and without RSVH. This provided a smaller disutility for respiratory morbidity (0.07) than in the current analysis (0.09–0.16 depending on RSVH status) applied for two years shorter duration and with costs and disutility applied according to RSVH status. The morbidity rates and utility values used in the current analysis reflect the substantial evidence supporting the association of RSVH in early childhood (including specifically in 32–35wGA infants in SPRINGCitation37) with long-term respiratory morbidity that can persist into adulthoodCitation2–4,Citation66. Indeed, 12 of the 20 health economic models identified in the systematic review considered long-term respiratory sequelae, notably all those published in the last five yearsCitation36,Citation52,Citation67,Citation68. Long-term respiratory morbidity and utility scores were identified as important contributors to the ICUR in the DSA. Importantly, however, even when this sequela was restricted to 13 or 6 years, palivizumab remained cost-effective ($38,391/QALY and $53,728/QALY, respectively). summarises the differences in the key variables as discussed above.
Table 7. Comparison of key parameters in the Canadian cost-utility analysisCitation17 with CRST versus the current analysis with IRST (base case analyses).
A clinically relevant component of this new cost-utility analysis was the inclusion of MARI, which is now widely recognized as a significant contributor to the overall burden of RSVCitation1. None of the 20 analyses identified by the systematic review included formal assessment of MARI. A key challenge to including MARI (which may explain its absence from earlier analyses) is the limited data on rates in 32–35wGA infants and the efficacy of palivizumab in its prevention. For this reason, MARI was explored in two scenarios, both of which found palivizumab to remain highly cost-effective versus no prophylaxis in moderate- and high-risk infants identified by the IRST ($30,120/QALY and $32,390/QALY). It is also reassuring that the calculated rate of MARI used for non-prophylaxed infants of 12.2% was not too dissimilar from the rate of 9.5% in the placebo arm of the nirsevimab preterm (29–34 completed wGA) studyCitation69.
Extensive sensitivity analyses including DSA, PDSA and PSA were conducted to deal with uncertainty associated with key model inputs. Data limitations included the availability of short- and long-term QoL outcomes for infants born 32–35wGA, with the DSA and PDSA identifying long-term utility scores as a key driver of the ICUR. The Greenough et al.Citation42 data are almost 20 years old but arguably remain the best available source for utilities following RSVH in premature infants and are used as the basis of many published assessmentsCitation17,Citation36,Citation52. More recent estimates of the impact of RSV on QoL are available; however, they are less applicable to our population of interest than the older data. For example, two recent studies by Hodgson et al.Citation70 and Diez-Gandia et al.Citation71 were not restricted to infants (rather children less than five and two years, respectively), included term as well as preterm children, and, importantly, did not provide a disutility for RSV hospitalized infants, rather the QoL of all RSV-positive children regardless of disease severity. The estimate of the disutility during hospitalization is based on data from 2005; a recent US systematic review of studies that derived utility lost per RSV episode identified only 7 such studiesCitation72, none of which provided more relevant data than that used herein. It is also important to note that this disutility is applied only for the duration of the hospital stay, which is for an average of six days. Therefore, it does not impact the outputs of the model to any significant degree but does reflect the clinical situation. Utility data related to long-term morbidity following RSV-LRTI are also lacking, so a surrogate value was used from a follow-up of children with asthmaCitation43, which was the same approach taken in the cost-utility analysis by Sanchez-Luna et al.Citation36. Another limitation is that while gestational age-specific data are available for long-term respiratory morbidity (SPRINGCitation37) and the efficacy of palivizumabCitation28–30 in its prevention for up to six years, beyond this time point more general sources (Sigurs et al.Citation2,Citation39,Citation40) and data extrapolation had to be employed. However, given the increasingly recognized temporal association between RSV LRTI and long-term morbidityCitation2–4,Citation37,Citation66 and the documented beneficial effects of palivizumab in this regard up to age three yearsCitation29–31, we believe that our approach is clinically justified. It would also have been preferable if there were available one Canadian source of data for palivizumab efficacy at preventing RSVH and hospital resource use, although the three key sources used (PICNICCitation7, CARESSCitation27, and QuebecCitation10,Citation28) were all large, well conducted studies in the relevant population. That the base case was well below $50,000/QALY (point estimate: $29,789; mean from PSA: $30,373) and that the model was validated against outcomes from FLIP-2Citation58, afford confidence in the reliability of the results. Moreover, the base case considered the list price of palivizumab, and local discounts will inevitably reduce costs and improve the ICUR associated with the use of palivizumab, particularly in combination with local policies recommending vial sharing () Citation62,Citation63. Further benefits of prophylaxis not captured in this cost-utility analysis include avoiding the mental health impact on families/carers of having a child with severe RSV-LRTI and the additional costs associated with managing patients from indigenous and remote communitiesCitation12–14,Citation72,Citation73.
The current model can readily be adapted for cost analyses of palivizumab in other countries, particularly those with a similar economic status and RSV seasonality; it would principally involve updating to local costs and including country-specific data on RSVH rates, as available. There are several new preventive interventions for RSV on the near horizon, including the extended half-life monoclonal antibodies nirsevimab (which is already licenced in Europe) and clesrovimab and maternal and childhood vaccinesCitation74. These newer interventions have the potential to provide protection to all infants, although differences in their immunization coverage as well as financial, logistical and manufacturing constraints, and, importantly, parental choice, will likely mean that a variety of options will be needed to fully address the burden of RSVCitation75–77. The overall structure and decision tree of the current model could be adapted for economic evaluations of these newer therapies.
Conclusions
This current analysis demonstrates that selectively targeting palivizumab to those Canadian 32–35wGA infants at moderate- and high-risk of severe RSV-LRTI using the IRST (or CRST) is a highly cost-effective strategy versus no prophylaxis. Adoption of a RST within prophylaxis policies for 32–35wGA infants will help to ensure the more equitable use of palivizumab across Canada.
Transparency
Declaration of financial/other relationships
XCE and BP have received research funding and/or compensation as advisor/lecturer from AstraZeneca and Sanofi. BRG, IK and JF employer has received payment from AstraZeneca for work on various projects. JET has nothing to disclose. The funding body had no role in the design and development of the cost-utility model, the interpretation of the data or writing the manuscript. Peer reviewers on this manuscript have received an honorarium from JME for their review work but have no other relevant financial relationships to disclose.
Author contributions
All authors contributed to the study conception and design, the development of the publication, maintained control over the final content, and approved publication.
Previous presentations
Information on the model and its results have been previously presented as abstracts and posters at Scientific and Medical meetings.
Supplemental Material
Download PDF (503.8 KB)Supplemental Material
Download PDF (580.7 KB)Acknowledgements
The authors would like to thank Krista Lanctôt (Medical Outcomes and Research in Economics Research Group, Sunnybrook Health Sciences Centre, University of Toronto, Toronto, Ontario, Canada) for organising additional analyses of the CARESS database.
Data availability statement
All data generated or analysed during this study are included in this published article [and its supplementary information files].
References
- Li Y, Wang X, Blau DM, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. Lancet. 2022;399(10340):2047–2064.
- Sigurs N, Aljassim F, Kjellman B, et al. Asthma and allergy patterns over 18 years after severe RSV bronchiolitis in the first year of life. Thorax. 2010;65(12):1045–1052.
- Coutts J, Fullarton J, Morris C, et al. Association between respiratory syncytial virus hospitalization in infancy and childhood asthma. Pediatr Pulmonol. 2020;55(5):1104–1110.
- Fauroux B, Simões EAF, Checchia PA, et al. The burden and long-term respiratory morbidity associated with respiratory syncytial virus infection in early childhood. Infect Dis Ther. 2017;6(2):173–197.
- Shi T, Ooi Y, Zaw EM, et al. Association between respiratory syncytial virus-associated acute lower respiratory infection in early life and recurrent wheeze and asthma in later childhood. J Infect Dis. 2020;222(Suppl 7):S628–S633.
- Berry CE, Billheimer D, Jenkins IC, et al. A distinct low lung function trajectory from childhood to the fourth decade of life. Am J Respir Crit Care Med. 2016;194(5):607–612.
- Law B, Langley J, Allen U, et al. The pediatric investigators collaborative network on infections in Canada study of predictors of hospitalization for respiratory syncytial virus infection for infants born at 33 through 35 completed weeks of gestation. Pediatr Infect Dis J. 2004;23(9):806–814.
- Lanari M, Anderson EJ, Sheridan-Pereira M, et al. Burden of respiratory syncytial virus hospitalisation among infants born at 32–35 weeks’ gestational age in the Northern hemisphere: pooled analysis of seven studies. Epidemiol Infect. 2020;148:e170.
- Thampi N, Knight BD, Thavorn K, et al. Health care costs of hospitalization of young children for respiratory syncytial virus infections: a population-based matched cohort study. CMAJ Open. 2021;9(4):E948–E956.
- Papenburg J, Saleem M, Teselink J, et al. Cost-analysis of withdrawing immunoprophylaxis for respiratory syncytial virus in infants born at 33–35 weeks gestational age in Quebec: a multicenter retrospective study. Pediatr Infect Dis J. 2020;39(8):694–699.
- Rafferty E, Paulden M, Buchan S, et al. Evaluating the individual healthcare costs and burden of disease associated with RSV across age groups. Pharmacoeconomics. 2022;40(6):633–645.
- Carbonell-Estrany X, Dall’Agnola A, Fullarton JR, et al. Interaction between healthcare professionals and parents is a key determinant of parental distress during childhood hospitalisation for respiratory syncytial virus infection (European RSV outcomes study [EROS]). Acta Paediatr. 2018;107(5):854–860.
- Leidy NK, Margolis MK, Marcin JP, et al. The impact of severe respiratory syncytial virus on the child, caregiver, and family during hospitalization and recovery. Pediatrics. 2005;115(6):1536–1546.
- Mitchell I, Defoy I, Grubb E. Burden of respiratory syncytial virus hospitalizations in Canada. Can Respir J. 2017;2017:4521302.
- Robinson JL. Preventing respiratory syncytial virus infections. Paediatr Child Health. 2011;16(8):487–490.
- Sampalis JS, Langley J, Carbonell-Estrany X, et al. Development and validation of a risk scoring tool to predict respiratory syncytial virus hospitalization in premature infants born at 33 through 35 completed weeks of gestation. Med Decis Making. 2008;28(4):471–480.
- Smart KA, Paes BA, Lanctôt KL. Changing costs and the impact on RSV prophylaxis. J Med Econ. 2010;13(4):705–708.
- Robinson JL, Le Saux N, Canadian Paediatric Society, Infectious Diseases and Immunization Committee, et al. Preventing hospitalizations for respiratory syncytial virus infection. Paediatr Child Health. 2015;20(6):321–333.
- National Advisory Committee on Immunization. Recommended use of palivizumab to reduce complications of respiratory syncytial virus infection in infants; [accessed 2023 Mar 15]. Available from: https://www.canada.ca/en/public-health/services/publications/vaccines-immunization/palivizumab-respiratory-syncitial-virus-infection-infants.html#a9
- Jalink M, Langley JM. The palivizumab patchwork: variation in guidelines for respiratory syncytial virus prevention across Canadian provinces and territories. Paediatr Child Health. 2021;26(2):e115–e120.
- Blanken MO, Paes B, Anderson EJ, et al. Risk scoring tool to predict respiratory syncytial virus hospitalisation in premature infants. Pediatr Pulmonol. 2018;53(5):605–612.
- Husereau D, Drummond M, Augustovski F, et al. Consolidated health economic evaluation reporting standards 2022 (CHEERS 2022) explanation and elaboration: a report of the ISPOR CHEERS II good practices task force. Value Health. 2022;25(1):10–31.
- Canadian Agency for Drugs and Technologies in Health. Guidelines for the economic evaluation of health technologies. 4th ed.; [cited March 2017; accessed 2022 Aug 31]. Available from: https://www.cadth.ca/sites/default/files/pdf/guidelines_for_the_economic_evaluation_of_health_technologies_canada_4th_ed.pdf
- Caro JJ, Briggs AH, Siebert U, et al. ISPOR-SMDM modeling good research practices task force. Modeling good research practices–overview: a report of the ISPOR-SMDM modeling good research practices task force -1. Value Health. 2012;15(6):796–803.
- Mitchell I, Paes BA, Li A, et al. CARESS: the Canadian registry of palivizumab. Pediatr Infect Dis J. 2011;30(8):651–655.
- Lanctôt K, Saleem M, Kim D. Canadian RSV evaluation study of Synagis® [CARESS] annual report 2016–2017. Toronto (ON): Medical Outcomes and Research in Economics; 2018.
- CARESS final data for 32-35 wGA infants. Sunnybrook health sciences centre. Toronto (ON): Sunnybrook; 2022.
- Simoes E, Groothuis JR, Carbonell-Estrany X, et al. Palivizumab prophylaxis, respiratory syncytial virus, and subsequent recurrent wheezing. J Pediatr. 2007;151(1):34–42.
- Blanken MO, Rovers MM, Molenaar JM, et al. Respiratory syncytial virus and recurrent wheeze in healthy preterm infants. N Engl J Med. 2013;368(19):1791–1799.
- Yoshihara S, Kusuda S, Mochizuki H, et al. Effect of palivizumab prophylaxis on subsequent recurrent wheezing in preterm infants. Pediatrics. 2013;132(5):811–818.
- Papenburg J, Defoy I, Massé E, et al. Impact of the withdrawal of palivizumab immunoprophylaxis on the incidence of respiratory syncytial virus (RSV) hospitalizations among infants born at 33 to 35 weeks’ gestational age in the province of Quebec, Canada: the RSV-Quebec study. J Pediatric Infect Dis Soc. 2021;10(3):237–244.
- Wang D, Cummins C, Bayliss S, et al. Immunoprophylaxis against respiratory syncytial virus (RSV) with palivizumab in children: a systematic review and economic evaluation. Health Technol Assess. 2008;12(36):1–86. iii,ix–x.
- Wang D, Bayliss S, Meads C. Palivizumab for immunoprophylaxis of respiratory syncytial virus (RSV) bronchiolitis in high-risk infants and young children: systematic review and additional economic modelling of subgroup analyses. Health Technol Assess. 2011;15(5):iii–iiv. 1–124.
- Carbonell-Estrany X, Simões EAF, Dagan R, et al. Motavizumab for prophylaxis of respiratory syncytial virus in high-risk children: a noninferiority trial. Pediatrics. 2010;125(1):e35–e51.
- Ambrose CS, Anderson EJ, Simões EA, et al. Respiratory syncytial virus disease in preterm infants in the U.S. born at 32-35 weeks gestation not receiving immunoprophylaxis. Pediatr Infect Dis J. 2014;33(6):576–582.
- Sanchez-Luna M, Burgos-Pol R, Oyagüez I, et al. Cost-utility analysis of palivizumab for respiratory syncytial virus infection prophylaxis in preterm infants: update based on the clinical evidence in Spain. BMC Infect Dis. 2017;17(1):687.
- Carbonell-Estrany X, Pérez-Yarza EG, Sanchez García L, et al. Long-term burden and respiratory effects of respiratory syncytial virus hospitalization in preterm infants-the SPRING study. PLoS One. 2015;10(5):e0125422.
- Sigurs N, Bjarnason R, Sigurbergsson F, et al. Asthma and immunoglobulin E antibodies after respiratory syncytial virus bronchiolitis: a prospective cohort study with matched controls. Pediatrics. 1995;95(4):500–505.
- Sigurs N, Bjarnason R, Sigurbergsson F, et al. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am J Respir Crit Care Med. 2000;161(5):1501–1507.
- Sigurs N, Gustafsson PM, Bjarnason R, et al. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. Am J Respir Crit Care Med. 2005;171(2):137–141.
- Weiner LB, Masaquel AS, Polak MJ, et al. Cost-effectiveness analysis of palivizumab among pre-term infant populations covered by Medicaid in the United States. J Med Econ. 2012;15(5):997–1018.
- Greenough A, Alexander J, Burgess S, et al. Health care utilisation of prematurely born, preschool children related to hospitalisation for RSV infection. Arch Dis Child. 2004;89(7):673–678.
- Chiou CF, Weaver MR, Bell MA, et al. Development of the multiattribute pediatric asthma health outcome measure (PAHOM). Int J Qual Health Care. 2005;17(1):23–30.
- Ontario Ministry of Health. Schedule of benefits. Physician services under the health insurance act; [cited 2022 July 01; accessed 2022 Aug 31]. Available from: https://www.health.gov.on.ca/en/pro/programs/ohip/sob/physserv/sob_master.pdf
- Canadian Institute for Health Information. Focus on the emergency department. Ottawa (ON): CIHI; 2020 [accessed 2022 Aug 31]. Available from: https://www.cihi.ca/sites/default/files/document/hospital-spending-highlights-2020-en.pdf
- Ismaila AS, Sayani AP, Marin M, et al. Clinical, economic, and humanistic burden of asthma in Canada: a systematic review. BMC Pulm Med. 2013;13(1):70.
- Canada Revenue Agency; [accessed 2022 Jun 20]. Available from: https://www.canada.ca/en/revenue-agency/services/tax/businesses/topics/payroll/benefits-allowances/automobile/automobile-motor-vehicle-allowances/automobile-allowance-rates.html
- Statistics Canada. Payroll employment, earnings and hours, and job vacancies; [cited 2021 Oct; accessed 2022 Jun 20]. Available from: https://www150.statcan.gc.ca/n1/daily-quotidien/211223/dq211223b-eng.htm
- Statistics Canada. Labour force characteristics, monthly, seasonally adjusted and trend-cycle, last 5 months; [accessed 2022 Jun 20]. Available from: https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1410028701&pickMembers%5B0%5D=1.1&pickMembers%5B1%5D=3.1&pickMembers%5B2%5D=4.8&pickMembers%5B3%5D=5.1&cubeTimeFrame.startMonth=08&cubeTimeFrame.startYear=2021&cubeTimeFrame.endMonth=12&cubeTimeFrame.endYear=2021&referencePeriods=20210801%2C20211201%20
- Statistics Canada. Consumer Price Index, monthly, not seasonally adjusted [accessed 2022 Jun 20]. Available from: https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1810000401
- Statistics Canada. Canadian Vital statistics, birth database. Birth statistics for the years 2016–2020. Ottawa (Canada): Statistics Canada.
- Narayan O, Bentley A, Mowbray K, et al. Updated cost-effectiveness analysis of palivizumab (Synagis) for the prophylaxis of respiratory syncytial virus in infant populations in the UK. J Med Econ. 2020;23(12):1640–1652.
- Briggs AH, Weinstein MC, Fenwick EAL, et al. Model parameter estimation and uncertainty analysis: a report of the ISPOR-SMDM modeling good research practices task force working group–6. Med Decis Making. 2012;32(5):722–732.
- Vreman RA, Geenen JW, Knies S, et al. The application and implications of novel deterministic sensitivity analysis methods. Pharmacoeconomics. 2021;39(1):1–17.
- The IMpact-RSV Study Group. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics. 1998;102(3 Pt 1):531–537.
- Notario G, Vo P, Gooch K, et al. Respiratory syncytial virus-related hospitalization in premature infants without bronchopulmonary dysplasia: subgroup efficacy analysis of the IMpact-RS V trial by gestational age group. Pediatric Health Med Ther. 2014;5:43–48.
- Treasury Board of Canada. Canadian cost-benefit analysis guide – regulatory proposals; [2007; accessed 2022 Jun 20]. Available from: https://www.tbs-sct.gc.ca/rtrap-parfa/analys/analys-eng.pdf
- Figueras-Aloy J, Carbonell-Estrany X, Quero-Jiménez J, et al. FLIP-2 study: risk factors linked to respiratory syncytial virus infection requiring hospitalization in premature infants born in Spain at a gestational age of 32 to 35 weeks. Pediatr Infect Dis J. 2008;27(9):788–793.
- Figueras-Aloy J, Manzoni P, Paes B, et al. Defining the risk and associated morbidity and mortality of severe respiratory syncytial virus infection among preterm infants without chronic lung disease or congenital heart disease. Infect Dis Ther. 2016;5(4):417–452.
- Lanctôt KL, Masoud ST, Paes BA, et al. The cost-effectiveness of palivizumab for respiratory syncytial virus prophylaxis in premature infants with a gestational age of 32-35 weeks: a Canadian-based analysis. Curr Med Res Opin. 2008;24(11):3223–3237.
- Griffiths EA, Vadlamudi NK. CADTH’s $50,000 cost-effectiveness threshold: fact or fiction? Value Health. 2016;19(7):A488–A489.
- Ontario Ministry of Health. Respiratory syncytial virus prophylaxis for high-risk infants program reference manual; 2019 [accessed 2022 Aug 31]. Available from: https://www.health.gov.on.ca/en/pro/programs/drugs/funded_drug/pdf/rsv_info.pdf
- British Columbia Provincial Health Services Authority. BC RSV immunoprophylaxis program. Administrative manual and decision support tool 2021/2022; [accessed 2022 Aug 31]. Available from: https://www.childhealthbc.ca/sites/default/files/b.c.-rsv-manual-2021-22cc.pdf
- Paes B, Fullarton JR, Rodgers-Gray BS, et al. Adoption in Canada of an International Risk Scoring Tool to predict respiratory syncytial virus hospitalization in moderate-to-late preterm infants. Curr Med Res Opin. 2021;37(7):1149–1153.
- Sampalis JS. Morbidity and mortality after RSV-associated hospitalizations among premature Canadian infants. J Pediatr. 2003;143(5 Suppl):S150–S156.
- Sørensen KG, Øymar K, Dalen I, et al. Asthma, atopy and lung function in young adults after hospitalisation for bronchiolitis in infancy: impact of virus and sex. BMJ Open Resp Res. 2022;9(1):e001095.
- Blanken MO, Frederix GW, Nibbelke EE, et al. Cost-effectiveness of rule-based immunoprophylaxis against respiratory syncytial virus infections in preterm infants. Eur J Pediatr. 2018;177(1):133–144.
- Hansen R, McLaurin K, Sullivan S. Cost-effectiveness of palivizumab prophylaxis by gestational and chronologic age among infants at increased risk of hospitalization for respiratory syncytial virus. AMCP managed care and specialty pharmacy annual meeting 2017. Denver, CO United States. J Manag Care Spec Pharm. 2017;23(3-A SUPPL):S80.
- Griffin MP, Yuan Y, Takas T, et al. Single-dose nirsevimab for prevention of RSV in preterm infants. N Engl J Med. 2020;383(5):415–425.
- Hodgson D, Atkins KE, Baguelin M, et al. Estimates for quality of life loss due to respiratory syncytial virus. Influenza Other Respir Viruses. 2020;14(1):19–27.
- Díez-Gandía E, Gómez-Álvarez C, López-Lacort M, et al. The impact of childhood RSV infection on children’s and parents’ quality of life: a prospective multicenter study in Spain. BMC Infect Dis. 2021;21(1):924.
- Mitchell I, Paes BA, Lanctôt KL, et al. RSV hospitalization in aboriginal infants in the Canadian registry of Synagis® (CARESS) following prophylaxis (2005–2012). Paediatr Child Health. 2013;18(suppl_A):12A.
- Banerji A, Lanctôt KL, Paes BA, et al. Comparison of the cost of hospitalization for respiratory syncytial virus disease versus palivizumab prophylaxis in Canadian inuit infants. Pediatr Infect Dis J. 2009;28(8):702–706.
- PATH. RSV vaccine and mAb snapshot; 2023 Jan [accessed 2023 Mar 14]. Available from: https://www.path.org/resources/rsv-vaccine-and-mab-snapshot/
- Bont L, Weil Olivier C, Herting E, et al. The assessment of future RSV immunizations: how to protect all infants? Front Pediatr. 2022;10:981741.
- Zheng Z, Weinberger DM, Pitzer VE. Predicted effectiveness of vaccines and extended half-life monoclonal antibodies against RSV hospitalizations in children. NPJ Vaccines. 2022;7(1):127. .
- Zheng Z. Challenges in maximizing impacts of preventive strategies against respiratory syncytial virus (RSV) disease in young children. Yale J Biol Med. 2022;95(2):293–300. eCollection 2022 Jun.