Abstract
Background
Pulmonary arterial hypertension (PAH), a rare vasculopathy progressively leading to right heart failure and death, is associated with considerable economic burden. Oral prostacyclin pathway agents (PPAs) like selexipag and treprostinil address an underlying PAH pathway, yet are often under-utilized. Data on head-to-head cost comparison of various PPAs is lacking.
Methods
In this retrospective study using a large health claims database, we compared the per-patient-per-year (PPPY) costs and healthcare resource utilization (HRU) among PAH patients taking either oral selexipag, inhaled treprostinil or oral treprostinil in the United States between July 2015 and March 2020. Patients with ≥1 prescription for one of the drugs of interest, ≥1 in-patient pulmonary hypertension (PH) diagnosis, or ≥ 2 outpatient PH diagnoses were included in this study. Baseline differences between the three groups were adjusted using an inverse probability of treatment weighting approach. 411 patients were selected for the final study cohorts.
Results
All-cause hospitalization costs were highest for oral treprostinil ($39,983) compared to oral selexipag ($20,635) and inhaled treprostinil ($16,548; p = .037). Total PAH-related medical costs were 40% lower for patients on oral selexipag compared to patients on oral and inhaled treprostinil ($24,351 vs. $40,398 and $40,339, respectively; p = .006). PAH-related outpatient visits were lowest for patients on oral selexipag (14 PPPY visits) compared to oral treprostinil (16 PPPY visits) and inhaled treprostinil (22 PPPY visits; p = .001).
Conclusions
Compared to oral and inhaled treprostinil, oral selexipag may incur lower medical costs and reduce PAH related outpatient visits for patients with PAH.
Introduction
Pulmonary arterial hypertension (PAH, Group 1 pulmonary hypertension)Citation1, is a rare, and progressive disease. PAH is characterized by pulmonary arterial remodelingCitation2,Citation3, increased arterial blood pressure, and pulmonary vascular resistance, progressively leading to right-heart failure, and deathCitation1. The prevalence of PAH ranges from 47.6 to 54.7 patients per million in EuropeCitation4, and 12.4 patients per million in the USCitation5. Around 500–1,000 new cases are diagnosed each year in the USCitation6. The long-term prognosis in PAH remains poorCitation7, although advances in PAH diagnostics guidelinesCitation1, more therapeutic optionsCitation8, and improved patient support over the last two decades have improved patients’ life expectancyCitation9.
Currently available interventions for PAH regulate three different pathways: (1) the prostacyclin pathway (prostacyclin pathway agents [PPAs]), (2) the endothelin pathway (endothelin receptor antagonists [ERAs]), and (3) the nitric oxide pathway (phosphodiesterase type 5 inhibitors [PDE-5is] or a soluble guanylate cyclase stimulator [sGCS]). PPAs have been recommended for patients with idiopathic, heritable or drug-induced PAH who are at intermediate-high or high risk of death while on ERAs or PDE-5i drugsCitation10.
Evidence from clinical trials and observational studies strongly support the efficacy of combination therapy in PAHCitation11. ERAs, and PDE-5is are the most commonly used first-line therapies due to their established clinical efficacy and good tolerabilityCitation12,Citation13. In patients with an inadequate clinical response to initial therapy, an additional agent targeting the prostacyclin pathway should be consideredCitation14. However, the use of the first generation of PPAs (parenteral treprostinil and parenteral epoprostenol) has been challenging due to the requirement of continuous parenteral administration and their associated complications such as line infections and related sepsisCitation1,Citation15–17.
Two inhaled PPAs, treprostinil and iloprost are currently available in the US, but the inhalation procedure is cumbersome and time consumingCitation18 and their approval was based on the results of short duration trialsCitation19,Citation20. This challenge is mitigated with oral formulations of PPAs. The oral form of treprostinil was approved by the US Food and Drug Administration (FDA) in 2013 for the treatment of PAH (Group 1) to improve exercise capacityCitation21. Patients treated with treprostinil showed a 26% lower risk of clinical worseningCitation22, and improved exercise intoleranceCitation23 compared to placebo. Treprostinil is titrated based on response and tolerability without a dose ceiling.
Selexipag, an oral PPA, was approved by the FDA in 2015 to delay disease progression and reduce the risk of hospitalization for PAHCitation24. In the GRIPHON trial, selexipag reduced the risk of morbidity and mortality events vs placebo by 40%Citation25. The addition of selexipag to an ERA or PDE5 in patients who are at intermediate-low risk of death; and addition of oral treprostinil to ERA or PDE5i/riociguat monotherapy is recommended to reduce the risk of morbidity/mortality events, as per the 2022 ESC/ERS guidelines for PHCitation10.
There are no head-to head clinical studies between the various PPAs. Considerable economic burden is associated with the management of PAHCitation26. This study aimed to provide a comparison of direct costs and HRU including hospitalization, emergency department and outpatient visits during follow-up among patients with PAH on oral selexipag, inhaled treprostinil and oral treprostinil in the US.
Methods
Data source
Longitudinal patient data were sourced from the Optum’s de-identified Clinformatics Data Mart (CDM) Database (2007–2021), an adjudicated administrative health claims database covering 14 million annual US lives and is a mix of commercially insured and Medicare members. The database was selected due to its longitudinal nature and comprehensive coverage of claims required to meet the study objectives. Individual patient consent was not required as the data only contains de-identified health information that, as described by the HIPPA Privacy Rule, is not Protected Health Information. It includes outpatient pharmacy dispensing claims (coded with National Drug Codes (NDC), inpatient and outpatient medical claims which provide procedure codes (coded in CPT-4, HCPCS, ICD-9-PCS or ICD-10-PCS) and diagnosis codes (coded in ICD-9-CM or ICD-10-CM) and member enrollment dates. Detailed list of all ICD-9-CM, ICD-10-CM, CPT-4, and NDC used in this study have been given in Supplementary Table 1.
Study design
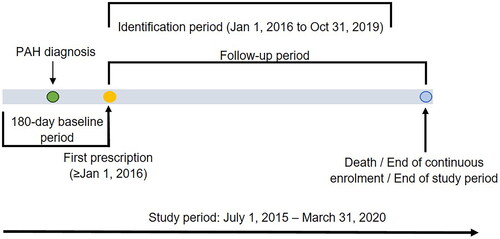
This retrospective study of longitudinal claims data was carried out from 1 July 2015 to 31 March 2020, including the baseline period (for demographic and clinical information), the identification period (to identify patients of interest), and the follow-up period (for costs and HRU measurements). Details of the study design are illustrated in .
Patients were indexed on the first target treatment during the identification period (1 January 2016 through 31 October 2019) and the first prescription date was considered as the index date. The study and identification period were chosen to avoid possible confounding with the COVID-19 pandemic. The baseline period was 180-days pre-index with continuous medical and pharmacy enrollment. Follow-ups were carried out post-index until death, end of continuous enrollment or end of the study period. The outcomes of interest were compared between treatment groups after adjusting for differences using the inverse probability of treatment weighting (IPTW) approachCitation27,Citation28. The data used in this study were deidentified in compliance with the Health Insurance Portability and Accountability ActCitation29, thus institutional review board approval was not required.
Patient selection
Individuals ≥18 years of age at index with pharmacy claims for at least one prescription for one of the three drugs (i.e. oral selexipag, oral treprostinil, inhaled treprostinil) in the identification period, continuous medical and pharmacy enrollment during baseline period, and at least one documented diagnosis of PH (ICD-9-CM: 416.0, 416.8; ICD-10-CM: I27.0, I27.2, I27.20, I27.21, I27.89) in an inpatient or emergency room (ER) setting, or at least two documented diagnoses of PH in an outpatient setting during the baseline period were included in the study. Patients were excluded from the analysis if they had a prescription of any prior PPAs, and/or a claim associated with PH group 3–5 of the Venice Clinical Classification and Dana Point classificationsCitation30 during the baseline period.
Study outcome measures
Patient demographics, background PAH medications, and comorbidities were assessed during the baseline period. All-cause and PAH-related HRU (defined as a claim containing either a PH diagnosis or treatment), including number of hospitalizations (in-patient [IP] stays), intensive care unit (ICU) admissions (subset of hospitalizations), length of hospital stay, outpatient (OP), and emergency room (ER) visits, were assessed over the entire observation period. The rate of HRU events were calculated on a total number of events per-person-per-year (PPPY) basis. IP stays included claims for acute care and skilled nursing facilities, and length of IP stay was calculated on a per-stay basis across all patients as well as only for those with a stay. OP visits included ER visits, laboratory tests, and visits related to routine medical care. Total medical costs, all-cause and PAH-related, were defined as sum of costs toward hospitalization, ER visits, and OP visits, and did not include OP pharmacy costs. PPPY medical costs were estimated using a generalized linear regression model from the index date to the end of follow-up for each patient. Time to first hospitalization, both all-cause and PAH-related are also reported.
Data analysis
Direct comparisons were conducted for HRU and costs between the three treatment groups. In order to reduce potential bias due to confounding by indication, IPTW was used to adjust for measured confounders. A gradient boosted model (GBM) using the TWANG R packageCitation28 and MNPS SAS macro by the RAND CorporationCitation27 was used to calculate the propensity scores for each patient in the three treatment cohorts. While the traditional approach for calculating propensity scores is to use logistic regression, that was not possible in this case since there are three treatment groups. GBM is a classification technique that comes from machine learning. It uses iterative regression trees to classify patients based on a set of covariates . As summarized in McCaffrey et al.Citation28, it is particularly suitable for calculating a propensity score in cases with more than two treatment conditions. The number of trees for the gradient boosted model, interaction depth, and shrinkage were set to 5,000, 3, and 0.01 respectively. The dependent variable in the model had three levels, indicating whether the patient initiated oral selexipag, inhaled treprostinil or oral treprostinil. Independent variables in the model included demographic characteristics, the use of ERA, and drugs acting on the nitric oxide pathway in the baseline period (combination, monotherapy, or none), Charlson Comorbidity Index (CCI), PAH associated comorbidities, and other comorbidities at baseline.
The average treatment effect (ATE) weight was then derived by inversing the propensity scores by the patients’ probability of receiving the treatment they received relative to the probability of receiving the target treatment. The propensity score distributions among different treatment cohorts were then explored to check the comparability of the treatment groups. Sizeable overlaps among the groups illustrated satisfactory overlap in covariate distributions and indicated that the groups are comparable. In addition, one-way ANOVAs were conducted to test for differences between the three treatment groups among the covariates before and after weighting.
Statistical analysis
Descriptive statistics were reported as means and standard deviation (SD) for continuous variables, and frequencies and proportions for categorical, and dichotomous variables. The number and percentage of patients with missing values were reported for each variable. Total number of hospitalizations, ER visits, OP visits and total medical costs during follow-up were reported on a PPPY basis at the level of the aggregate group to adjust for varying lengths of follow-up time. Systematic differences between the treatment groups were evaluated by a weighted one-way ANOVA test for continuous variables and weighted Chi-square tests for categorical variables.
Weighted multivariable analyses were carried out to model the number of events and the costs as a function of the independent variable (oral selexipag vs. inhaled treprostinil vs. oral treprostinil) and covariates. As the HRU outcomes are right skewed, generalized estimating equations (GEE) using a negative binominal distribution with a logarithm link function and robust standard errors clustered on the patient to account for differing follow-up time was used. Similarly, the cost data is right skewed with a long tail, therefore generalized linear models (GLM) using a gamma distribution and logarithm link was employed. The mean difference of PPPY, 95% CIs, and p values were reported.
Time to first hospitalization was assessed by Kaplan–Meier survival analysis and Cox proportional hazard models. All statistical analyses were performed using SAS/STAT Software, version 9.4 (SAS Institute Inc.). No adjustment for multiple testing was performed. p Values less than .05 were considered statistically significant.
Results
Patient selection and study population
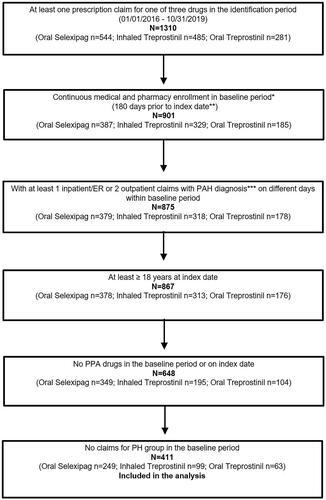
A total of 1,310 patients with at least one prescription for oral selexipag, inhaled treprostinil, or oral treprostinil were identified between 1 January 2016 and 31 October 2019. Of the identified patients, 901 patients had continuous medical and pharmacy enrollment for at least 180 days prior to their index prescription. The final cohorts, obtained after exclusion based on pre-defined criteria, consisted of 411 patients, which included 249 in the oral selexipag group, 99 in the inhaled treprostinil group, and 63 in the oral treprostinil group, respectively ().
Figure 2. Patient attrition flow chart. *Baseline period is considered as 180 days prior to index date; **Index date is the first date of use of respective study drug in the identification period; ***PAH diagnosis codes are ICD-9-CM: 416.0, 416.8; ICD-10-CM: I27.0, I27.2, I27.20, I27.21, I27.89. N. Abbreviations. n, sample size; ER, emergency room; PAH, pulmonary arterial hypertension; PA, prostacyclin pathway agent; PH, pulmonary hypertension.
Baseline characteristics of patient population
Prior to applying the weights, there were differences in several baseline characteristics between the three groups (). Compared to the inhaled and oral treprostinil groups before weighting, the oral selexipag group had slightly younger patients (47% below 65 years in oral selexipag group vs. 33% in the inhaled treprostinil group, and 37% in the oral treprostinil group), a higher proportion of females (72.7% in oral selexipag group vs. 65.7% in the inhaled treprostinil group vs. 66.7% in the oral treprostinil group), and higher proportion of patients on commercial insurance (30% in oral selexipag group vs. 26% in inhaled treprostinil group, and 21% in oral treprostinil group). The majority of the patients (52% of overall patient population) were based in the Southern part of the US (). The mean time to end of follow-up time for the overall patient population was 534 days (p = .79).
Table 1. Baseline demographic characteristics of study groups before and after inverse probability of treatment weights.
Prior to weighting, around 88% of the patients in the oral selexipag group, and 87% patients in the oral treprostinil groups reported the use of an ERA and/or PDE-5i/sGC as background medication, which was significantly higher compared to the proportion of patients in the inhaled treprostinil group (68%; p < .001) (). Over 29% of the study population had at least one of three key PAH associated comorbidity (p = .38; ).
Table 2. Background medication use at baseline before and after inverse probability of treatment weights.
Table 3. Baseline comorbidities before and after inverse probability of treatment weights.
After weighting, differences in baseline parameters were no longer statistically significant (). The mean time to end of follow-up for the study population after weighting was 539 days for the oral selexipag group, 536 days for inhaled treprostinil, and 526 days for oral treprostinil (p = .98).
Total medical costs (weighted population)
The mean PPPY all-cause total medical costs for oral selexipag, oral treprostinil and inhaled treprostinil patient groups were $59,090, $77,380, and $70,293 respectively (). However, the difference in mean medical costs amongst the groups were not statistically significant (p = .32).
Figure 3. Mean all-cause medical costs of patient population (weighted population). Mean PPPY costs calculated from index date to end of initial treatment period. Overall medical costs are the sum of hospitalization costs, outpatient costs and ER costs. ICU costs are a subset of hospitalization costs. Error bars and data in parentheses represent 95% confidence intervals (CI). Abbreviations. ICU, intensive care unit; PPPY, per patient per year; USD, United States dollars; ER, emergency room. *indicates statistically significant difference (p < .05) between interventions for the outcomes.
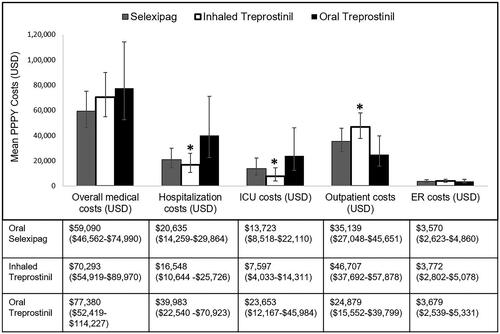
Of the total all-cause medical cost components, hospitalization costs were lower for patients receiving oral selexipag ($20,635) and inhaled treprostinil ($16,548) than those receiving oral treprostinil ($39,983; p = .037). Mean all-cause costs specific to the ICU were higher (p = .035) for patients receiving oral treprostinil ($23,653), followed by oral selexipag ($13,723) and inhaled treprostinil ($7,597). Patients in the inhaled treprostinil group had the highest PPPY mean all-cause outpatient cost ($46,707), followed by oral selexipag ($35,139), and oral treprostinil ($24,879; p = .025) ().
PAH-related PPPY mean total medical costs were significantly different between the three treatment groups (p = .006). The PAH-related total mean medical cost for oral selexipag was $24,351, for inhaled treprostinil was $40,339, and for oral treprostinil was $40,398 (p = .006) (). PAH-related hospitalization costs were not significantly different between the three groups (p = .061), but the subset of costs associated with PAH-related ICU admissions were highest for the oral treprostinil group ($12,767), followed oral selexipag ($5,066), and then inhaled treprostinil ($2,043; p = .0117).
Figure 4. Mean PAH-related medical costs of patient population (weighted population). Mean PPPY costs calculated from index date to end of initial treatment period. ICU costs are a subset of hospitalization costs. Error bars and data in parentheses represent 95% confidence intervals (CI). Abbreviations. ICU, intensive care unit; PPPY, per patient per year; USD, United States dollars; ER, emergency room; PAH, pulmonary arterial hypertension. *indicates statistically significant difference (p <.05) between interventions for the outcomes.
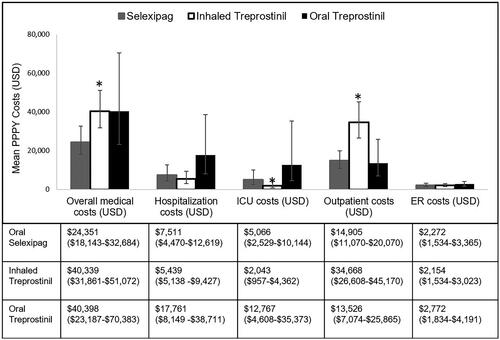
Mean PAH-related outpatient costs were significantly different (p < .0001). The inhaled treprostinil group had the highest mean PPPY PAH-related outpatient cost ($34,668), followed by oral selexipag ($14,905) and oral treprostinil ($13,526) ().
Healthcare resource utilization (weighted population)
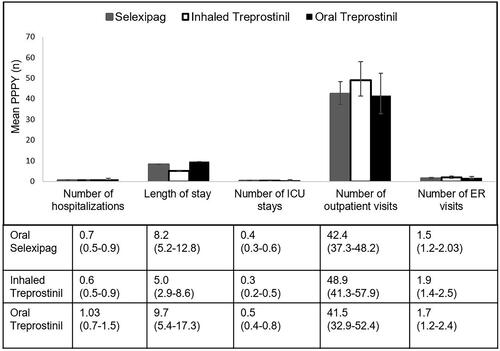
No significant differences were observed for mean all-cause PPPY number of hospitalizations (p = .053), all-cause ICU stays (p = .08), all-cause hospital length of stay per patient (p = .10) () or per all-cause hospitalized patient (p = .69; meanselex=10.9 days, meaninh_trep=9.4, meanoral_trep=10.3), all-cause outpatient visits (p = .31), or all-cause ER visits (p = .56) ().
Figure 5. Mean all-cause HRU of patient population (weighted population). Mean PPPY (number) calculated from index date to end of initial treatment period. Number of ICU stays are a subset of number of hospitalizations. Error bars and data in parentheses represent 95% confidence intervals (CI). Abbreviations. ICU, intensive care unit; PPPY, per patient per year; USD, United States dollars; ER, emergency room; PAH, pulmonary arterial hypertension.
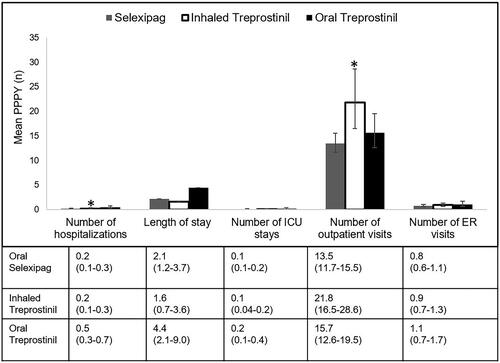
Differences between PAH-related HRU were observed between the three groups. Patients treated with oral treprostinil showed a higher mean number of PAH-related hospitalizations (0.5 PPPY) compared to oral selexipag (0.2 PPPY) and inhaled treprostinil (0.2 PPPY; (p = .007) (). The mean number of PAH-related ICU stays, PAH-related hospital length of stay per patient (p = .10) or per PAH-related hospitalized patient (p = .95; meanselex=10.8 days, meaninh_trep=11.9, meanoral_trep=10.7) were not significantly different between the three groups. The mean number of PAH-related outpatient visits were significantly higher for the inhaled treprostinil group (22 PPPY visits) compared to oral selexipag (14 PPPY) and oral treprostinil (16 PPPY; p = .001) (). There were no significant differences in PAH-related ER visits between the groups.
Figure 6. Mean PAH-related HRU of patient population (weighted population). Mean PPPY (number) calculated from index date to end of initial treatment period. Number of ICU stays are a subset of number of hospitalizations. Error bars and data in parentheses represent 95% confidence intervals (CI). Abbreviations. ICU, intensive care unit; PPPY, per patient per year; USD, United States dollars; ER, emergency room; PAH, pulmonary arterial hypertension. *indicates statistically significant difference (p <.05) between interventions for the outcomes.
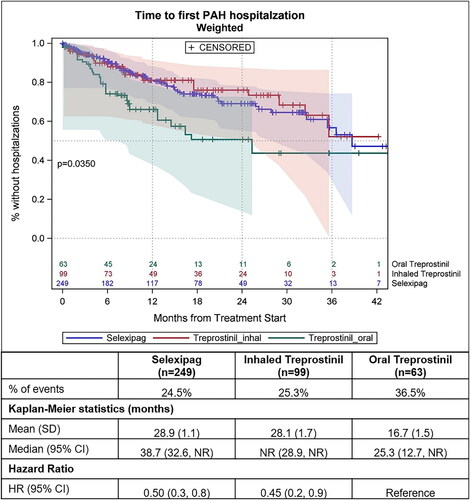
The median time to all-cause hospitalization was not different between the groups (p = .1823; ). The median time to PAH-related hospitalization was significantly shorter for patients on oral treprostinil (25 months) compared to those taking inhaled treprostinil (median not reached) and taking oral selexipag (39 months; p = .0350; ). For patients taking oral selexipag and inhaled treprostinil, the risk of all-cause hospitalization was reduced by 32% and 31% respectively (HRselex= 0.68 [95%CI: 0.45, 1.01; p = .06], HRinh_trep= 0.69 [95%CI: 0.40, 1.17]; p = .170), and the risk of PAH-related hospitalization was reduced by 50% and 55% respectively (HRselex= 0.50 [95%CI: 0.30, 0.85; p = .011], HRinh_trep= 0.45 [95%CI: 0.23, 0.87]; p = .017) compared to patients taking oral treprostinil ( and ).
Figure 7. Kaplan–Meier curves showing time to first all-cause hospitalization after propensity score weighting. Follow-up length is from the index date till the earliest of first all-cause hospitalization, death, end of continuous enrollment or end of study period p <.05 is considered statically significant. Shaded areas in the figure represent 95% Hall–Wellner confidence bands. Abbreviations. CI, 95% confidence interval; n, number of patients; SD, standard deviation.
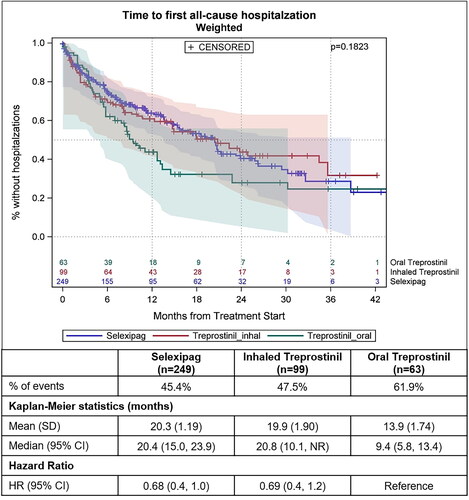
Figure 8. Kaplan–Meier curves showing time to first PAH-related hospitalization after propensity score weighting. Follow-up length is from the index date till the earliest of first PAH-related hospitalization, death, end of continuous enrollment or end of study period. p <.05 is considered statically significant. Shaded areas in the figure represent 95% Hall–Wellner confidence bands. CI, 95% confidence interval; n, number of patients; SD, standard deviation.
Discussion
In this study, the all-cause mean total medical costs for PAH patients on the three prostacyclin therapy cohorts ranged between $59,090 PPPY for selexipag to $77,380 PPPY for oral treprostinil, while the mean PAH-related medical costs ranged between $24,351 PPPY for selexipag to $40,398 PPPY for oral treprostinil. Hospitalization costs varied between $20,635 to $39,983 PPPY, which correlates with the findings of a previous study where the mean cost of the initial hospitalization for PAH patients was reported to be $30,286Citation31.
We observed that, descriptively, patients on oral selexipag incur 24% lower all-cause medical costs compared to patients on oral treprostinil, 16% lower all-cause medical costs compared to patients on inhaled treprostinil, and 40% lower PAH-related medical costs compared to patients on oral and inhaled treprostinil. The difference in medical costs between both oral selexipag and inhaled treprostinil compared to oral treprostinil was driven by differences in hospitalization costs, which were nearly 60% lower for inhaled treprostinil patients and 50% lower for selexipag patients compared to oral treprostinil. The latter is consistent with findings of a previous real-world study where oral selexipag resulted in significantly lower risks of hospitalization compared to oral treprostinilCitation32.Alternatively, outpatient costs for patients on inhaled treprostinil were highest ($46,707) amongst the three interventions in this study, possibly due to the additional medical supplies needed for administration, and the consequent inclusion these costs under the medical benefit.
The differences in PAH-related total medical costs were also reflected in PAH-related utilization estimates in this study. The risk for PAH-related hospitalization and PAH-related hospitalizations were lower for both oral selexipag and inhaled treprostinil compared to oral treprostinil. Furthermore, there were only two fewer PAH-related outpatient visits for oral selexipag compared to oral treprostinil, but eight and six fewer visits per-patient per-year, respectively, compared to inhaled treprostinil–a significant burden to the patient. Results of this comparative study are consistent with findings of the pivotal GRIPHON clinical trial on selexipag, which showed that 40% reduction in primary endpoint events (p < .0001). Selexipag’s beneficial effect was primarily attributed to the reduction in PAH-related hospitalizations (14% hospitalization in treatment group vs. 19% in placebo) and lower rates of other disease-progression eventsCitation25.
A possible reason for the differences in HRU between oral selexipag and inhaled treprostinil in our study is that an unknown proportion of patients treated with inhaled treprostinil may have interstitial lung disease (PH-ILD; WHO Group 3). This is because it is not possible to verify the clinical subtype of PH due to the lack of specificity of ICD codes to PH groups and due to ILD being composed of multiple diseases making it difficult to define PH-ILD using administrative claims data. Furthermore, inhaled treprostinil is the only drug indicated in PH-ILD to improve exercise abilityCitation19, which may explain the smaller difference in hospitalization outcome between oral selexipag and inhaled treprostinil compared to that observed between oral selexipag and oral treprostinil.
In a retrospective cohort study of the IBM Watson MarketScan database, Dean and colleagues analyzed costs and HRU of 130 patients treated with oral treprostinil and 126 patients treated with selexipagCitation33. They reported that the adjusted PAH-related mean number of inpatient visits was not significantly different between oral selexipag and oral treprostinil (0.1 vs 0.1) during the post index periodCitation33. Whereas in the present study, oral selexipag was associated with a reduction in PAH-related mean number of PPPY inpatient visits compared to oral treprostinil (0.19 vs 0.47). This difference between findings can be attributed to the methods used in the analysis of inpatient visits. The study by Dean et al. had a limited follow-up time to six monthsCitation33, Limited follow-ups can potentially underestimate the benefits of the treatment, considering the extended titration period for certain therapies (two to three months or more), and longer median time to hospitalization preventing the observations of adverse events. There is no minimum follow-up time in our study; patients were followed until the end of continuous enrollment, death, or March 2020 (end of study period).
The findings of this real-world study highlight the differences in costs and HRU between the PPAs currently prescribed for patients with PAH, with comparatively lower rates of hospitalization and lower medical costs incurred by patients treated with oral selexipag. These findings are consistent with previous studiesCitation32,Citation34, and adds to the evidence base to help physicians and policy makers in their clinical decision making.
The current study has the following limitations that are inherent to retrospective analyses using claims data; therefore, the comparisons should be interpreted with these considerations. Observable differences between the three treatment groups were accounted for by weighting, however there are unmeasured confounders that could influence treatment choice that we were not able to account for in this study. Therefore, we stress that the results of this analysis are not to be considered causal, but rather correlational in nature. Administrative claims databases may have database errors or omissions, coding errors by providers or administrators, or misdiagnoses, limiting data accuracy. Although the databases list the prescribed medication, the information on whether the medication was taken by the patient is unavailable. Information on disease severity in terms of functional capacity, 6-min walk distance, serum N-terminal pro-brain natriuretic peptide or hemodynamic values, which may influence treatment choice and outcomes, and is therefore an unmeasured confounder, is not available in claims databases. Furthermore, diagnostic coding for PAH under ICD-10-CM, prior to October 2017, was marked by the challenge that diagnostic codes existed for PH but not specifically for PAH (i.e. PH Type 1), leading to misidentification of some patients, and misclassification of ILD as PAH may be a cause of this limitation. There are almost four and two times as many patients taking oral selexipag compared to oral and inhaled treprostinil, respectively. This imbalance and, in the case of oral treprostinil, relatively small sample size, may affect the estimates of cost and HRU for the latter two categories. Additionally, presence of comorbidities and PH diagnosis are not clinically validated. Finally, the cost estimates available in the CDM database have been standardized and should not be interpreted as the actual cost of care.
Conclusions
The hospitalization, ICU, and ER component costs of selexipag and inhaled treprostinil were often trending together with inhaled treprostinil frequently showing a nominally lower cost. However, the outpatient costs for inhaled treprostinil overshadowed the savings from the other healthcare settings. Because of its overall medical cost savings and comparable or lower resource use, oral selexipag should be preferred as a PPA relative to oral treprostinil specifically, as it can help in alleviating the burden of PAH.
Transparency
Declaration of financial/other interests
EP, XL and RP are employed by Putnam PHMR and received funding from Janssen Pharmaceutical to conduct the study. YT, AB and SP are all employed by Janssen Pharmaceutical and own stock. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
AB, YT, SP, RP and EP designed the study, EP and XL performed the analysis, and all authors contributed to interpreting the results. The main manuscript text was written by EP and revised by EP, AB, and YT. All authors reviewed the manuscript.
Previous presentations
This research has not been previously presented.
Supplemental Material
Download PDF (321.8 KB)Acknowledgements
The authors would like to acknowledge Swati Sucharita Dash, from Molecular Connections Analytics Pvt Ltd, Bangalore, India for writing assistance in preparation of the initial draft and creation of figures included in the manuscript. Molecular Connections Analytics Pvt Ltd was contracted and paid for by Putnam PHMR.
Additional information
Funding
References
- Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the joint task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016;37(1):67–119.
- Tuder RM, Marecki JC, Richter A, et al. Pathology of pulmonary hypertension. Clin Chest Med. 2007; 28(1):23–42, vii.
- Guignabert C, Tu L, Le Hiress M, et al. Pathogenesis of pulmonary arterial hypertension: lessons from cancer. Eur Respir Rev. 2013;22(130):543–551.
- Leber L, Beaudet A, Muller A. Epidemiology of pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension: identification of the most accurate estimates from a systematic literature review. Pulm Circ. 2021;11(1):2045894020977300.
- Frost AE, Badesch DB, Barst RJ, et al. The changing picture of patients with pulmonary arterial hypertension in the United States: how REVEAL differs from historic and non-US contemporary registries. Chest. 2011;139(1):128–137.
- American Lung Association. Learn about pulmonary arterial hypertension. [cited 2022 Apr 20]. Available from: https://www.lung.org/lung-health-diseases/lung-disease-lookup/pulmonary-arterial-hypertension/learn-about-pulmonary-arterial-hypertension
- Farber HW, Miller DP, Poms AD, et al. Five-year outcomes of patients enrolled in the REVEAL registry. Chest. 2015;148(4):1043–1054.
- Montani D, Chaumais MC, Guignabert C, et al. Targeted therapies in pulmonary arterial hypertension. Pharmacol Ther. 2014;141(2):172–191.
- Benza RL, Miller DP, Barst RJ, et al. An evaluation of long-term survival from time of diagnosis in pulmonary arterial hypertension from the REVEAL registry. Chest. 2012;142(2):448–456.
- Humbert M, Kovacs G, Hoeper MM, et al. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022;43(38):3618–3731.
- Galiè N, Channick RN, Frantz RP, et al. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur Respir J. 2019;53(1):1801889.
- Zheng YG, Ma H, Hu EC, et al. Oral targeted therapies in the treatment of pulmonary arterial hypertension: a meta-analysis of clinical trials. Pulm Pharmacol Ther. 2014; 29(2):241–249.
- Song C, Kunovszki P, Beaudet A. Comparison of healthcare encounters and drug persistence in patients with pulmonary arterial hypertension receiving oral selexipag, inhaled iloprost, or parenteral treprostinil: a retrospective database analysis. J Health Econ Outcomes Res. 2022;9(1):151–160.
- Momoi M, Hiraide T, Shinya Y, et al. Impact of additional selexipag on prostacyclin infusion analogs in patients with pulmonary arterial hypertension. Respir Med Case Rep. 2022;36:101592.
- Kallen AJ, Lederman E, Balaji A, et al. Bloodstream infections in patients given treatment with intravenous prostanoids. Infect Control Hosp Epidemiol. 2008;29(4):342–349.
- Humbert M, Sitbon O, Simonneau G. Treatment of pulmonary arterial hypertension. N Engl J Med. 2004;351(14):1425–1436.
- Barst RJ, Rubin LJ, Long WA, et al. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. N Engl J Med. 1996;334(5):296–301.
- Farber HW, Gin-Sing W. Practical considerations for therapies targeting the prostacyclin pathway. Eur Respir Rev. 2016;25(142):418–430.
- Waxman A, Restrepo-Jaramillo R, Thenappan T, et al. Inhaled treprostinil in pulmonary hypertension due to interstitial lung disease. N Engl J Med. 2021;384(4):325–334.
- Nathan SD, Waxman A, Rajagopal S, et al. Inhaled treprostinil and forced vital capacity in patients with interstitial lung disease and associated pulmonary hypertension: a post-hoc analysis of the INCREASE study. Lancet Respir Med. 2021;9(11):1266–1274.
- US Food and Drug Administration. Drug approval package: orenitram (treprostinil) extended release tablets [Internet]; [cited 2022 May 11]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2013/203496Orig1s000TOC.cfm
- White RJ, Jerjes-Sanchez C, Bohns Meyer GM, et al. Combination therapy with oral treprostinil for pulmonary arterial hypertension. A double-blind placebo-controlled clinical trial. Am J Respir Crit Care Med. 2020;201(6):707–717.
- McLaughlin VV, Benza RL, Rubin LJ, et al. Addition of inhaled treprostinil to oral therapy for pulmonary arterial hypertension: a randomized controlled clinical trial. J Am Coll Cardiol. 2010;55(18):1915–1922.
- US Food and Drug Administration. Actelion receives US FDA approval of uptravi (selexipag) for the treatment of pulmonary arterial hypertension | Fierce Pharma [Internet]; [cited 2022 Apr 20]. Available from: https://www.fiercepharma.com/pharma/actelion-receives-us-fda-approval-of-uptravi-selexipag-for-treatment-of-pulmonary-arterial
- Sitbon O, Channick R, Chin KM, et al. Selexipag for the treatment of pulmonary arterial hypertension. N Engl J Med. 2015;373(26):2522–2533.
- Phatak H, Pena JDO, Patel K, et al. PRO18 a systematic literature review of the economic burden and cost drivers in pulmonary arterial hypertension. Value in Health. 2021;24:S200–S201.
- McCaffrey DF, Burgette LF, Griffin BA, et al. Propensity scores for multiple treatments: a tutorial for the MNPS macro in the TWANG SAS macros [Internet]. RAND Corporation; 2015 Mar [cited 2022 May 5]. Available from: https://www.rand.org/pubs/tools/TL169z1.html
- McCaffrey DF, Griffin BA, Almirall D, et al. A tutorial on propensity score estimation for multiple treatments using generalized boosted models. Stat Med. 2013;32(19):3388–3414.
- Nass SJ, Levit LA, Gostin LO, Rule I of M (US) C on HR and the P of HITHP. HIPAA, the privacy rule, and its application to health research [Internet]. Beyond the HIPAA privacy rule: enhancing privacy, improving health through research. National Academies Press (US); 2009 [cited 2022 Jun 27]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK9573/
- Simonneau G, Robbins IM, Beghetti M, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2009; 54(1 Suppl):S43–S54.
- Canavan N. Rehospitalization is driving costs in pulmonary arterial hypertension. Am Health Drug Benefits. 2013;6(9):600–601.
- McConnell JW, Tsang Y, Pruett J, et al. Comparative effectiveness of oral prostacyclin pathway drugs on hospitalization in patients with pulmonary hypertension in the United States: a retrospective database analysis. Pulm Circ. 2020;10(4):2045894020911831.
- Dean BB, Saundankar V, Stafkey-Mailey D, et al. Medication adherence and healthcare costs among patients with pulmonary arterial hypertension treated with oral prostacyclins: a retrospective cohort study. Drugs Real World Outcomes. 2020;7(3):229–239.
- Solakidi A, Tzanetakos C, Beaudet A, et al. Cost-Effectiveness of selexipag for the treatment of pulmonary arterial hypertension (PAH) in Greece. Value in Health. 2017;20(9):A645.