Abstract
Aims
To evaluate the cost-effectiveness of standard-of-care treatment (SoC) to SoC in combination with omalizumab (OML + Soc) in patients with severe asthma using real-world prospective clinical data from four major medical centers in Turkey.
Materials and methods
Between February 2018 and November 2019, a total of 206 patients with severe asthma, including 126 of whom were in the OML + SoC group and 80 in the SoC group, were followed for 12 months to evaluate their asthma status and quality of life. Cost data for this patient-level economic evaluation were sourced from the MEDULA database of the hospitals and expressed in Turkish Lira (₺). Efficacy data were obtained by means of Turkish versions of the Asthma Control Test for asthma status, the 5-level EQ-5D-5L version (EQ-5D-5L), and the Asthma Quality of Life Scale for quality of life. A Markov model with 2-week cycles was specified, comparing costs and treatment effects of SoC vs. OML + SoC over a lifetime from the Turkish payer perspective.
Results
Per-patient costs were ₺23,607.08 in the SoC arm and ₺425,329.81 in the OML + Soc arm, for a difference of ₺401,722.74. Life years (LY) and quality-adjusted life years (QALY) were 13.60 and 10.08, respectively, in the SoC group; and 21.26 and 13.35, respectively, in the OML + SoC group, for differences of 7.66 LYs and 3.27 QALYs. This yielded an incremental cost-effectiveness ratio of an additional ₺52,427.04 to gain 1 LY and an incremental cost-utility ratio of an incremental ₺122,675.57 to gain 1 QALY; the latter being below the ₺156,948 willingness-to-pay threshold for Turkey referenced by WHO. One-way and multivariate sensitivity analyses confirmed the base-case results.
Conclusion
Whereas most economic evaluations are based on aggregate data, this independent cost-effectiveness analysis using prospective real-world patient-level data suggests that omalizumab in combination with standard of care is cost-effective for severe asthma from the Turkish public payer perspective.
PLAIN LANGUAGE SUMMARY
What is the context?
Severe asthma, a subset of difficult-to-treat asthma, refers to asthma that cannot be controlled despite adherence to optimized maximal therapy and treatment of contributing factors, or asthma that worsens when high-dose therapy is reduced.
Omalizumab is the first biologic therapy approved for the treatment of allergic asthma. Its main role is to prevent the release of various inflammation factors that cause severe asthma episodes.
Cost-effectiveness analysis is an economic method of determining how much more a new and better treatment costs relative to the current treatment in terms of how many life years (LY) and how many quality-adjusted life years (QALY) are gained with the new treatment. Cost-effectiveness results tell us how much more money is needed over the cost of the current treatment to achieve one additional LY, regardless of the quality of life, or one additional LY with good quality of life.
No cost-effectiveness data obtained from actual clinical patient data are available for Turkey.
Our study found that the addition of omalizumab to the current standard of care for severe asthma increases costs but also increases life years and quality-adjusted life years. The additional cost was less than what the World Health Organization assumes is reasonable for Turkey.
This study used actual clinical patient data and noted that asthma patients in the omalizumab group used fewer health services, had a better clinical course, had a better quality of life, and lived longer with their disease under control.
In severe asthmatic patients, adding omalizumab to standard-of-care, while more costly, yields better outcomes and is therefore cost-effective.
The cost-effectiveness estimates fall within the margins of being cost-responsible. The Turkish public payer should strongly consider making omalizumab available to all eligible patients. This will enable working-age patients to work, and contribute to their families, while also strengthening the Turkish economy.
Introduction
Asthma is a chronic respiratory disease affecting up to an estimated 18% of the population in different countries for as many as 358 million people worldwideCitation1. Its global prevalence rate increased by 12.6% from 1990 to 2015Citation2. In 2017, the prevalence of physician-diagnosed asthma in patients 15 years and older was 6.9%Citation3. Persistent severe asthma, defined as asthma with symptoms occurring more than twice a weekCitation4, affects a small proportion of the total asthma populationCitation5, however, is associated with lower quality of life and high direct and indirect costsCitation6–8.
The economic burden of asthma, and severe asthma in particular, differs from country to country in terms of the proportion of gross national product expended on asthma care, how asthma care is organized and delivered, how it is financed, and which pharmacotherapies are approved and coveredCitation9. This economic burden further varies as new treatment options are approved, government policies change, pricing dynamics in the healthcare market evolve, and the effectiveness of asthma control programs improveCitation10. Direct treatment costs are the largest driver of the economic burden, including the costs of pharmaceuticals. To these health expenditures should be added the cost of travel, loss of productivity, psychological impact, impaired quality of life, and decreased participation in family lifeCitation9,Citation11,Citation12; although their consideration may vary from one study to the next based on the perspective taken.
The long-term goal of asthma treatment is to maintain normal respiratory function without symptoms, exacerbations, or side effects through asthma control and rescue/relief therapy. Inhaled corticosteroids (ICS), long-acting β2-agonists (LABA), leukotriene receptor antagonists (LTRA), sustained-release theophylline, and long-acting muscarinic antagonists (LAMA) are drugs that provide asthma control. Short-acting formulations of β2-agonists (SABA), inhaled anticholinergic agents, theophylline, and oral β2-agonists, as well as magnesium and oral corticosteroids, are rescue/relief options to manage exacerbationsCitation12. While this long-standing standard of care (SoC) has been effective in mild and moderate asthma, it has been of limited effectiveness in managing severe allergic asthma. The advent of biological agentsCitation13 and in particular omalizumab, a humanized monoclonal antibody that specifically binds to free human immunoglobulin E (IgE) to affect the inflammatory processCitation14, has had a major impact on the management of severe allergic asthma in real-world clinical practiceCitation15–18.
Rising costs in small-molecule and biological agents used in asthma have compelled countries and healthcare systems to assess the economic implications and value of established and novel treatments. Studies conducted in various countries have demonstrated that the incremental costs of biological agents and the improvements in associated clinical outcomes are cost-effective under stated willingness-to-pay thresholdsCitation19–23. However, while omalizumab is within the scope of reimbursement in Turkey, no cost-effectiveness studies specific to Turkey have been published.
We report here on a real-world economic evaluation study using patient-level data that assessed the cost-effectiveness of 12 months of treatment with omalizumab in combination with SoC relative to SoC alone in patients with persistent severe asthma treated at four major medical centers in Turkey. This study intended to provide the Social Security Institution, which is the reimbursement agency in Turkey, with quantitative economic data for decision-making at the national level, and to provide an example that can be considered in the future appraisal of biological agents and reimbursement decision-making. In addition, being a study using real-world patient data in the setting of severe allergic asthma from four major medical centers, the study may provide guidance to other asthma treatment centers.
Methods
Patient population
The population consisted of patients with severe asthma and classified according to Turkish and international asthma severity guidelinesCitation12,Citation24: asthma that is uncontrolled despite adherence to maximally optimized high-dose ICS-LABA therapy and management of contributory factors, or that worsens when high dose treatment is decreased. In total, 206 eligible patients with severe allergic asthma age 19 and older consented to participate in the study, to be interviewed, and to have their clinical records retrieved: 50 at Hacettepe University Hospital, 111 at Ankara University Hospital, six at Gazi University hospital, and 39 at Atatürk Chest Diseases and Thoracic Surgery Training and Research Hospital. Of these, 71% resided in Ankara and 29% were referred from other provinces of Turkey. A total of 126 (61%) patients received omalizumab plus SoC (OML + SoC) and the remaining 80 (39%) received SoC (). Patients in both groups tended to be older, mainly female, having been diagnosed with asthma over a year ago, with a family history of asthma, and at least one comorbidity (all p > .05). Proportionately more patients in the OML + SoC group were atopic compared to the SoC group (63.5 and 53.8%, respectively, p = .046). Though not statistically significant, median IgE was lower in the OML + SoC (295.0 vs. 472.8 IU/ml), which is attributed to these patients being treated with omalizumab. Procedurally, consenting patients were interviewed (see below) and their medical records were retrieved.
Table 1. Patient characteristics.
Considering an estimated 8697 patients with several allergic asthma in Turkey in 2019, our sample of 206 patients enabled the detection of outcome parameters with an error margin of 6.95% and a confidence level of 95%; and or an error margin of 5.67%, and a confidence interval of 90%; and an error margin of 5% at a confidence interval of 85.3%Citation25.
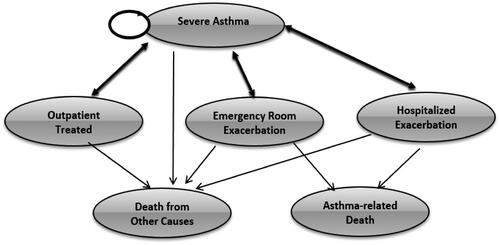
Model structure
Based on the literature review and expert clinical opinion, a state transition Markov model with six health states was specified () and operationalized in a bespoke model programmed in Microsoft Excel 2016 (Microsoft Corporation, Redmond, WA, USA). All patients start in the severe allergic asthma state; may stay in this state; may require outpatient treatment; may experience an exacerbation requiring an emergency room visit; may experience an exacerbation leading to hospitalization; or may die from other causes. Patients in the outpatient, emergency department, or hospitalization states may return to the severe allergic asthma state, after which they may or may not return to any of the 3 treatment states. These patients may also die, either from asthma-related or from other causes. Both death states were considered absorbing states in the model. The outpatient visits, emergency room visits, or hospitalizations reflect the healthcare resource utilization (HRU) as a result of worsening asthmatic disease and asthma exacerbations, while the severe allergic asthma and both death states reflect clinical states.
Per expert clinical opinion, cycle length was set at 2 weeks under the premise that this would capture singular clinical episodes (outpatient visit, emergency room visit, hospitalization), whereas a longer cycle length might include more than one clinical episode.
Model settings
The analyses were from the payer perspective, which in Turkey translates into the national health insurance plan. Transition probabilities and relative risks were sourced from the literatureCitation19. The simulated time horizon extended for 54 years from the minimum entry-into-study age of 19 until each individual in the model was assumed to have died. Thus, the maximum life expectancy was estimated to be 73 years. Note that this is not the average life expectancy in Turkey, which in 2019 was 77.54 yearsCitation26. The 73 years of life expectancy incorporates the years of life lost to asthma, which has been estimated to average 18.6 yearsCitation27. Outcomes and costs were discounted at an annual rate of 3%.
Data collection
Data collection was carried out in two different stages. In the first stage, cost data were collected, and in the second stage, effectiveness data were collected and analyses were carried out. Cost data were obtained from the hospital information system and covered the year preceding the study entry interview date of the patient. Effectiveness data were obtained face-to-face during routine clinic visits, or exceptionally by telephone when the patient could not be reached, with validated measurement tools; specifically, the Asthma Control Test (ACT) for treatment effectivenessCitation28. The EQ-5D-5L was used to measure the general quality of lifeCitation29, and the Asthma Quality of Life Questionnaire (AQLQ) for disease-specific quality of lifeCitation30. When EQ-5D-5L data were not available, AQLQ was cross-walked to estimate equivalents in EQ-5D-5L. We also retrospectively collected data on oral steroid use, unscheduled outpatient visits, emergency room visits, and hospitalizations based on statements and patient files and calculated transition probabilities for use in the scenario analyses.
Clinical inputs
Exacerbations and HRU
Each asthma exacerbation was classified in terms of the HRU required to manage it. A patient needing short-term high-dose oral steroids was defined as outpatient treatment. A patient presenting to the emergency room with a major exacerbation but not requiring hospitalization was considered an emergency room exacerbation. If the patient was admitted, it was considered an exacerbation requiring hospitalization. In the absence of Turkey-specific clinical trial data regarding the relative risks of asthma exacerbations, data from the PROSPERO study were applied ()Citation19.
Table 2. Clinical inputs.
Further, to evaluate outpatient visits or inpatient admissions, we reviewed all the invoices of the patients in our study. For example, if a patient was seen in a surgery outpatient clinic or the emergency department and was diagnosed with gastro-esophageal reflux disease (GERD), this patient would be recorded as having GERD as a comorbidity. However, as GERD and allergic asthma are unlikely to be related, the costs of the visit were considered extraneous and not included in our cost calculations. Alternately, if a patient in the omalizumab group was seen in the allergy and immunology outpatient clinic twice within a month’s time, as opposed to once for the monthly administration of omalizumab, and the ICD-10 code indicated an asthma-related condition, this additional was considered an unplanned outpatient treatment. If a patient presented to the emergency room with asthma exacerbation, dyspnea, or anaphylactic shock due to allergic asthma, this visit would be classified as disease-related and the costs were included.
Mortality
The Turkish Statistical Institute (TUIK) does not record data related to the emergency room or hospital death due to severe asthma exacerbation. We, therefore, sourced such data from literatureCitation32 and had these reviewed by clinical experts as to their applicability to Turkey. Age-specific vital statistics published by TUIK were used to estimate deaths due to other causesCitation33 ().
Health-related quality of life (HRQoL)
As there were no EQ-5D-5L index values for Turkey to calculate the benefit value, German coefficients were usedCitation31. No Turkey-specific utility values for the health states of unscheduled outpatient visits, emergency room visits, and hospitalizations are available. Therefore, we used utility data estimated by Lloyd et al.Citation34 for unscheduled admissions and hospitalizations; and, as no utility data could be found in the literature for emergency room visits due to severe asthma, the decrease in the benefit value was estimated by considering the research findings obtained by Lloyd et al.Citation34 and Willson et al.Citation35 ().
Cost inputs
Medical service costs (per patient)
We sourced the direct medical cost data from the Social Insurance Institution of Turkey, which is a governmental reimbursement agency by the ICD-10 code. For the base-case analysis, we obtained from the billing departments of each of the four hospitals (MEDULA costs, referring to the Health Practice Communiqué) the covered and reimbursable asthma care costs for the 12 months preceding enrollment in the study—calculated separately for each patient’s emergency visits, unscheduled visits, hospitalizations, and outpatient visits. The costs for asthma-related health care services (outpatient visit, emergency room visit, hospitalization) provided at other health care facilities than one of the four primary medical centers were estimated within the scope of the Health Practice Communiqué price list based on patient records, patient statements, and expert opinion. These estimated cost data were used in the scenario analyses.
The following inputs were used to estimate the 12-month costs of each patient’s HRU ():
Table 3. Cost inputs (₺).
Disease monitoring
Standard asthma treatment: costs of SoC asthma treatment in the absence of asthma exacerbations (treatment costs for the SoC arm).
OML + SoC asthma treatment: costs of asthma treatment in the absence of asthma exacerbations in which omalizumab complements SoC (treatment costs for the OML + SoC arm).
Unscheduled outpatient visits: costs of outpatient visits for asthma exacerbations requiring the administration of oral steroids but no emergency care (treatment costs for both the OML + SoC and SoC arms).Emergency room visits: costs of treatment for exacerbations requiring emergency treatment, including anaphylactic shock, dyspnea, and other asthma exacerbations requiring emergency treatment (treatment costs for both the OML + SoC and SoC arms)
Hospitalizations: costs of hospitalizations for exacerbations requiring hospitalization (treatment costs for both the OML + SoC and SoC arms)
Note that for a patient in the severe allergic asthma state without HRU the costs equaled the sum of the disease monitoring costs and the standard asthma treatment costs.
Medication costs (per patient)
The cost of medications administered during emergency room visits or hospitalizations was obtained from the invoices sent to MEDULA. For medications prescribed during outpatient visits, costs were calculated as follows. Per expert opinion, we assumed that all medications and dosages were to be taken for 12 months. In total, 71 different medications and/or regimens were thus identified across 206 patients. Retail sales prices were taken from the June 2019 price list of the Turkish Medicines and Medical Devices Agency. To these retail prices, we applied the discounts on original drugs (with or without generics), generic drugs, and all drugs over 20 years old imposed by the Social Security Institution Health Implementation Communiqué and published in the Official Gazette in 2019. Since 2006, the Social Security Institution is the sole reimbursement agency in Turkey.
Analyses
Base-case analyses
We calculated the cost of SoC, the cost of OML + Soc, and the difference between both. We estimated the life years (LY) and quality-adjusted life years (QALY) for the Soc and OML + Soc groups and the difference between both. We then calculated the incremental cost-effectiveness ratios using the LY and the QALY metrics, the estimate the additional cost required to gain, respectively, 1 LY and 1 QALY.
Scenario analyses
First, variations in discount rates, utility values, and time horizons were applied in scenario analyses. Discount rates, mainly set by consensus, vary from jurisdiction to jurisdiction, and often within jurisdictions as well. Since there is no discount rate determined for Turkey, the 3% discount rate referenced by WHO was usedCitation36 but It has also been argued that different discount rates might apply to costs and outcomes. We used the German coefficient for the EQ-5D-5L, though we also considered adopting the coefficient for The Netherlands. Depending on various factors, treatments may vary in duration and this may affect the time horizon. Second, as some patients may not respond to omalizumab, we asked specialist physicians to review 12-month asthma control data in patients in the OML + SoC arm and identify non-responders. These were subsequently removed and a scenario analysis with revised transition probabilities was performed. In a final scenario analysis, we included the estimated costs that were calculated according to the Health Practice Communiqué price list for services provided at other healthcare facilities as recalled by patients.
Sensitivity analyses
One-way (OWSA) and probabilistic sensitivity analyses (PSA) were carried out to determine how the impact of uncertainty in the parameters. In the one-way sensitivity analysis, 20% upper and lower limit values were used and the impact on cost-effectiveness results were estimated and represented in a Tornado Diagram. The fixed 20% upper and lower limit approach was preferred over an approach mixing statistical estimates of precision, such as for instance the 95% confidence interval because few such data were available for key parameters. In the PSA, a Monte Carlo simulation with 1000 iterations was executed.
Ethics approval
This study was approved by the Clinical Research Ethics Committee of Gazi University Hospital (decision 582, dated 27 November 2017).
Results
Base-case analysis
Patients treated with omalizumab plus SoC showed an overall survival of 21.26 years compared to 13.60 for patients receiving SoC only, for an incremental survival benefit of 7.67 life years (LY) (). After adjustment for quality of life, QALY estimates were 13.35 for the patients in the omalizumab plus SoC arm and 10.08 for the patients in the SoC arm, for a QALY differential of 3.27 QALYs. The costs for emergency room visits, hospitalizations, and unscheduled outpatient treatments (including the cost of using short and high-dose oral corticosteroids) in the SoC group were higher compared to the OML + SoC group. While the standard treatment cost in severe asthmatic patients was ₺23,607.08, when the cost of the omalizumab medication was added to the comparatively lower treatment and disease management costs for the OML + SoC patients, the cost of patients receiving treatment in this group increased by ₺401,722.74 to ₺425,329.81. The absolute and quality-adjusted life year differentials favoring OML + SoC yet costs favoring SoC yielded an ICER value of an incremental cost of ₺52,427.04 per LY gained (g) and an ICER value of an incremental cost of ₺122,675.57 per QALYg.
Table 4. Base-case results.
There is no specified willingness-to-pay threshold for Turkey. Consistent with WHO guidanceCitation37,Citation38, we applied 1 and 3 times the GDP as a threshold. The LY ICER of an incremental ₺52,427.04 to gain 1 LY was marginally (i.e. 0.01%) above the 2019 GDP of ₺52,316 and well below the 3XGDP of ₺156,948 to gain 1 LY. The QALY ICER value of ₺122,675.57 per QALYg exceeded the 2019 GDP of ₺52,316 but was below the 3xGDP of ₺156,948 per QALYg; suggesting that OML + SoC is a cost-effective treatment for severe asthma.
Scenario analyses
In a first scenario analysis (), we applied four sets of hypothetical discount rates for costs and outcomes. The lowest QALY ICER value was obtained when costs were discounted by 4% and outcomes by 1.5% (₺81,361.36/QALYg), compared to the QALY ICER of ₺103,942.01/QALYg when no discounting was applied and ₺112,955.84 when both costs and outcomes were discounted at 1.5%. When the utility value obtained from the EQ-5D-5L scale is calculated using the Dutch index scores, the ICER value increases to ₺145,355.04/QALYg. Compared to the base-case time horizon of 54 years starting at age 19, 30 years of treatment is associated with an ICER of ₺134,625.17/QALYg, 10 years of treatment translates into an ICER of ₺246,747.13/QALYg, and when treatment is stopped after 1 year, the ICER rises to ₺633,840.81/QALYg. Running the model for the OML complete response group with the transition probabilities of Turkey yields an ICER value of ₺76,293.46/QALYg. Applying the transition probabilities of Turkey as input parameters resulted in an ICER value of ₺96,717.41/QALYg. When the input parameter of the OML complete responder patient group was used, the ICER value was calculated as ₺104,340.03/QALYg. In the case of adding the estimation data on the costs, the ICER was determined as ₺121,955.65/QALYg ().
Table 5. Scenario analysis results.
Sensitivity analyses
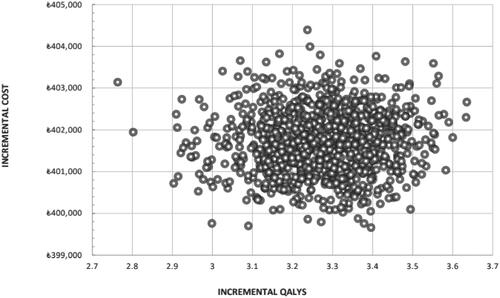
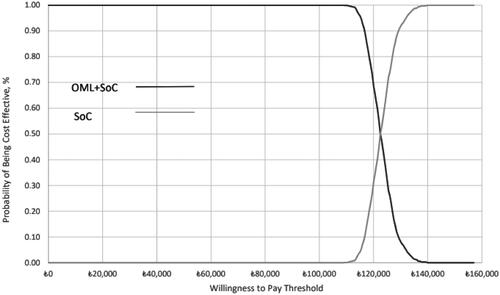
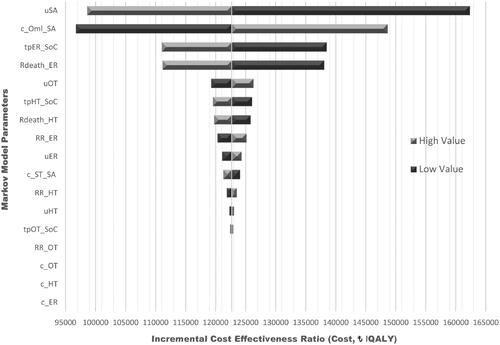
OWSAs () revealed that severe asthma utility values were the most influential parameter: a decrease in the utility values is associated with an increase in the QALY ICER up to ₺162,330.78/QALYg, whereas when the utility value increases, the QALY ICER decreases to ₺98,591.09/QALYg. The second influential parameter was the disease management costs in the OML + SoC group, with ICERs up to ₺148,648.14/QALYg. In PSAs, the average increase in direct medical costs with the addition of omalizumab to SoC was ₺401,728.28, and the average gain in QALY was 3.27, yielding a PSA ICER of ₺122,675.30/QALYg. All 1000 iterations yielded estimates in the northeast quadrant of the cost-effectiveness plane (). The cost-effectiveness curve () demonstrates that OML + SoC has a 50% likelihood of being cost-effective at a willingness-to-pay threshold of ₺122,500 rising to a 100% likelihood at a threshold of ₺140,000/QALYg.
Figure 2. Tornado diagram of one-way sensitive analyses. Abbreviations. uSA, utility of severe asthma; c_OML_SA, severe asthma OML cost; tpER_SoC, transition probabilities emergency room ex. on standard therapy; Rdeath_ER, emergency room ex. mortality rate; uOT, utility of outpatient treated; tpHT_SoC, transition probabilities hospitalized ex. on standard therapy; Rdeath_HT, hospitalized ex. mortality rate; RR_ER, relative risk for emergency room ex.; c_ST_SA, severe asthma standard therapy cost; uER, utility of emergency room ex.; RR_HT, relative risk for hospitalized ex.; uHT, utility of hospitalized ex.; tpOT_SoC, transition probabilities outpatient treated on standard therapy; RR_OT, relative risk for outpatient treated; c_OT, outpatient treated cost; c_HT, hospitalized ex. Cost; c_ER, emergency room ex. cost.
Discussion
This analysis based on real-world patient-level data from four major medical centers in Turkey evaluated the clinico-economic benefit of adding omalizumab to SoC in the treatment of adult patients with severe allergic asthma. The principal findings are 3-fold. First, compared to SoC, adding omalizumab to SoC was associated with decreases in the use of oral corticosteroids, asthma medication inhalers, and short-acting rescue medication. Second, in terms of healthcare utilization, the omalizumab regimen was also associated with lower rates of hospitalization, emergency room visits, and unscheduled outpatient visits. Third, these reductions in medication use and health care resource consumption offset—in part but not totally—the incremental cost of adding omalizumab to SoC, yet did so within boundaries of cost-effectiveness set at 3× the GDP for Turkey.
When evaluating cost-effectiveness, a key question is whether the treatment being evaluated yields clinically relevant benefits over the comparator at a justifiable cost. Specific to the present study, a related important question is whether the clinical benefits observed in this Turkish real-world sample are aligned with those observed in other observational studies. This was the case in our study as the findings are consistent with those from a systematic review and a related meta-analysis of 42 real-world studies on omalizumab in severe allergic asthmaCitation39–42. These studies firmly established the short-term effectiveness of omalizumab over the first year of treatment, provided strong evidence of long-term effectiveness up to 4 years, and emergent effectiveness evidence beyond. The meta-analysis, which per data availability was limited to assessments at 4–6, 12, and 24 months, consistently revealed large proportions of treatment responders, as well as improvements in respiratory function and asthma symptom control, reductions in oral and inhaled corticosteroid use, reductions in exacerbations and hospitalizations—all contributing to improvements in disease-specific quality of life.
Our study calculated costs as real and estimated. The salient finding is that both calculations showed that healthcare utilization and associated costs with regard to unscheduled outpatient visits, emergency room visits, and hospitalization were lower. Planned outpatient visits were higher in cost but this is attributable to the need for scheduled injections of omalizumab as per the therapy protocol and therefore the need for a planned visit. In a Polish multicenter study by Różyk et al. positive improvement was observed in all indicators except routine physician examinations 52 weeks after starting omalizumab therapy—here too, attributable to the number of visits for omalizumab injectionsCitation43. In a US cost-effectiveness study of omalizumab vs. standard therapy from the payer perspective, the per-person hospitalization, emergency room visit, and unscheduled outpatient visit costs requiring corticosteroids were lower in patients treated with omalizumab than in asthmatic patients receiving standard therapy. Here too, the per-person cost for routine visits was higher for omalizumab-treated patientsCitation44.
Patients receiving the omalizumab regimen achieved an additional 3.27 QALYs compared to those treated with standard therapy. This QALY benefit is higher than the 1–2 QALY gains in prior studiesCitation6,Citation21,Citation22,Citation45. Further, the cost-effectiveness of omalizumab in terms of asthma exacerbations avoided or improved ACT scores have been demonstrated as wellCitation23. Two studies in Japan concluded that omalizumab was not cost-effective, though these findings should be interpreted within the context of the local healthcare systemCitation46,Citation47.
The scenario analyses showed that assumptions other than omalizumab treatment duration did not materially affect the cost-effectiveness results. This was the case across varying reduction rates, EQ-5D-5L index scores, patient costs, transition probabilities, and response to omalizumab. The ICER value decreased by ∼₺18,000 to ₺104,304 in a remodeling exercise that removed those patients who had a partial or no response to omalizumab therapy. Though specific data are not available, we hypothesize that patients with eosinophilic asthma were the most likely partial responders. At the time of our study, omalizumab was the de facto standard of care for both allergic and eosinophilic asthma. Between 2015 and 2018 four monoclonal drugs were approved specifically for eosinophilic asthma, but these were not on the Turkish formulary and in the Turkish reimbursement system at the time of our study. In late 2021 another monoclonal drug was approved, which has no allergic or eosinophilic phenotype or biomarker limitation on its label. It will be important to replicate and extend our study once these agents are available in Turkey.
A constraint in our study was the unavailability of transition possibilities specific to Turkey and the need to apply transition probabilities from external sources. Turkey has a different health system structure compared to countries for which cost-effectiveness studies are available. Health services (including secondary and tertiary care) are highly accessible in Turkey and the absence of a formal referral chain increases the number of patients who enter secondary care when they have health problems. We also acknowledge that the inadequate management of asthma in primary care in Turkey increases the entry of asthma patients into inpatient or outpatient secondary care. Hence, we followed the expert opinion that the rates of outpatient visits, emergency room visits, and hospitalizations should be evaluated in a scenario analysis using inputs reflecting the Turkish health system. This lowered the ICER to ₺96,717.41 per QALY. Sensitivity analyses revealed that most inputs were robust to variation with omalizumab remaining within the willingness-to-pay threshold—except for the 20% increase in utility value for severe asthma. A willingness-to-pay threshold exceeding ₺144,000 was estimated to achieve 100% cost-effectiveness for the omalizumab regimen. Lastly, as noted also in other studiesCitation34,Citation35, there is a time-dependency to our findings: when the time horizon in the analysis decreases from 54 years to 30 years the OML + SoC ICER of ₺134,625.17 per QALYg exceeds the willingness-to-pay threshold; with the corresponding estimates being ₺246,747.13 per QALY gained for 10 years and ₺633,840.81 per QALY gained for 1 year. Given its high cost, early discontinuation as opposed to life-long treatment is a very high burden to providers and their payers.
Our study has limitations while also identifying areas for future research. Although all patients were treated in accordance with the GINA guidelinesCitation1, their treating physicians may have considered additional factors. This may explain why, in this real-world study of routine clinical practice, some differences were noted between the patients in the OML + SoC and the SoC groups. Patients who resided in other provinces of Turkey were initially at one of the four sites in our study but visited local hospitals when experiencing an exacerbation. As we could not retrieve cost data from these local hospitals, we had to apply general cost estimates while also needing to rely on patient recall of such events. The EQ-5D-5L does not have weights for Turkey. We applied coefficients for Germany because of the relative similarity between both countries in such indicators as chronic disease prevalence, population structure, life expectancy at birth and at age 65, and death rates. Increasing the number of iterations in the probabilistic sensitivity analyses might narrow the precision of estimates. We cannot exclude the possibility of bias inherent to observational studies like ours. Budget permitting, future studies will benefit from clinical validation and programming validation.
In conclusion, this independent cost-effectiveness study for Turkey found that adding omalizumab to standard therapy in severe allergic asthma is associated with a gain of 3.27 QALYs at a relative additional cost of ₺122,675 per QALY gained. This underscores omalizumab therapy’s clinical, quality of life, and economic benefit in the treatment of Turkish patients suffering from severe allergic asthma.
Transparency
Declaration of funding
This research was supported by a Tubitak grant from The Scientific and Technological Research Council of Türkiye. This research was conducted independently and without funding from Novartis.
Declaration of financial/other relationships
IO, SB, and GK participated in an Advisory Board sponsored by Novartis. IO, SB, GK, FOE, and ED received honoraria for lectures from Novartis. DT, MT, AFK, TH, OA, and KKI declare no conflict of interest. IA holds equity in Matrix45, LLC, which has been contracted by Novartis for work on omalizumab in various indications. By company policy, associates of Matrix45 cannot hold equity in sponsor organizations, nor provide services or receive compensation independently from sponsor organizations. Matrix45 provides its services on a non-exclusivity basis.
Author contributions
DT designed the study, collected the data, analyzed the data, and developed an initial draft of the manuscript. data analysis and wrote the article. MT supervised the study in all its phases. IA advised on the structure of the manuscript and edited the manuscript. The remaining authors provided access to patients and facilitated the implementation of the study. DT and IA constituted the writing committee. All other authors reviewed the manuscript for scientific and technical merit. All authors approved the submission version of the manuscript and agreed to submit to the journal.
Acknowledgements
The authors would like to thank Job FM van Boven (University Medical Center Groningen, The Netherlands) for his guidance during the Tubitak fellowship of Tugay that formed the basis of the study reported in this manuscript. The authors also would like to thank Maarten Postma (University Medical Center Groningen, The Netherlands) for his support of the Tubitak fellowship.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Previous presentations
Oral presentation, Turkish Thoracic Society, 25th Annual National Congress, Antalya, Turkey, 24–28 May 2022.
Data availability statement
Requests to be submitted for review to Deniz Tugay.
References
- Global Initiative for Asthma. Available from: https://ginasthma.org/
- GBD 2015 Chronic Respiratory Disease Collaborators. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir Med. 2007;5(9):691–706.
- Üner S, Balcılar M, Ergüder T, editors. Türkiye Hanehalki Sağlik Araştirmasi Bulaşici Olmayan Hastaliklarin Risk Faktörleri. Ankara: Dünya Sağlık Örgütü Avrupa Bölge Ofisi, T.C. Sağlık Bakanlığı; 2018.
- Allergy & Asthma Network. How severe is my asthma: classifying asthma severity. Available from: https://allergyasthmanetwork.org/news/how-severe-is-my-asthma/
- Von Bülow A, Kriegbaum M, Backer V, et al. The prevalence of severe asthma and low asthma control among Danish adults. J Allergy Clin Immunol Pract. 2014;2(6):759–767.
- Belhassen M, Demoly P, Bloch-Morot E, et al. Costs of perennial allergic rhinitis and allergic asthma increase with severity and poor disease control. Allergy. 2017;72(6):948–958.
- Nordon C, Grimaldi-Bensouda L, Pribil C, et al. Clinical and economic burden of severe asthma: a French cohort study. Respir Med. 2018;144:42–49.
- Serra-Batlles J, Plaza V, Morejón E, et al. Costs of asthma according to the degree of severity. Eur Respir J. 1998;12(6):1322–1326.
- Nunes C, Pereira AM, Morais-Almeida M. Asthma costs and social impact. Asthma Respir Pract. 2017;6:2–11.
- Weiss KB, Gergen PJ, Hodgson T. An economic evaluation of asthma in the United States. N Engl J Med. 1992;326(13):862–866.
- Bateman ED, Hurd SS, Barnes PJ, et al. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J. 2008;31(1):143–178.
- Çelik EG, editors. Astım Tanı ve Tedavi Rehberi 2020 Güncellemesi. Ankara: BULUŞ Tasarım ve Matbaacılık Hizmetleri San. Tic.; 2020.
- Ichikawa T, Sugiura H. Essence of the Japanese guidelines for adult asthma. In: Yokoyama A, editor. Advances in asthma: pathophysiology, diagnosis and treatment. Singapore: Springer; 2019. p. 117–131.
- Matsumoto H. Treatment with anti-IgE monoclonal antibody and free IgE. In: Yokoyama A, editor. Advances in asthma: pathophysiology diagnosis and treatment. Singapore: Springer; 2019. p. 145–156.
- Abraham I, Alhossan A, Lee C, et al. “Real-life” effectiveness studies of omalizumab in adult patients with severe allergic asthma: systematic review. Allergy. 2016;71(5):593–610.
- Alhossan A, Lee C, MacDonald K, et al. “Real-life” effectiveness studies of omalizumab in adult patients with severe allergic asthma: meta-analysis. J Allergy Clin Immunol Pract. 2017;5(5):1362–1370.e2.
- Faulkner K, MacDonald K, Abraham I, et al. “Real-world” effectiveness of omalizumab: an updated meta-analysis of research on adults with severe allergic asthma. Expert Rev Clin Immunol. 2021;17(1):73–83.
- MacDonald KM, Kavati A, Ortiz B, et al. Short- and long-term real-world effectiveness of omalizumab in severe allergic asthma: systematic review of 42 studies published 2008–2018. Expert Rev Clin Immunol. 2019;15(5):553–569.
- Sullivan PW, Li Q, Bilir PS, et al. Cost-effectiveness of omalizumab for the treatment of moderate-to-severe uncontrolled allergic asthma in the United States. Curr Med Res Opin. 2020;36(1):23–32.
- Levy AN, Ruiz AJ, Soler NG, et al. Cost-effectiveness of omalizumab in severe persistent asthma in Spain: a real-life perspective. J Asthma. 2015;52(2):205–210.
- van Nooten F, Stern S, Braunstahl G, et al. Cost-effectiveness of omalizumab for uncontrolled allergic asthma in The Netherlands. J Med Econ. 2013;16(3):342–348.
- Brown R, Turk F, Dale P, et al. Cost-effectiveness of omalizumab in patients with severe persistent allergic asthma. Allergy. 2007;62(2):149–153.
- Vennera MC, Valero A, Uria E, et al. Cost-effectiveness analysis of omalizumab for the treatment of severe persistent asthma in real clinical practice in Spain. Clin Drug Investig. 2016;36(7):567–578.
- Global Asthma Network. Global initiative for asthma. Global strategy for asthma management and prevention. Chicago (IL): Global Initiative for Asthma; 2022.
- Select Statistical Service. Population proportion–sample size. Available from: https://select-statistics.co.uk/calculators/sample-size-calculator-population-proportion/
- Macrotrends. Turkey life expectancy 1950–2023. Available from: https://www.macrotrends.net/countries/TUR/turkey/life-expectancy
- Rahavi H, Taft AS, Mirzaei M. Years of life lost due to asthma in a population-based 10-year study in Yazd, Iran. Lung India. 2018;35(6):472–475.
- Asthma Control Test. Available from: www.asthmacontroltest.com
- Available from: https://euroqol.org/support/how-to-obtain-eq-5d/
- Özgen Alpaydın A, Yorgancıoğlu A. Validity and reliability of “asthma quality of life questionnaire” in a sample of Turkish adult asthmatic patients. Tüberküloz ve Toraks Dergisi. 2011;59(4):321–327.
- EQ-5D. Index value set calculators. SPSS_value set – Germany – EQ-5D-5L [cited 2017 Sept 28]. Available from: https://euroqol.org/support/analysis-tools/index-value-set-calculators/
- Watson L, Turk F, James P, et al. Factors associated with mortality after an asthma admission: a national United Kingdom database analysis. Respir Med. 2007;101(8):1659–1664.
- Turkish Statistical Institute. TÜİK istatistik veri portal. Available from: data.tuik.gov.tr/Kategori/GetKategori?p=nufus-ve-demografi-109&dil=2
- Lloyd A, Price D, Brown R. The impact of asthma exacerbations on health-related quality of life in moderate to severe asthma patients in the UK. Prim Care Respir J. 2007;16(1):22–27.
- Willson J, Bateman ED, Pavord I, et al. Cost-effectiveness of tiotropium in patients with asthma poorly controlled on inhaled glucocorticosteroids and long-acting b-agonists. Appl Health Econ Health Policy. 2014;12(4):447–459.
- Edejer TT, Baltussen R, Adam T, et al., editors. Cost-effectiveness and strategic planning (WHO-CHOICE). Available from: https://www.who.int/choice/cost-effectiveness/en/
- Evans DB, Lim SS, Adam T, et al. Evaluation of current strategies and future priorities for improving health in developing countries. BMJ. 2005;331(7530):1457–1461.
- Edoka IP, Stacey NK. Estimating a cost-effectiveness threshold for health care decision-making in South Africa. Health Policy Plan. 2020;35(5):546–555.
- Norman G, Faria R, Paton F, et al. Omalizumab for the treatment of severe persistent allergic asthma: a systematic review and economic evaluation. Health Technol Assess. 2013;17(52):1–342.
- Lai T, Wang S, Xu Z, et al. Long-term efficacy and safety of omalizumab in patients with persistent uncontrolled allergic asthma: a systematic review and meta-analysis. Sci Rep. 2015;5:8191.
- McQueen RB, Sheehan DN, Whittington MD, et al. Cost-effectiveness of biological asthma treatments: a systematic review and recommendations for future economic evaluations. Pharmacoeconomics. 2018;36(8):957–971.
- Agache I, Beltran J, Akdis C, et al. Efficacy and safety of treatment with biologicals (benralizumab, dupilumab, mepolizumab, omalizumab and reslizumab) for severe eosinophilic asthma. A systematic review for the EAACI guidelines – recommendations on the use of biologicals in severe asthma. Eur J Allergy Clin Immunol. 2020;75(5):1023–1042.
- Jahnz-Różyk K, Lis J, Warchol M, et al. Clinical and economic impact of a one-year treatment with omalizumab in patients with severe allergic asthma within a drug programme in Poland. BMC Pulm Med. 2018;18(1):48.
- Campbell JD, Spackman DE, Sullivan SD. The costs and consequences of omalizumab in uncontrolled asthma from a USA payer perspective. Allergy. 2010;65(9):1141–1148.
- Levy ML, Andrews R, Buckingham R. Why asthma still kills: the national review of asthma deaths confidential enquiry report. London: Royal College of Physicians; 2014.
- Wu AC, Paltiel D, Kuntz KM, et al. Cost-effectiveness of omalizumab in adults with severe asthma: results from the asthma policy model. J Allergy Clin Immunol. 2007;120(5):1146–1152.
- Morishima T, Ikai H, Imanaka Y. Cost-effectiveness analysis of omalizumab for the treatment of severe asthma in Japan and the value of responder prediction methods based on a multinational trial. Value Health Reg Issues. 2013;2(1):29–36.