Abstract
Objective
To evaluate the cost-effectiveness of supplemental breast imaging modalities for women with heterogeneously and extremely dense breasts and average or intermediate risk of breast cancer (BC) in the USA, and analyze capacity requirements for supplemental magnetic resonance imaging (MRI) and contrast-enhanced mammography (CEM).
Methods
Clinical and economic outcomes for supplemental imaging modalities including full- and abbreviated-protocol MRI (Fp-MRI, Ab-MRI), CEM, and ultrasound (U/S) as add-on to x-ray mammography (XM) or digital breast tomosynthesis (DBT), were compared to XM or DBT alone, in a decision tree linked to a Markov chain validated by comparison with a microsimulation analysis. A Delphi panel supplemented model input parameters from the literature. A capacity model evaluated the number of additional daily scans and scanners required for Fp-MRI and CEM.
Results
Compared to XM or DBT alone, all supplemental imaging protocols were cost-effective. Both Fp- and Ab-MRI, and to a lesser extent CEM and U/S, yielded superior clinical outcomes to XM or DBT. Compared to XM alone, U/S and Ab-MRI had the lowest incremental cost-effectiveness ratios (ICER). For U/S, the ICER was $23,394 for the average-risk population and $13,241 for the intermediate-risk population. For CEM, the ICER was $38,423 and $23,772, respectively. For the extremely dense subpopulation with intermediate risk, supplemental screening requirements could be accommodated by conducting one Fp-MRI scan per day per existing general scanner.
Conclusions
While ultrasound had the lowest ICER, MRI and CEM demonstrated the best clinical outcomes, compared to XM or DBT alone for women with dense breasts and intermediate and high risk. Existing MRI scanner capacity has the potential to meet most of the supplemental screening needs of this population.
Introduction
Breast cancer is the world’s most common cancer, and the leading cause of cancer death among women – accounting for 2.3 million diagnoses and 685,000 deaths in 2020Citation1. While breast cancer detection is a topic of high public awareness and prioritization, the specific risks women with dense breast tissue face are relatively overlooked. This is despite the fact that approximately 35% of women in the USA aged 40–74 have heterogeneously dense breasts (Breast Imaging Reporting and Data System [BI-RADS] category C) and 8% have extremely dense breasts (BI-RADS category C)Citation2,Citation3.
Women with dense breasts face a “four-fold challenge” related to accurate detection of breast cancer. Firstly, greater breast density is associated with elevated cancer risk. Women with dense breasts face a 3–5-fold higher risk of developing breast cancer than those with non-dense breastsCitation4,Citation5. Secondly, breast malignancies are more likely to be missed with routine screening modalities, such as x-ray mammography (XM) and digital breast tomosynthesis (DBT) – due to reduced sensitivity in dense breast tissueCitation6–9. Thirdly, while guidelines recommend supplemental screening for women with dense breastsCitation10, there is a lack of clarity on which supplemental modalities are preferred, and there is restricted access to supplemental modalities. Finally, breast density assessment and reporting in XM is inconsistent across the USA. Currently, 38 states are required to inform women about their breast density, but which patients are informed and the type of information received is not standardized across statesCitation11. A national requirement by the FDA has been recently issued where, by 2024, all states will need to send federal density notification statements (“not dense” or “dense”) to patientsCitation12. This has the potential to enhance the low understanding of elevated risk among women with dense breastsCitation13.
Add-on modalities to XM or DBT have demonstrated improved sensitivity in dense breasts and include contrast-enhanced breast magnetic resonance imaging (MRI – in its full [Fp-MRI] or abbreviated [Ab-MRI] protocols), contrast-enhanced mammography (CEM), and ultrasound (U/S)Citation14–17. However, no supplemental imaging modality is recognized as being standard of careCitation18 and no consensus exists among guidelines on what the preferred supplemental modality should be. Guidelines state that there is insufficient evidence on relative screening accuracy of supplemental imaging modalities and a lack of cost-effectiveness studies across all available supplemental modalitiesCitation10,Citation19–22. A recent umbrella literature reviewCitation23 found the systematic literature too heterogeneous to enable comparisons between modalities on specificity but concluded that, regardless of modality, all women with dense breasts may benefit from supplemental screening after mammography or DBT. Moreover, individual studies and another recent systematic literature review have suggested improved cancer detection rates (CDR) for contrast-enhanced modalities, and especially for MRICitation24–26.
The evidence on the cost-effectiveness of add-on modalities to XM and DBT for the dense breast population is sparse, results are mixed, and stratification by breast cancer risk is usually not consideredCitation27. Thus, there is a substantial barrier to informed decision-making around reimbursement for supplemental imaging modalities. To date, the cost-effectiveness of supplemental MRI after XM has only been reported for the following populations:
Extremely dense breast population with average breast cancer riskCitation28,Citation29;
Intermediate risk of breast cancer and dense breasts in its fullCitation30 and abbreviatedCitation31 protocols; and
Intermediate risk of breast cancer and extremely dense breasts in its abbreviated protocolCitation32.
To our knowledge, the cost-effectiveness of other supplemental modalities (to either XM or DBT) or for other breast cancer risk subpopulations has not been reported.
There is also little information on the investment requirements necessary to develop capacity for supplemental screening for women with dense breasts. While CEM is sometimes believed to be a more affordable and standardizable option than MRICitation33, with a lower sensitivity but a higher specificityCitation25, this hypothesis has not been robustly tested across supplementary screening modalities.
This study, in the setting of the USA, compares the cost-effectiveness of all currently available supplemental imaging modalities to XM or DBT (Fp-MRI, Ab-MRI, CEM, U/S) with XM or DBT alone for women with dense breasts and average or intermediate breast cancer risk (as defined by ACR guidelines). A separate analysis calculates capacity investment (number of additional scans per existing scanner and number of additional scanners) that would be required to enable supplemental screening with Fp-MRI and CEM.
Materials and methods
Cost-effectiveness analysis
Population
Asymptomatic women in the USA with dense (meaning heterogeneously or extremely dense henceforth) breasts between 40–74 years of age annually invited to screeningCitation27 were studied, with two subpopulations according to ACR breast cancer risk classificationCitation34,Citation35: average risk (women with unknown personal or family history of breast cancer, or <15% lifetime risk of breast cancer), and intermediate risk (women with a personal history or first-degree family history, or who have 15–20% lifetime risk of breast cancer).
Model structure
A decision tree () linked to a Markov chain () modeled the screening pathway for asymptomatic women with dense breasts. The decision tree compared clinical outcomes of supplemental screening after a negative XM or DBT against outcomes of XM or DBT alone. It accounted for all potential diagnostic work-up outcomes (true positive, false positive, true negative, false negative) based on input values for sensitivity and specificity associated with a particular imaging modality.
Figure 1. Decision tree structure. Abbreviations: DBT, digital breast tomosynthesis; D, disease; T, test; +, positive; ‒, negative; TP, true positive; FP, false positive; FN, false negative; TN, true negative. T1m, tumor size ≤1 mm; T1a, tumor size >1 mm and ≤5 mm; T1b, tumor size >5 mm and ≤10 mm; T1c, tumor size >10 mm and ≤20 mm; T2, tumor size >20 mm and ≤50 mm; T3 tumor size >50 mm. Notes: T + and T‒ represent screening modality outcomes. TP, FP, FN, and TN represent pathological (biopsy) diagnosis outcomes. Supplemental imaging modalities under study: Ab-MRI, full MRI, CEM, and U/S. Dashed line (+/‒) indicates that the supplemental modality screening can be implemented or not depending on the result from the XM/DBT screening. When not implemented, the diagnostic outcomes FN/TN come directly from XM/DBT screening. When implemented, the diagnostic outcomes FN/TN come after the supplemental modality screening. The lower arm represents standard of care (i.e. screening with XM or DBT alone) and does not include supplemental modality screening. Each diagnosis outcome was directly associated with a terminal node in the decision tree and was each linked to a health state in the Markov model, as illustrated in . True positive screening outcomes were related to detected breast cancer of a particular tumor size following the tumor, node, and metastasis (TNM) system for staging breast cancerCitation36. False positives and true negatives were interpreted as no breast cancer. False negatives were assumed to relate to undetected breast cancer.
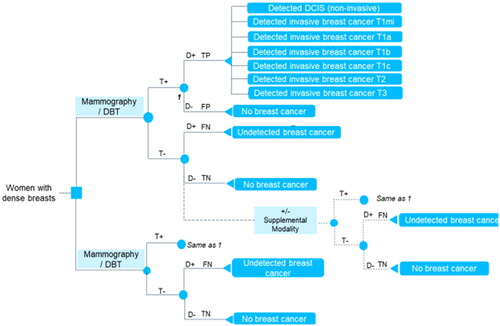
Figure 2. Markov model structure. Notes: adapted from Kaiser, DietzelCitation30. aRe-enter the decision tree after two cycles (some will develop cancer). bRe-enter the decision tree after two cycles (some undetected will be rescreened – and potentially detected at larger size). Interval cancers aim to capture the number of cancers that occur between screening intervals for undetected and no breast cancer individuals. DCIS, ductal carcinoma in situ, T1mi, tumor size ≤1mm; T1a, tumor size >1mm and ≤5 mm; T1b, tumor size >5mm and ≤10 mm; T1c, tumor size >10mm and ≤20 mm; T2, tumor size >20mm and ≤50 mm; T3 tumor size >50mm.
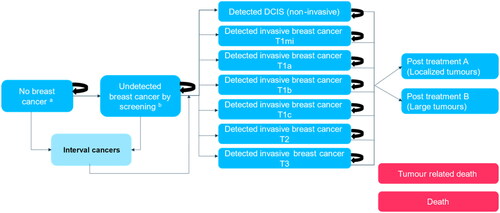
Each diagnostic outcome was directly associated with a terminal health state also represented in the Markov chain. True positive screening outcomes were related to detected breast cancer of a particular tumor size following the tumor, node, metastasis (TNM) system for staging breast cancerCitation36. False positives and true negatives were interpreted as lack of breast cancer. False negatives were assumed to relate to undetected breast cancer.
The Markov chain allows for recursiveness of the health states from the decision tree and captures long-term clinical and economic outcomes of screening. Annual cycle length was deployed, and half-cycle correction was applied. An annual screening frequency was modeled, as this is the standard recommendation from breast experts in the USA for women of 40 years old and aboveCitation19,Citation37. Full screening participation was assumed. XM or DBT was repeated every screening round and only women who had a negative XM or DBT result underwent supplemental screening. After a screening round, it was assumed women remained in the breast cancer health state or transited to another health state. Individuals with no breast cancer (true negatives or false positives) could develop cancers in subsequent rounds. Individuals with breast cancer were distributed between health states according to cancer tumor size (true positives) or were moved to the undetected breast cancer health state (false negatives).
When a cancer was first detected in one of the screening rounds, individuals were assumed to remain in that health state for one cycle and to transit to a post-treatment stage A (treatment for localized tumors) or B (extensive and systemic treatment of large tumors)Citation30,Citation31 in the subsequent cycle. A 100% treatment success rate was assumed. Screen-undetected cancers could be detected in subsequent screening rounds, when they were assumed to have grown to a larger tumor sizeCitation38,Citation39. It was assumed individuals could transit to the absorbing state of death (due to breast cancer or due to any other cause) at any time.
Model input parameters
Screening accuracy and epidemiology data
Sensitivity and specificity values for standalone XM and DBT, and supplemental screening modalities after a negative XM or DBT for women with dense breasts by average and intermediate breast cancer risk subpopulations were taken from the literature. Given the lack of sensitivity and specificity data for the intermediate-risk subpopulation for most supplemental screening modalities after negative XM, and for the average-risk subpopulation after negative DBT, a combined data point for the average and intermediate-risk subpopulation was used per each first-line screening modality (XM or DBT).
Delphi panel
An expert elicitation exercise was conducted to validate literature derived sensitivity and specificity values for screening modalities in the combined average/intermediate risk population (validation of Supplementary Table S1-1). Given the sparsity of the data in the existing literatureCitation23 and the lack of a common baseline that would allow sensitivity and specificity comparisons across modalities, a set of values that would better represent clinical practice experience than the literature was developed for a model scenario analysis.
Six experts were selected according to their knowledge and involvement in breast screening accuracy in the real world. They also had to meet the following expert’s participation criteria:
Role/expertise should align with one or more of the following:
Clinical expertise in breast imaging,
Clinical trial investigator for imaging modalities in breast cancer screening,
Researcher with experience conducting or evaluating breast cancer screening accuracy studies (observational) for imaging modalities,
Published peer-reviewed papers/technical reports/conference proceedings on the screening accuracy for breast cancer imaging modalities, or
Teaches medical students/residents/fellows on screening accuracy evidence for imaging modalities in breast cancer screening.
Five or more years of experience in their area of expertise.
Be extremely/very familiar with screening test accuracy measures.
One to 20 years of experience reading exams from supplemental screening imaging modalities following a normal mammogram or DBT:
Be fairly/very confident in providing an expert opinion on their supplemental screening modality of choice, for the screening of women with dense breasts.
Publication requirements:
Be aware of ongoing/published research on supplemental breast cancer screening for women with dense breasts, and
Published more than one empirical peer-reviewed article on the screening performance of breast imaging modalities.
Experts were asked to rank supplemental modalities in terms of screening accuracy and provide sensitivity and specificity estimates for values seen in clinical practice, including relative differences between screening modalities according to whether they were supplemental to XM or DBT. Expert estimates were collected independently and consolidated by Wickenstones. Consensus was prospectively defined as being achieved when 80% of the experts were in agreementCitation40,Citation41. A virtual meeting with all the available experts was conducted at the end to sign off on consensus achieved. See Supplementary Section 1 for further details on the Delphi Panel methodology.
Utilities
Utility values were used to estimate the health-related quality-of-life (HRQoL) of women in each health state (Supplementary Section 2). HRQoL differences related to tumor size upon detection were considered by assuming utility decrements by tumor size as follows: ductal carcinoma in situ (DCIS); ≤1 mm; >1 mm and ≤5 mm; >5 mm and ≤10 mm; >10 mm and ≤20 mm; >20 mm and ≤50 mm; and >50 mm. A reduction in HRQoL of 0.01 for undergoing screening was assumed to account for discomfort that women may experience due to screening proceduresCitation42. An additional 0.01 decrement in utility was assumed to account for cancers not detected by screening (false negatives). Utility decrements were also implemented to account for reduced HRQoL after treatment of localized tumors and extensive and systemic treatment of large tumorsCitation30,Citation31.
Health-care resource utilization
Screening costs were estimated using the Medicare Physician Fee Schedule Look-up tool [https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PFSlookup]Citation43. Using the Current Procedural Terminology (CPT) codes for each screening modality, the look-up tool provides the 2021 national average rate. Tumor-size related treatment costs were collected from the existing literatureCitation44 (Supplementary Section 2). No additional costs were assumed for post-treatment health states. Undetected breast cancers were assumed to incur no costs until the cancer is detected. All costs were adjusted to reflect 2021 prices.
Transition probabilities
Transition probabilities were based on the number of screen detected and clinically detected cancers (proxy for interval cancers) by TNM staging for supplemental MRI and first-line XM obtained from unreported data in Geuzinge et al.Citation28 (personal communication, see acknowledgments). Mortality rates by tumor size were calculated using the NHS predict tool (https://breast.predict.nhs.uk/tool)Citation30,Citation31 (Supplementary Table S2-1).
Cost-effectiveness implementation
The cost-effectiveness of screening modalities was analyzed for a simulated sample of 1,000 asymptomatic women with dense breasts undertaking screening. Clinical and health economic outcomes were reported. Costs and benefits beyond 1 year were discounted at a rate of 3%Citation45,Citation46. A lifetime horizon was assumed, and a healthcare perspective was adopted. Deterministic and probabilistic sensitivity analysis were performed.
First, the cost-effectiveness of supplemental modalities as an add-on to XM or DBT was calculated. Second, a cost-effectiveness analysis was performed for relevant pair-comparisons across all supplemental imaging modalities when applied after negative XM or DBT. The results were reported for the average and the intermediate breast cancer risk subpopulations and for relevant health states. Results for each health state of the Markov chain are reported in Supplementary Section 8.
The model was validated comparing the Markov model results to an adapted microsimulation model (Supplementary Section 6).
Capacity analysis
A capacity model was developed to estimate additional daily scans per existing scanner needed to implement supplementary MRI or CEM screening for women with dense breasts. In addition, the number of new scanners needed for breast cancer screening was calculated. It was assumed that the scanner infrastructure could be grouped into: (a) existing dedicated breast scanners, used only for breast imaging; (b) existing general scanners, used for various types of imaging, including breast imaging; and (c) new dedicated scanners, where payers may need to invest in extra scanners not included in the existing infrastructure. The relative proportion of general scanners extended to breast imaging was varied, and the corresponding investment needed and extra number of scans needed were recorded.
Capacity model input parameters are listed in Supplementary Section 3.
The capacity model presents results over a 15-year time frame for the extremely-dense (8% of women in the USA) and heterogeneously-dense (35% of women in the USA) breast populationCitation2 from 40–74 years of age. Results are shown for the combined average and intermediate risk populations as well as for each risk subgroup, where the proportion of individuals in the average and intermediate subgroups was taken from the literatureCitation47. For the main analysis, it was assumed that 40% of general MRI scanners could be extended for breast imagingCitation48 and that 3% were existing dedicated scanners only used for breast imagingCitation49. For CEM, the AMR Imaging H 1/19 reported that 0.15% of mammography scans were currently contrast-enhanced in the USA, resulting in 20 scanners.
Results
Cost-effectiveness analysis
Supplemental imaging as an add-on to XM or DBT
Add-on to XM
For women with dense breasts, all assessed supplemental imaging modalities improved clinical outcomes compared to XM alone, for both the average () and the intermediate () breast cancer risk subpopulations (with the exception of number of false positives). They were able to detect more invasive cancers, low- and high-grade DCIS, and reduce breast cancer deaths, the number of false negatives and the total number of undetected cancers (false negatives and interval cancers). These results were particularly prominent for contrast-enhanced modalities (Fp-MRI, Ab-MRI, and CEM) in the intermediate-risk subpopulation.
Table 1. Clinical and economic outcomes by supplemental imaging modality as an add-on to XM (annual screening) for the dense breast population and average breast cancer risk subpopulation per 1,000 screenings and lifetime horizon.
Table 2. Clinical and economic outcomes by supplemental imaging modality as an add-on to XM (annual screening) for the dense breast population and intermediate breast cancer risk subpopulation per 1,000 screenings and lifetime horizon.
Over a lifetime horizon, the simulated long-term total costs and effects were higher for all supplemental imaging modalities than those for XM alone. For both average () and intermediate () breast cancer risk subpopulations, the lowest incremental cost-effectiveness ratio (ICER) was for U/S ($23,394 and $13,241, respectively) followed by Ab-MRI ($38,423 and $23,772, respectively). The corresponding ICERs of all supplemental imaging modalities were below a willingness-to-pay (WTP) threshold of $100,000 per quality-adjusted life years (QALY), which is the standard health-benefit price benchmark used by the United States Institute for Clinical and Economic Review in its assessmentsCitation50.
The Markov model results for supplemental Fp-MRI as add-on to XM and XM alone were validated using a Microsimulation Screening Analysis (MISCAN)Citation51. Biennial screening and a mixed-risk population (combined average and intermediate populations) was assumed to align with the assumptions of the MISCAN model. Supplementary Section 5 shows results for different screening frequencies. Overall, results in both models show the same directionality with some differences in magnitudes due to structural modeling differences. The Markov model yielded higher incremental QALYs and costs, resulting in a lower ICER ($19,737) than the MISCAN model ($24,940) (Supplementary Section 6).
Add-on to DBT
Similar results were seen when DBT was the primary imaging modality instead of XM, for the average () and intermediate () breast cancer risk subpopulations. All clinical outcomes were improved when adding a supplemental modality to DBT, except for false positives. The lowest ICER was for U/S followed by Ab-MRI. The corresponding ICERs of all supplemental imaging modalities were below a willingness-to-pay (WTP) threshold of $100,000 per QALYCitation50 and, therefore, supplemental imaging as an add-on to XM was consistently cost-effective.
Table 3. Clinical and economic outcomes by supplemental imaging modality as an add-on to DBT (annual screening) for the dense breast population and average breast cancer risk subpopulation per 1,000 screenings and lifetime horizon.
Table 4. Clinical and economic outcomes by supplemental imaging modality as an add-on to DBT (annual screening) for the dense breast population and intermediate breast cancer risk subpopulation per 1,000 screenings and lifetime horizon.
It is notable that the number of detected cancers when using standalone DBT was lower than the number detected by standalone XM. This is due to the sensitivity values found in the existing literature (39% for DBT and 59% for XM).
Supplemental imaging modalities as add-ons to XM and DBT – sensitivity analysis and scenarios
Deterministic and probabilistic sensitivity analyses were performed to evaluate the reliability of results (Supplementary Section 4). Variables were breast cancer prevalence and incidence, undetected breast cancer mortality and utility, the discount rates for costs and effects, the cost of screening modalities, and the age at model start. The scenario analyses substantiated the conclusions of the main analyses.
Additional variables that could influence the model results were explored, i.e. changing the screening frequency, excluding DCIS, accounting for decline of breast density with age, assuming false positives decrements, and accounting for equipment costs. The ICERs for these scenarios for supplemental imaging modalities as an add-on to XM and for the average breast cancer risk subpopulation are reported in Supplementary Section 5. Despite ICERs being higher for all the scenarios compared to the base case model, they all sustained the conclusions of the main analyses.
Cost-effectiveness analysis using Delphi panel clinical experience estimates for sensitivity and specificity
compares ICER results for supplemental screening modalities after XM when using sensitivity and specificity values derived from the existing literature against clinical experience estimates (estimated using a Delphi panel methodology, see Supplementary Tables S1-1 and S1-2, respectively) by breast cancer risk subpopulations.
Figure 3. ICER results by supplemental imaging modality as add-on to XM when using sensitivity and specificity literature derived values vs clinical practice estimated values. (a) Average risk subpopulation; (b) Intermediate risk subpopulation. Abbreviations. Fp-MRI, full-protocol magnetic resonance imaging; Ab-MRI, abbreviated MRI; CEM, contrast-enhanced mammography; ICER, incremental cost-effectiveness ratio.
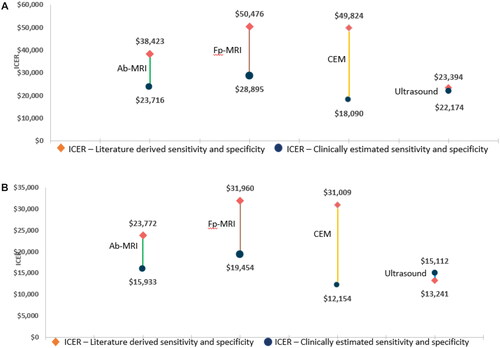
Using clinical experience estimates of sensitivity and specificity values yielded lower ICERs for all supplemental modalities (Fp-MRI, Ab-MRI, CEM, U/S), for both the average- and the intermediate-risk subpopulations, except for U/S in the intermediate risk subpopulation. In particular, the ICER for CEM decreased by more than 60% in both subpopulations, and the ICER for Fp-MRI decreased by around 40%. The difference for Ab-MRI was a bit smaller than for the other modalities (ICER decreased by 38% for the average-risk subpopulation and by 24% for the intermediate-risk subpopulation).
The main drivers for the differences between the literature and Delphi-derived values were the less positive clinical experience estimate of the sensitivity of standalone XM (30%) compared to the literature derived value (59%), the less positive sensitivity and specificity estimates for supplemental U/S (50% sensitivity and 85% specificity against 55% sensitivity and 94% specificity literature values), and the decrease in specificity for MRI (87% clinical practice values against 92% literature derived value) (see Supplementary Section 1).
Clinical outcome results for supplemental screening after XM, and economic and clinical outcomes results for supplemental screening modalities after DBT, using the clinical experience estimates, are reported in Supplementary Tables S5-2 and S5-3, respectively.
Capacity analysis
shows capacity results for Fp-MRI and CEM for the three scenarios under study for the extremely and the heterogeneously dense breast population.
Table 5. Capacity results by various availability scenarios of scanners and dense breast population: supplemental Ab-MRI and CEM as an add-on to XM.
A total of 2,321,688 women in the extremely dense breast group and a total of 10,157,386 mammogram-negative women in the heterogeneously dense group were modeled over a 15-year time horizon. This was based on the screening accuracy of XM and the prevalence of extremely (8%) and heterogeneous (35%) dense breasts among women in the USA. Forty percent of general MRI scanners were assumed to be extended for breast imaging use.
For the extremely dense breast with average and intermediate risk population, approximately three extra scans (two for the average risk and one for the intermediate risk subpopulations) per existing general scanner per day would be required to meet the 2,321,688 additional scans per year in the USA (if existing dedicated breast Fp-MRI scanners and general scanners combined were to be used [, 4th column]). In contrast, if only CEM were to be used to supplement XM, an additional 1,994 CEM scanners would be needed with investment costs of $826 million.
The results for the heterogeneously dense breast with average and intermediate risk population show that if dedicated breast Fp-MRI scanners and general scanners were to be used, 12 extra scans per existing general scanner per day (eight for the average risk and four for the intermediate risk subpopulations) or, alternatively, an additional investment of 2,901 dedicated scanners (with investment costs of $2,224 million) would be required to meet the 10,157,386 additional scans per year in the USA. If only CEM were to be used to supplement XM, an additional 8,790 CEM scanners would be needed, with investment costs of $3,640 million.
The above results are for the entire dense breast population from 40–74 years of age. When considering the dense breast population over 65 years of age (Supplementary Table S5-3), where breast density prevalence is assumed to decline and a proportion of patients no longer require supplemental screening for MRI, there was a reduction in the additional Fp-MRI scanners needed and the number of additional Fp-MRI scans. Moreover, if using abbreviated protocols, investment requirements would further decrease.
Discussion
To our knowledge, this is the first study analyzing the cost-effectiveness of all available supplemental imaging modalities (Fp-MRI, Ab-MRI, CEM, U/S) as add-ons to XM or DBT in women with dense breasts, by breast cancer risk subpopulations (average and intermediate risk). In the absence of a clinical trial comparing the screening efficiency of all currently available supplemental modalities for XM and DBT, the present study provides robust evidence on clinical and economic outcomes.
Cost-effectiveness of modalities
Over a lifetime horizon, all the supplemental imaging modalities were consistently and robustly cost-effective, when compared to XM or DBT alone, within a WTP threshold of $100,000/QALYCitation50. In general, supplemental modalities were able to detect more cancers than XM or DBT, as well as enabling fewer breast cancer deaths, and the occurrence of false negative results and undetected cancers.
Each supplemental imaging modality presented a unique combination of clinical and economic strengths and weaknesses. Clinical outcomes were particularly improved for supplemental contrast-enhanced modalities (Fp-MRI, Ab-MRI, CEM) and, within this group of modalities, false positives and false negatives were lower with supplemental MRI than with CEM. Contrast-enhanced modalities, especially MRI, were able to detect more cancers, particularly those of smaller tumor size (below 20 mm) compared to ultrasound. Detecting cancers at an earlier tumor stage led to more cancer deaths averted when using contrast-enhanced modalities than when using ultrasound after XM. Ultrasound was the supplemental modality with the highest number of undetected cancers, false negatives, and cancer deaths, but the lowest number of false positives.
However, despite MRI having higher sensitivity than CEMCitation52, the number of cancers detected (including DCIS) was similar for both; suggesting that CEM should be considered as an alternative option when MRI is not available or contra-indicated. From a purely economic perspective, U/S seemed more advantageous as it showed the lowest ICER. Consequently, the preferred supplemental screening modality after a negative XM or DBT could be determined according to the WTP threshold selected. Without adjusting for local system specificities, supplemental U/S might be preferred in healthcare systems that operate within WTP thresholds below $25,000/QALY. Ab-MRI may be preferred in those operating within WTP thresholds between $20,000 to 40,000/QALY.
While costs for the contrast-enhanced modalities were higher than for U/S, these costs were offset by higher QALY gains, that were driven by superior clinical outcomes. Compared to U/S, all contrast-enhanced supplemental modalities, especially Ab-MRI, had an increased CDR for invasive and precancerous (low- and high-grade DCIS) lesions, a reduction in the number of undetected and false-negative results, and a higher number of cancer deaths averted. The predicted clinical benefits for supplemental contrast-enhanced modalities were significant for both average- and intermediate-risk subpopulations. These clinical benefits may be more important than formal ICER considerations alone, especially from the patient perspective and in the context of population-based screening programs.
XM and DBT have lower sensitivity and, therefore, a higher false-negative detection rate and higher costs in women with dense breasts versus non-dense breastsCitation6–9. This study shows that when using supplemental screening, in particular MRI, the number of false negatives significantly decreases, being almost zero in some scenarios.
The Delphi panel concluded that literature-derived sensitivity and specificity values were not representative of clinical experience in the USA. Scenario analyses suggested that, when using the alternative Delphi-derived estimates for sensitivity and specificity, all supplemental imaging modalities (excluding U/S) had substantially lower ICERs than when using literature derived values. The Delphi panel participants confirmed that some screening accuracy estimates in the literature, as reported in a recent SLRCitation23, may lack clinical validity and could lead to overestimation of health gains attributable to modalities such as U/S and DBT.
Capacity requirements
The required investment in capacity in the USA to enable greater MRI adoption in the screening of women with dense breasts was calculated assuming that 40% of existing general MRI machines were also used for breast indications. For the extremely dense breast population in the USA, approximately three extra Fp-MRI scans per existing general scanner per day (two for the average risk and one for the intermediate risk subpopulations) would be required to meet the 2,321,688 additional scans per year required, with no need to invest in new dedicated Fp-MRI capacity. For the heterogeneously dense breast population, 12 additional MRI scans per existing general scanner per day (eight for the average risk and four for the intermediate risk subpopulations), with an investment of 2,901 dedicated scanners (with additional cost of $2,224 million), would be required to meet the 10,157,386 additional scans per year. In contrast, a greater investment would be needed for CEM in both dense breast subpopulations (1,994 CEM scanners with investment costs of $826 million for the extremely dense population; and 8,790 more CEM scanners with investment costs of $3,640 million for the heterogeneously dense population).
The capacity results assumed a 100% screening participation to give a conservative estimate of possible capacity requirements. The number of additional scans and scanners would decrease if applying the actual screening participation rate in the USA, which is around 76%Citation53.
Some studies have suggested that CEM may be less costlyCitation54 and more accessible than MRICitation55,Citation56, with potentially faster examination and reading times. Capacity modeling in the present study suggests that, when existing facilities are considered, wide-scale CEM may be more challenging to implement in practice than is typically anticipated as more scanners at higher additional costs than MRI would be needed. Not all existing mammography units can be adapted or upgraded to deliver contrast-enhanced imaging – only certain mammography suites can be upgraded, and this varies across models. Therefore, the cost of a purpose-built contrast-enhanced, mammography-capable facility may not be that much different from the cost of acquiring a new MRI facility.
Future perspectives
In some studies, supplemental MRI has been found to increase false positive diagnosesCitation57, potentially leading to overdiagnosis and overtreatment of breast cancer at early stagesCitation58. Recent improvements to MRI, such as application of artificial intelligence (AI) to MRICitation59–61, the use of AI for effective triage of negative examinationsCitation61–63, or the use of incident MRI screening roundsCitation64 could reduce false-positive rates and, consequently, the associated costs. That said, while overtreatment is a concern, in many cases, false positives could potentially be reclassified as true positives based on histopathology of the lesion – with MRI better able to detect high-risk proliferative precancerous lesions than XM or DBTCitation65.
The present study suggests that real-world evidence with clinical validity could provide support for women’s access to supplemental screening which can detect more breast malignancies, at earlier stages, thereby improving disease prognosis and saving women’s lives. Greater certainty around the clinical and economic benefits of supplemental screening could also drive awareness of the need for mandatory breast density reporting and justify the necessary investments in capacity to optimize screening practice.
Study limitations
The limitations of the current study include incomplete data on the screening accuracy of all screening modalities in the intermediate- and average-risk populations. For this analysis the screening accuracy estimates were assumed to be equal across the risk populations. This assumption was validated using a Delphi panel of experts. Likewise, screening accuracy estimates were assumed to be equal for prevalent and incident rounds, due to a lack of available data for all modalities.
The performance of screening modalities could vary between incident and prevalent rounds and, thus, this could be a weakness of this study’s economic evaluation. We also made a simplifying assumption that breast cancer incidence was constant regardless of age, due to lack of data in the risk subgroups. While utility decrements associated with biopsy are anticipated, these would have a minimal impact on overall outcomes over a lifetime horizon. Consequently, they were not considered in this analysis. While the $100,000/QALY threshold is used by some US organizations (e.g. ICER)Citation50, there is no standardized criteria for “cost-effectiveness” across the US system. Consequently, the ICER thresholds presented in this study should be considered indicative; however, it should be noted that results in the present study were consistently below that threshold. The Markov model results for Fp-MRI supplemental to XM alone were validated using a MISCAN. Despite the above limitations, both techniques showed aligned cost-effectiveness results.
Conclusion
When used alone, XM and DBT are sub-optimal for screening women with dense breasts in the average and intermediate breast cancer risk subpopulations. Supplemental screening modalities (Ab-MRI, Fp-MRI, CEM and U/S) were cost-effective and led to better clinical outcomes compared to XM or DBT alone. While U/S would be the preferred supplemental modality from a purely economic perspective, MRI yielded the best clinical outcomes, with the highest number of cancers detected and cancer deaths averted, and the lowest number of false negative diagnoses and undetected cancers. CEM should be the recommended modality when MRI facilities are unavailable, as it yields similar clinical outcomes. From a capacity perspective, investment in scanner infrastructure was more favorable for MRI than for CEM.
Transparency
Declaration of funding
Bayer AG has initiated, organized, and funded this project including payment of agencies involved in the development and execution of this project and honoraria to clinical experts.
Declaration of financial/other relationships
MB and FL are employed by Bayer AG. IS, BS, ÖÅ, AC, and JH are employed by Wickenstones Ltd., the agency involved in the development and execution of this study. EM and GN are clinical experts.
Author contributions
All authors were involved with all aspects of this work (including conception and design; analysis and interpretation of the data, and drafting and review of the paper) and agree to be accountable for all aspects of the work.
Reviewer disclosures
Peer reviewers on this manuscript have received an honorarium from JME for their review work but have no other relevant financial relationships to disclose.
Supplemental Material
Download MS Word (348.4 KB)Acknowledgements
The authors are thankful to the authors of Geuzinge et al.Citation28 and especially to Nicolien van Ravesteyn and Harry J. de Koning for providing modeling advice and inputs for transition probabilities and model validation through MISCAN.
References
- World Health Organization. Breast cancer. 2021. https://www.who.int/news-room/fact-sheets/detail/breast-cancer
- Sprague BL, Gangnon RE, Burt V, et al. Prevalence of mammographically dense breasts in the United States. J Natl Cancer Instit. 2014;106(10):dju255. doi: 10.1093/jnci/dju255.
- Radiology ACo. Breast imaging reporting and data system (BI-RADS) ultrasound. Reston (VA): American College of Radiology; 2003.
- Vachon CM, van Gils CH, Sellers TA, et al. Mammographic density, breast cancer risk and risk prediction. Breast Cancer Res. 2007;9(6):217. doi: 10.1186/bcr1829.
- Eriksson L, Czene K, Rosenberg LU, et al. Mammographic density and survival in interval breast cancers. Breast Cancer Res. 2013;15(3):R48. doi: 10.1186/bcr3440.
- Mandelson MT, Oestreicher N, Porter PL, et al. Breast density as a predictor of mammographic detection: comparison of interval- and screen-detected cancers. J Natl Cancer Inst. 2000;92(13):1081–1087. doi: 10.1093/jnci/92.13.1081.
- Hadadi I, Rae W, Clarke J, et al. Diagnostic performance of adjunctive imaging modalities compared to mammography alone in women with Non-Dense and dense breasts: a systematic review and Meta-Analysis. Clin Breast Cancer. 2021;21(4):278–291. doi: 10.1016/j.clbc.2021.03.006.
- Boyd NF, Guo H, Martin LJ, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356(3):227–236. doi: 10.1056/NEJMoa062790.
- Wanders JO, Holland K, Veldhuis WB, et al. Volumetric breast density affects performance of digital screening mammography. Breast Cancer Res Treat. 2017;162(1):95–103. doi: 10.1007/s10549-016-4090-7.
- Mann RM, Athanasiou A, Baltzer PAT, et al. Breast cancer screening in women with extremely dense breasts recommendations of the european society of breast imaging (EUSOBI). Eur Radiol. 2022;32(6):4036–4045. doi: 10.1007/s00330-022-08617-6.
- DenseBreast-info. State legislation map. 2023. https://densebreast-info.org/legislative-information/state-legislation-map/
- DenseBreast-info. FDA National Reporting Standard. 2023. https://densebreast-info.org/legislative-information/national-reporting-standard/
- Beidler LB, Kressin NR, Wormwood JB, et al. Perceptions of breast cancer risks among women receiving mammograph screening. JAMA Netw Open. 2023;6(1):e2252209. doi: 10.1001/jamanetworkopen.2022.52209.
- Wu T, Warren LJ. The added value of supplemental breast ultrasound screening for women with dense breasts: a single center Canadian experience. Can Assoc Radiol J. 2022;73(1):101–106. doi: 10.1177/08465371211011707.
- Mann RM, Kuhl CK, Moy L. Contrast-enhanced MRI for breast cancer screening. J Magn Reson Imaging. 2019;50(2):377–390. doi: 10.1002/jmri.26654.
- Cozzi A, Magni V, Zanardo M, et al. Contrast-enhanced mammography: a systematic review and Meta-Analysis of diagnostic performance. Radiology. 2022;302(3):568–581. doi: 10.1148/radiol.211412.
- Comstock CE, Gatsonis C, Newstead GM, et al. Comparison of abbreviated breast MRI vs digital breast tomosynthesis for breast cancer detection among women with dense breasts undergoing screening. JAMA. 2020;323(8):746–756. doi: 10.1001/jama.2020.0572.
- von Euler-Chelpin M, Lillholm M, Vejborg I, et al. Sensitivity of screening mammography by density and texture: a cohort study from a population-based screening program in Denmark. Breast Cancer Res. 2019;21(1):111. doi: 10.1186/s13058-019-1203-3.
- Vegunta S, Kling JM, Patel BK. Supplemental cancer screening for women with dense breasts: guidance for health care professionals. Mayo Clin Proc. 2021;96(11):2891–2904. doi: 10.1016/j.mayocp.2021.06.001.
- EviCore H. Breast imaging guidelines. Version 1.0.2022. 2022. https://www.evicore.com/-/media/files/evicore/clinical-guidelines/evicore_breast_v102022_final_eff01012022_pub09272021.pdf
- Schünemann HJ, Lerda D, Quinn C, et al. Breast cancer screening and diagnosis: a synopsis of the european breast guidelines. Ann Intern Med. 2020;172(1):46–56. doi: 10.7326/M19-2125.
- Institute for Q, Efficiency in Health C. IQWiG Executive Summaries of Final Reports. Systematic Guideline Search and Appraisal, as Well as Extraction of Relevant Recommendations, for the DMP "Breast Cancer". Cologne, Germany: Institute for Quality and Efficiency in Health Care (IQWiG) Copyright © 2014 by the Institute for Quality and Efficiency in Healthcare (IQWiG). 2014.
- Lobig F, Caleyachetty A, Forrester L, et al. Performance of supplemental imaging modalities for breast cancer in women with dense breasts: findings from an umbrella review and primary studies analysis. Clinical Breast Cancer. 2023;23(5):478–490. doi: 10.1016/j.clbc.2023.04.003.
- Hussein H, Abbas E, Keshavarzi S, et al. Supplemental breast cancer screening in women with dense breasts and negative mammography: a systematic review and Meta-Analysis. Radiology. 2023;306(3):221785. doi: 10.1148/radiol.221785.
- Fallenberg EM, Schmitzberger FF, Amer H, et al. Contrast-enhanced spectral mammography vs. mammography and MRI - clinical performance in a multi-reader evaluation. Eur Radiol. 2017;27(7):2752–2764. doi: 10.1007/s00330-016-4650-6.
- Endrikat J, Schmidt G, Haverstock D, et al. Sensitivity of Contrast-Enhanced breast MRI vs X-ray mammography based on cancer histology, tumor grading, receptor status, and molecular subtype: a supplemental analysis of 2 large phase III studies. Breast Cancer (Auckl). 2022;16:11782234221092155. doi: 10.1177/11782234221092155.
- Siu AL, U.S. Preventive Services Task Force. Screening for breast cancer: u.S. Preventive services task force recommendation statement. Ann Intern Med. 2016;164(4):279–296. doi: 10.7326/M15-2886.
- Geuzinge HA, Bakker MF, Heijnsdijk EA, et al. Cost-Effectiveness of magnetic resonance imaging screening for women with extremely dense breast tissue. J Natl Cancer Inst. 2021;113(11):1476–1483. doi: 10.1093/jnci/djab119.
- Bakker MF, de Lange SV, Pijnappel RM, et al. Supplemental MRI screening for women with extremely dense breast tissue. N Engl J Med. 2019;381(22):2091–2102. doi: 10.1056/NEJMoa1903986.
- Kaiser CG, Dietzel M, Vag T, et al. Cost-effectiveness of MR-mammography vs. conventional mammography in screening patients at intermediate risk of breast cancer - A model-based economic evaluation. Eur J Radiol. 2021;136:109355. doi: 10.1016/j.ejrad.2020.109355.
- Tollens F, Baltzer PAT, Dietzel M, et al. Cost-Effectiveness of digital breast tomosynthesis vs. Abbreviated breast MRI for screening women with intermediate risk of breast Cancer-How Low-Cost must MRI be? Cancers. 2021;13(6):1241. doi: 10.3390/cancers13061241.
- Wang J, Greuter MJW, Vermeulen KM, et al. Cost-effectiveness of abbreviated-protocol MRI screening for women with mammographically dense breasts in a national breast cancer screening program. Breast. 2022;61:58–65. doi: 10.1016/j.breast.2021.12.004.
- Jochelson MS, Lobbes MBI. Contrast-enhanced mammography: state of the art. Radiology. 2021;299(1):36–48. doi: 10.1148/radiol.2021201948.
- Mainiero MB, Moy L, Baron P, et al. ACR appropriateness criteria(®) breast cancer screening. J Am Coll Radiol. 2017;14(11s):S383–s390. doi: 10.1016/j.jacr.2017.08.044.
- Saslow D, Boetes C, Burke W, et al. American cancer society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57(2):75–89. doi: 10.3322/canjclin.57.2.75.
- Amin MB, Greene FL, Edge SB, et al. The eighth edition AJCC cancer staging manual: continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin. 2017;67(2):93–99. doi: 10.3322/caac.21388.
- Skaane P, Bandos AI, Gullien R, et al. Comparison of digital mammography alone and digital mammography plus tomosynthesis in a population-based screening program. Radiology. 2013;267(1):47–56. doi: 10.1148/radiol.12121373.
- Welch HG, Prorok PC, O'Malley AJ, et al. Breast-Cancer tumor size, overdiagnosis, and mammography screening effectiveness. N Engl J Med. 2016;375(15):1438–1447. doi: 10.1056/NEJMoa1600249.
- Weedon-Fekjaer H, Lindqvist BH, Vatten LJ, et al. Breast cancer tumor growth estimated through mammography screening data. Breast Cancer Res. 2008;10(3):R41. doi: 10.1186/bcr2092.
- Diamond IR, Grant RC, Feldman BM, et al. Defining consensus: a systematic review recommends methodologic criteria for reporting of delphi studies. J Clin Epidemiol. 2014;67(4):401–409.
- Yhec YHEC. Delphi Method [online] York 2016. https://yhec.co.uk/glossary/delphi-method/
- Akansel N, Gülşen M, Gültaş M. Influence of discomfort tolerance of women who undergo mammography on the perceived pain intensity due to the procedure. Eur J Breast Health. 2021;17(1):68–75. doi: 10.4274/ejbh.2020.6068.
- CMS. Medicare Physician Fee Schedule (MPFS) Look-up tool]. 2022. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PFSlookup
- Blumen H, Fitch K, Polkus V. Comparison of treatment costs for breast cancer, by tumor stage and type of service. Am Health Drug Benefits. 2016;9(1):23–32.
- Viscusi K. Discouting health effects for medical decisions. New York: Press Syndicate of the University of Cambridge; 1996.
- Shepard DS. Cost-effectiveness in health and medicine. By M.R. Gold, J.E siegel, L.B. Russell, and M.C. Weinstein (eds). New York: oxford university press, 1996. J Mental Health Policy Econ. 1999;2(2):91–92. doi: 10.1002/(SICI)1099-176X(199906)2:2<91::AID-MHP46>3.0.CO;2-I.
- Kerlikowske K, Chen S, Golmakani MK, et al. Cumulative advanced breast cancer risk prediction model developed in a screening mammography population. J Natl Cancer Inst. 2022;114(5):676–685. doi: 10.1093/jnci/djac008.
- IMV. Global imaging market outlook report. IMV Medical Information Division. 2019.
- IMV. MR Market Outlook Report © 2022 IMV, part of Science and Medicine Group. 2022.
- ICER. 2020-2023 Value Assessment Framework. Institute for Clinical and Economic Review. 2020.
- Sankatsing VD, Heijnsdijk EA, van Luijt PA, et al. Cost-effectiveness of digital mammography screening before the age of 50 in The Netherlands. Int J Cancer. 2015;137(8):1990–1999. doi: 10.1002/ijc.29572.
- Taylor DB, Burrows S, Saunders CM, et al. Contrast-enhanced mammography (CEM) versus MRI for breast cancer staging: detection of additional malignant lesions not seen on conventional imaging. Eur Radiol Exp. 2023;7(1):8. doi: 10.1186/s41747-022-00318-5.
- Centers for Disease Control and Prevention. National health interview survey. Cancer Trends Progress Report. 2019.
- Patel BK, Gray RJ, Pockaj BA. Potential cost savings of Contrast-Enhanced digital mammography. AJR Am J Roentgenol. 2017;208(6):W231–w237. doi: 10.2214/AJR.16.17239.
- Phillips J, Steinkeler J, Talati K, et al. Workflow considerations for incorporation of Contrast-Enhanced spectral mammography into a breast imaging practice. J Am Coll Radiol. 2018;15(6):881–885. doi: 10.1016/j.jacr.2018.02.012.
- Kamal R, Mansour S, Farouk A, et al. Contrast-enhanced mammography in comparison with dynamic contrast-enhanced MRI: which modality is appropriate for whom? Egypt J Radiol Nucl Med. 2021;52(1):216. doi: 10.1186/s43055-021-00586-y.
- Houssami N, Ciatto S, Macaskill P, et al. Accuracy and surgical impact of magnetic resonance imaging in breast cancer staging: systematic review and meta-analysis in detection of multifocal and multicentric cancer. J Clin Oncol. 2008;26(19):3248–3258. doi: 10.1200/JCO.2007.15.2108.
- O'Flynn EA, Ledger AE, deSouza NM. Alternative screening for dense breasts: MRI. AJR Am J Roentgenol. 2015;204(2):W141–9. doi: 10.2214/AJR.14.13636.
- Witowski J, Heacock L, Reig B, et al. Improving breast cancer diagnostics with artificial intelligence for MRI. medRxiv. 2022.
- Verburg E, van Gils CH, Bakker MF, et al. Computer-Aided diagnosis in multiparametric magnetic resonance imaging screening of women with extremely dense breasts to reduce False-Positive diagnoses. Invest Radiol. 2020;55(7):438–444. doi: 10.1097/RLI.0000000000000656.
- Jiang Y, Edwards AV, Newstead GM. Artificial intelligence applied to breast MRI for improved diagnosis. Radiology. 2021;298(1):38–46. doi: 10.1148/radiol.2020200292.
- Verburg E, van Gils CH, van der Velden BHM, et al. Deep learning for automated triaging of 4581 breast MRI examinations from the DENSE trial. Radiology. 2022;302(1):29–36. doi: 10.1148/radiol.2021203960.
- Jing X, Wielema M, Cornelissen LJ, et al. Using deep learning to safely exclude lesions with only ultrafast breast MRI to shorten acquisition and reading time. Eur Radiol. 2022;32(12):8706–8715. doi: 10.1007/s00330-022-08863-8.
- Veenhuizen SGA, de Lange SV, Bakker MF, et al. Supplemental breast MRI for women with extremely dense breasts: results of the second screening round of the DENSE trial. Radiology. 2021;299(2):278–286. doi: 10.1148/radiol.2021203633.
- Kuhl CK, Keulers A, Strobel K, et al. Not all false positive diagnoses are equal: on the prognostic implications of false-positive diagnoses made in breast MRI versus in mammography/digital tomosynthesis screening. Breast Cancer Res. 2018;20(1):13. doi: 10.1186/s13058-018-0937-7.
