Abstract
Aim
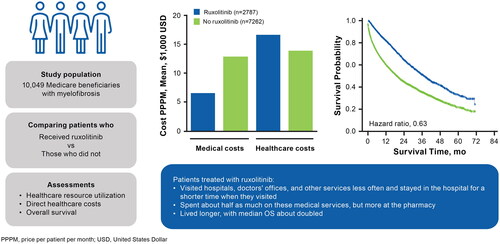
This study evaluated real-world healthcare resource utilization (HCRU), direct costs, and overall survival (OS) of patients who were Medicare beneficiaries and were newly diagnosed with myelofibrosis (MF) who filled ≥1 prescription of ruxolitinib versus those who did not.
Patients and Methods
This was a study of the US Medicare fee-for-service database. Beneficiaries were aged ≥65 years with an MF diagnosis (index) between January 1, 2012 − December 31, 2017. Data were summarized descriptively. OS was estimated using Kaplan-Meier analysis.
Results
Patients with ≥1 prescription fill of ruxolitinib (n = 2,787) had lower mean rates (per patient per month [PPPM]) versus patients who did not fill a prescription for ruxolitinib (n = 7,262) for hospitalizations (0.16 vs 0.32), length of inpatient stay (0.16 vs 2.44 days), emergency department visits (0.10 vs 0.14), physician office visits (4.68 vs 6.25), skilled nursing facility stays (0.02 vs 0.12), home health/durable medical equipment services (0.32 vs 0.47), and hospice visits (0.30 vs 1.70). Monthly medical costs were numerically lower in patients who had ≥1 fill of ruxolitinib versus those who did not fill a prescription for ruxolitinib ($6,553 vs $12,929), largely driven by inpatient costs ($3,428 vs $6,689). Pharmacy costs were $10,065 and $987 in patients who filled versus did not fill ≥1 prescription for ruxolitinib, respectively; total PPPM all-cause healthcare costs were $16,618 and $13,916, respectively. The median OS was 37.5 and 18.7 months for the cohorts of patients who filled versus did not fill ≥1 prescription for ruxolitinib, respectively (hazard ratio = 0.63, 95% CI = 0.59 − 0.67).
Conclusions
Ruxolitinib is associated with reduced HCRU and direct costs of medical care in addition to increased survival, suggesting it to be a cost-effective advance for patients with MF.
PLAIN LANGUAGE SUMMARY
Myelofibrosis is a rare bone marrow cancer. People with this disease do not live as long as the general population. They have difficult symptoms, can tire easily, and may have a large spleen that can be uncomfortable. Ruxolitinib is a treatment for myelofibrosis that can improve symptoms and help patients live longer.
This study asked how treating patients with ruxolitinib affected three things. (1) How often do they go to a healthcare provider? (2) How much do they spend on their healthcare? (3) How long do they live? The authors looked at Medicare records to answer these questions.
The study found that treated patients visited hospitals, doctors’ offices, and other services less often. When they did require hospital care, they stayed in the hospital for a shorter amount of time. As a result, treated patients spent about half as much on these services. However, patients treated with ruxolitinib spent more at the pharmacy. Finally, treated patients lived about twice as long as those who were never treated with ruxolitinib. These findings suggest that ruxolitinib is worthwhile for patients with myelofibrosis.
© 2023 The Author(s). Published by Informa UK Limited, trading as Taylor & Francis Group
Introduction
Myelofibrosis (MF) is a myeloproliferative neoplasm (MPN) defined by progressive bone marrow fibrosis and marrow failure and often associated with splenomegaly due to extramedullary hematopoiesis and burdensome constitutional symptoms, severely compromising quality-of-lifeCitation1–5. MF can arise either de novo (as primary MF [PMF]) or as a transformation from preceding polycythemia vera (PV) or essential thrombocythemia (ET [post-PV or post-ET MF])Citation3.
Traditionally, patients with MF had limited treatment options, namely cytoreductive agents, such as hydroxyurea, immunomodulatory agents, and androgens; splenectomy and splenic irradiation for the management of splenomegaly; and transfusions and erythropoiesis-stimulating agents for management of anemiaCitation6. However, these therapies do not substantively modify the natural course of disease progressionCitation7. Allogeneic hematopoietic-cell transplantation (alloHCT) is the only curative treatment for MF and confers long-term survival in patients with intermediate- to high-risk MF, but at a cost of early transplant-related mortality, resulting in inferior survival at 1 year after transplantationCitation8. Additionally, alloHCT is not available for most patients aged ≥65 years due to increased comorbidity burden associated with advanced age in general and MF diagnosis specifically, and Medicare in the United States does not cover alloHCT as a treatment for MF in this populationCitation9–11. For these reasons, healthcare resource utilization (HCRU) and associated costs have been reported to be higher among patients with MF compared with the general population and patients with the other MPNs, PV and ET, who receive treatment to control their diseaseCitation10.
Ruxolitinib, a JAK1/JAK2 inhibitor, was the first drug approved by the US Food and Drug Administration (FDA) in November 2011 for the treatment of adult patients with intermediate- to high-risk MF based on data from the phase 3 COMFORT trialsCitation12–15. In each study, patients treated with ruxolitinib showed improvements in splenomegaly and MF-related symptomsCitation13,Citation14. In a pooled analysis of data from these trials, patients treated with ruxolitinib showed improved OS compared with patients receiving placebo or best available therapyCitation15. Following FDA approval, median overall survival (OS) in real world settings has increased among all patients with MF, in particular increasing from 13–34 months to >44 months among patients ≥65 years of age, with further significant improvements among patients exposed versus unexposed to ruxolitinibCitation16,Citation17. However, real-world evidence evaluating the impact of ruxolitinib on HCRU and survival in all patients with MF, regardless of hematologic malignancy, is limited. Using data from the US Medicare fee-for-service (FFS) claims database, this retrospective observational study assessed HCRU, direct costs, and OS in patients newly diagnosed with intermediate- to high-risk MF who were Medicare beneficiaries and either filled or did not fill ≥1 prescription of ruxolitinib.
Methods
Study design and patients
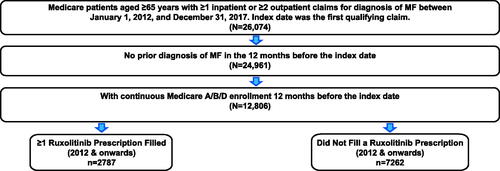
A retrospective analysis of the US Medicare FFS claims database (Parts A/B/D) was performed to identify patients with ≥1 inpatient or ≥2 outpatient claims with an International Classification of Disease, 9th or 10th Revision, Clinical Modification diagnosis code for MF from January 1, 2012, through December 31, 2017. Eligible patients were ≥65 years old (and therefore intermediate-1 or higher risk based on age) with a minimum of 12 months of preindex continuous medical and pharmacy enrollment (). Patients with a diagnosis of MF before the preindex period or who received alloHCT were excluded. The follow-up period was a variable length time period that began on the index date (date of MF diagnosis as indicated by the first qualifying MF claim) and ended at the earliest of the following: end of patient insurance enrollment, end of overall study period (December 31, 2018), or death, whichever occurred first. Patients were divided into two groups: (1) patients who filled ≥1 prescription for ruxolitinib (per relevant National Drug Code and Healthcare Common Procedure Coding System code at any time during the variable follow-up period) and (2) patients who did not fill a prescription for ruxolitinib during the variable follow-up period.
Outcomes
Baseline clinical characteristics described for these cohorts included medical history and comorbid conditions (including prior history of PV and ET), baseline Charlson Comorbidity Index (CCI) score using the Deyo adaptation (evaluated during the 12 months before the index event)Citation18, and duration of follow-up. All-cause HCRU outcomes for any reason (not strictly for treatment of MF) included number and duration of inpatient hospitalizations; number of emergency department (ED) visits, hospital outpatient visits, and physician office visits; stays in a skilled nursing facility (SNF); uses of home health and durable medical equipment (DME) services; and number of hospice visits. All-cause healthcare costs, including total healthcare costs, medical costs, and pharmacy costs, were evaluated. Total healthcare costs were calculated as the sum of medical and pharmacy costs. Medical costs comprised all costs incurred from all-cause HCRU outcomes listed above that were paid by Medicare. Pharmacy costs comprised expenditures for drugs that were reimbursed through the Part D pharmacy benefit. Expenditures were reported in US dollars ($) and standardized to 2022 values using the Consumer Price Index, a measure of inflation calculated by the US Bureau of Labor Statistics and provided by the Federal Reserve Bank of MinneapolisCitation19. OS was evaluated from the index date until death or end of data availability. Patients without a death date were censored at disenrollment or end of study period, whichever occurred first.
Statistical analysis
Cost data and HCRU were expressed as aggregated as per patient per month (PPPM) and summarized using the mean, median, 95% CI, and standard deviation (SD). These data were described by descriptive statistics. Median OS, 1- and 2-year survival rate, and risk of mortality were estimated using Kaplan-Meier and Cox proportional hazards regression analyses, adjusting for demographic and baseline clinical characteristics (age, sex, race, region, dual and low-income subsidy eligibility, CCI score, clinical conditions of interest [including PV, ET, hypertension, hyperlipidemia, diabetes, anemia, transfusion use], and all-cause total healthcare costs during the 12-month preindex period).
Results
Patient demographics and baseline characteristics
This analysis of 10,049 patients included 2,787 who filled ≥1 prescription for ruxolitinib and 7,262 who did not fill a prescription for ruxolitinib ( and ). Overall, the mean (SD) age at index was 77.0 (7.6) years, and 51% of patients were male. Mean patient age (≥1 prescription fill, 76.1 years; no prescription fill, 78.4 years), sex (male, 53% vs 50%), and race (White, 90% vs 87%) were similar between the two cohorts. A greater percentage of patients who filled versus did not fill ≥1 prescription for ruxolitinib were previously diagnosed with PV (22% vs 8%) or ET (27% vs 18%). Mean CCI score was lower in patients who did versus did not fill a prescription for ruxolitinib (3.5 vs 4.7). Mean (SD) duration of follow-up was 726 (530) days for patients who filled ≥1 prescription for ruxolitinib and 501 (505) days for patients who did not fill a prescription for ruxolitinib.
Table 1. Patient demographics and clinical characteristics at MF diagnosis.
Healthcare resource utilization
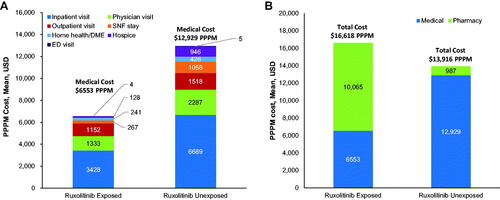
Among patients who filled ≥1 prescription for ruxolitinib, the mean (95% CI) rate of hospitalizations PPPM was 0.16 (0.15–0.17) days, compared with 0.32 (0.30–0.34) days in the cohort that did not fill a prescription for ruxolitinib. Among hospitalized patients, mean (95% CI) length of inpatient stay PPPM was shorter (0.16 [0.15–0.17] vs 2.44 [2.30–2.59], respectively; ). The mean (95% CI) rates of ED visits PPPM (0.10 [0.09–0.11] vs 0.14 [0.13–0.15]) and physician office visits PPPM (4.68 [4.56–4.80] vs 6.25 [6.10–6.40]) were lower in the cohort of patients who did versus did not fill a prescription for ruxolitinib. The mean (95% CI) rates of stays in SNFs PPPM (0.02 [0.02–0.03] vs 0.12 [0.10–0.13]), utilization of home health and DME service PPPM (0.32 [0.30–0.34] vs 0.47 [0.44–0.49]), and hospice visits PPPM (0.30 [0.26–0.35] vs 1.70 [1.56–1.85]) were also lower in the cohort of patients who did versus did not fill a prescription for ruxolitinib. The mean (95% CI) rate of outpatient visits PPPM was greater for patients who filled ≥1 prescription of ruxolitinib (1.88 [1.81–1.94]) versus patients who did not (1.69 [1.64–1.73]).
Table 2. HCRU of patients who filled and did not fill ≥1 prescription of ruxolitinib.
Costs
Patients who filled ≥1 prescription for ruxolitinib had numerically lower monthly medical costs compared with patients who did not fill a prescription for ruxolitinib (). Mean (95% CI) direct medical costs PPPM were $6,553 (6,149–6,956) for patients who filled ≥1 prescription for ruxolitinib and $12,929 (12,398, 13,461) for patients who did not. These costs PPPM were primarily driven by inpatient hospitalizations ($3,428 [3,095–3,761] vs $6,689 [6,278–7,101]), followed by physician office visits ($1,333 [1,271–1,395] vs $2,287 [2,180–2,394]), and outpatient visits ($1,152 [1,091–1,214] vs $1,518 [1,447–1,589]). However, pharmacy costs PPPM were numerically greater in patients who filled versus did not fill ≥1 prescription for ruxolitinib ($10,065 [9,839–10,292] vs $987 [927–1,045]); therefore, the all-cause direct total healthcare cost PPPM for patients who filled ≥1 prescription for ruxolitinib was $16,618 (16,160–17,076) compared with $13,916 (13,381–14,451) for patients who did not fill a prescription for ruxolitinib ().
Overall survival
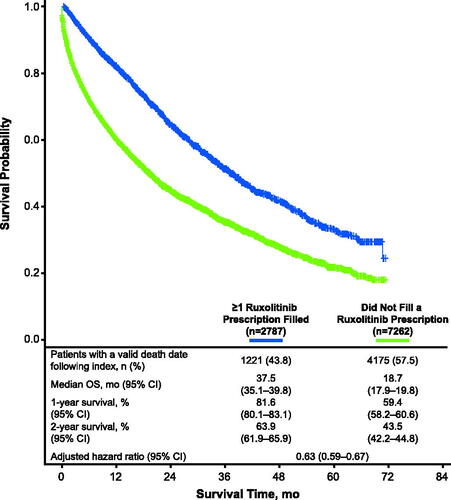
Valid death dates were available for 1,221 (43.8%) and 4,175 patients (57.5%) from the cohorts of patients who filled and did not fill ≥1 prescription for ruxolitinib, respectively (). The 1-year survival rate (95% CI) was 81.6% (80.1 − 83.1%) for the cohort of patients who filled ≥1 prescription for ruxolitinib and 59.4% (58.2 − 60.6%) for the cohort of patients who did not fill a prescription for ruxolitinib, and 2-year survival rates were 63.9% (61.9 − 65.9%) and 43.5% (42.2 − 44.8%), respectively. Median OS (95% CI) for each cohort was 37.5 (35.1 − 39.8) months and 18.7 (17.9 − 19.8) months, respectively (p < 0.0001; ). Compared with the cohort of patients who did not fill a prescription for ruxolitinib, risk of mortality was significantly lower in the cohort of patients who filled ≥1 prescription for ruxolitinib (adjusted hazard ratio [HR] = 0.63; 95% CI = 0.59 − 0.67; p <.0001; ).
Figure 3. Survival outcomes. The reference for the HR was the cohort of patients who did not fill a ruxolitinib prescription. The HR was adjusted for age, sex, race, region, dual and LIS eligibility, CCI score, clinical conditions of interest (including PV, ET, hypertension, hyperlipidemia, diabetes, anemia, transfusion use), and all-cause total healthcare costs during the 12-month pre-index period. CCI, Charlson Comorbidity Index; ET, essential thrombocythemia; HR, hazard ratio; LIS, low-income subsidy; OS, overall survival; PV, polycythemia vera.
Discussion
In this study, Medicare beneficiaries with MF who filled ≥1 prescription for ruxolitinib used substantially fewer healthcare resources and had lower all-cause direct medical costs than those who did not receive ruxolitinib, with savings in medical costs nearly offsetting higher pharmacy costs. Additionally, OS was improved among beneficiaries who filled ≥1 prescription for ruxolitinib, with median length of OS doubled and a significant decrease in risk of mortality compared with beneficiaries who did not receive ruxolitinib.
To date, there are no peer-reviewed real-world analyses directly comparing HCRU and costs among patients with MF who were treated versus not treated with ruxolitinib. A recent investigation of commercial or Medicare claims data for patients with MF (2011–2018) included separate data summaries for all patients regardless of treatment and those treated with ruxolitinib ≥30 mg/day or <30 mg/dayCitation20. Although statistical comparisons between all patients and ruxolitinib-treated patients were not conducted, ED visits and inpatient visits in the 6 months following MF diagnosis were numerically less common in patients treated with ruxolitinib versus the all patient group, and there was a nonsignificant trend toward fewer ED and inpatient visits in patients treated with ruxolitinib ≥30 mg/day versus <30 mg/day. Patients treated with ruxolitinib ≥30 mg/day also had numerically lower total medical costs compared with the all-patient group and those treated with ruxolitinib <30 mg/day. Previous analyses of real-world HCRU and costs for the treatment of MF have shown the burden that the disease imposes on patients and the healthcare system. Studies of large claims databases in the United States and Canada indicated that patients with MF had a significantly greater number of comorbidities, HCRU, and medical and pharmacy costs than age- and sex-matched groupsCitation10,Citation11. Additionally, real-world evidence from a recent retrospective chart review showed that symptoms and spleen size improved in patients who restarted ruxolitinib after a treatment interruptionCitation21, which has implications for HCRU and patient benefit in this population.
In this analysis, beneficiaries who filled ≥1 prescription for ruxolitinib exhibited a numerically lower comorbidity burden than those who did not receive ruxolitinib; however, the mean CCI score among patients who filled ≥1 prescription (3.5) was higher than that reported in a real-world US insurance claims analysis of patients with MF by Mehta et al.Citation10 (2.1), indicating a higher comorbidity burden among Medicare beneficiaries. Indeed, the higher mean CCI among patients who did not receive ruxolitinib was reflected in greater HCRU and medical costs compared with Mehta et al. (number of hospitalizations PPPM = 0.06; duration of hospitalizations PPPM = 0.55; all-cause costs of hospitalizations, outpatient visits, and ED visits PPPM = $3,804). Furthermore, age >65 years, which is an independent negative prognostic factor for MFCitation22, is associated with increased costs of treatmentCitation11. Compared with Mehta et al.Citation10 in which only 28% of all patients with MF were ≥65 years, all Medicare beneficiaries were ≥65 years. Thus, higher comorbidity burden among patients who filled ≥1 prescription for ruxolitinib, which is prognostic for poorer outcomeCitation23,Citation24 and increased costs of treatmentCitation11, and higher patient age may account for why inpatient hospitalization costs, outpatient visits, and ED visits PPPM ($4,584) are slightly higher than figures from 2010 ($3,804), before approval of ruxolitinibCitation10. These factors may also account for the reduced median OS among patients who filled (37.5 months) and did not fill ≥1 prescription for ruxolitinib (18.7 months) in this Medicare population compared with their counterparts from pooled analysis of the COMFORT trials (ruxolitinib-exposed, 64 months; ruxolitinib-unexposed, 28 months) and the difference in HR for OS (pooled COMFORT trials, 0.35)Citation15.
This retrospective observational study was subject to certain limitations. In the FFS database, there could be miscoding and misdiagnosis, incomplete records, and differences between prescription refills and actual medication use, but these limitations would be expected to affect both cohorts. Confounding factors, such as mutation burden, which is inversely correlated with patient outcomesCitation25, were not available in the database and therefore not included in the analysis. Also, because availability of MF disease characteristics was limited in the FFS database, we could not control for these parameters in a propensity analysis. The study also excluded patients who received alloHCT, and thus could not determine whether ruxolitinib treatment delayed alloHCT and reduced associated HCRU and costs. This analysis was among Medicare beneficiaries, and other populations that may have unequal access to the healthcare system or lack insurance are thus less likely to be represented in the data. Finally, the objective of this analysis was to examine the effect of any versus no ruxolitinib treatment on HCRU and costs; thus, the effects of specific doses and treatment duration were not analyzed.
Conclusions
In this retrospective study, monthly HCRU and direct medical costs were substantially lower for Medicare beneficiaries with MF who filled ≥1 prescription for ruxolitinib compared with those who did not, with medical costs savings nearly offsetting increased pharmacy costs. Moreover, filling ≥1 prescription for ruxolitinib was associated with prolonged survival. These results support the claim that ruxolitinib is a cost-effective treatment for patients with MF in a real-world setting. Nevertheless, the treatment landscape of MF continues to evolve, and as new and potentially more expensive treatments are introduced to the market, there comes the potential that the cost-effectiveness of treatment in general may change. Follow-up cost-effectiveness analyses, including analyses that incorporate quality-adjusted life units and work productivity to incorporate the effect on quality-of-life and indirect costs of MF treatment, are warranted.
Transparency
Declaration of financial/other relationships
ATG has a consulting or advisory role with AbbVie, Bristol Myers Squibb, CTI BiopharmaConstellation, Novartis, PharmaEssentia, and Sierra Oncology; received research funding from Pfizer, CTI, Incyte Corporation, Roche/Genentech, Gilead Sciences, Imago Biosciences, Sierra Oncology, and Celgene; and has equity ownership in Samus Therapeutics. JY and SP are employees and shareholders of Incyte Corporation. RS was an employee of Incyte Corporation at the time of the study. AS, AX, and SK were employees of Avalere Health, a paid consultant of Incyte Corporation, at the time of the study.
Author contributions
ATG: Analysis and interpretation of data; critical revision of the manuscript for important intellectual content; supervision. JY: Concept of design; analysis and interpretation; drafting of the manuscript; critical revision of the manuscript for important intellectual content; statistical analysis; obtaining funding; administrative, technical, or logistic support; supervision. AS: Concept of design; acquisition of data; analysis and interpretation of data; statistical analysis; administrative, technical, or logistic support; supervision. AX: Concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; administrative, technical, or logistic support. SK: Acquisition of data; analysis and interpretation of data; critical revision of the manuscript for important intellectual content; statistical analysis. RS: Concept of design; analysis and interpretation; drafting of the manuscript; critical revision of the manuscript for important intellectual content; obtaining funding; administrative, technical, or logistic support; supervision. SP: Concept of design; analysis and interpretation; drafting of the manuscript; critical revision of the manuscript for important intellectual content; obtaining funding; administrative, technical, or logistic support; supervision
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
The Editor in Chief helped with adjudicating the final decision on this paper.
Geolocation information
United States.
Previous presentations
Based on data previously presented at the Academy of Managed Care Pharmacy annual meeting, April 12–16, 2021.
Acknowledgements
Writing assistance was provided by Joshua Solomon, PhD, an employee of ICON (Blue Bell, PA), and the study was funded by Incyte Corporation (Wilmington, DE).
Data availability statement
The data described in this paper are sourced from Centers for Medicare and Medicaid Services (CMS) Medicare FFS claims and enrollment data. The analytic file constructed for this analysis cannot be shared due to restrictions set forth in the governing Data Use Agreement with CMS. Researchers may request use of CMS data through the Research Data Assistance Center.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the world health organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–2405. doi: 10.1182/blood-2016-03-643544.
- Ross DM, Babon JJ, Tvorogov D, et al. Persistence of myelofibrosis treated with ruxolitinib: biology and clinical implications. Haematologica. 2021;106(5):1244–1253. doi: 10.3324/haematol.2020.262691.
- O'Sullivan JM, Harrison CN. Myelofibrosis: clinicopathologic features, prognosis, and management. Clin Adv Hematol Oncol. 2018;16:121–131.
- Mesa RA, Schwager S, Radia D, et al. The myelofibrosis symptom assessment form (MFSAF): an evidence-based brief inventory to measure quality of life and symptomatic response to treatment in myelofibrosis. Leuk Res. 2009;33(9):1199–1203. doi: 10.1016/j.leukres.2009.01.035.
- Song MK, Park BB, Uhm JE. Understanding splenomegaly in myelofibrosis: association with molecular pathogenesis. IJMS. 2018;19(3):898. doi: 10.3390/ijms19030898.
- Keohane C, Radia DH, Harrison CN. Treatment and management of myelofibrosis in the era of JAK inhibitors. Biologics. 2013;7:189–198.
- Abdel-Wahab OI, Levine RL. Primary myelofibrosis: update on definition, pathogenesis, and treatment. Annu Rev Med. 2009;60:233–245. doi: 10.1146/annurev.med.60.041707.160528.
- Gowin K, Ballen K, Ahn KW, et al. Survival following allogeneic transplant in patients with myelofibrosis. Blood Adv. 2020;4(9):1965–1973. doi: 10.1182/bloodadvances.2019001084.
- Devlin R, Gupta V. Myelofibrosis: to transplant or not to transplant? Hematology Am Soc Hematol Educ Program. 2016;2016(1):543–551. doi: 10.1182/asheducation-2016.1.543.
- Mehta J, Wang H, Fryzek JP, et al. Health resource utilization and cost associated with myeloproliferative neoplasms in a large United States health plan. Leuk Lymphoma. 2014;55(10):2368–2374. doi: 10.3109/10428194.2013.879127.
- Bankar A, Zhao H, Iqbal J, et al. Healthcare resource utilization in myeloproliferative neoplasms: a population-based study from Ontario, Canada. Leuk Lymphoma. 2020;61(8):1908–1919. doi: 10.1080/10428194.2020.1749607.
- JAKAFI® (ruxolitinib). Full prescribing information. Wilmington (DE): Incyte corporation; 2020.
- Verstovsek S, Mesa RA, Gotlib J, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366(9):799–807. doi: 10.1056/NEJMoa1110557.
- Harrison C, Kiladjian JJ, Al-Ali HK, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012;366(9):787–798. doi: 10.1056/NEJMoa1110556.
- Verstovsek S, Gotlib J, Mesa RA, et al. Long-term survival in patients treated with ruxolitinib for myelofibrosis: COMFORT-I and -II pooled analyses. J Hematol Oncol. 2017;10(1):156. doi: 10.1186/s13045-017-0527-7.
- Verstovsek S, Parasuraman S, Yu J, et al. Real-world survival of US patients with intermediate- to high-risk myelofibrosis: impact of ruxolitinib approval. Ann Hematol. 2022;101(1):131–137. doi: 10.1007/s00277-021-04682-x.
- Masarova L, Bose P, Pemmaraju N, et al. Improved survival of patients with myelofibrosis in the last decade: single-center experience. Cancer. 2022;128(8):1658–1665. doi: 10.1002/cncr.34103.
- Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8.
- Federal Reserve Bank of Minneapolis. Consumer Price Index, 1913. Minneapolis, MN. 2023 https://www.minneapolisfed.org/about-us/monetary-policy/inflation-calculator/consumer-price-index-1913-
- Copher R, Kee A, Gerds A. Treatment patterns, health care resource utilization, and cost in patients with myelofibrosis in the United States. Oncologist. 2022;27(3):228–235. doi: 10.1093/oncolo/oyab058.
- Gerds AT, Yu J, Scherber RM, et al. Ruxolitinib re-treatment in patients with myelofibrosis: real-world evidence on patient characteristics and outcomes. Acta Haematol. 2022;145(4):448–453. doi: 10.1159/000520440.
- Cervantes F, Dupriez B, Pereira A, et al. New prognostic scoring system for primary myelofibrosis based on a study of the international working group for myelofibrosis research and treatment. Blood. 2009;113(13):2895–2901. doi: 10.1182/blood-2008-07-170449.
- Newberry KJ, Naqvi K, Nguyen KT, et al. Comorbidities predict worse prognosis in patients with primary myelofibrosis. Cancer. 2014;120(19):2996–3002. doi: 10.1002/cncr.28857.
- Breccia M, Bartoletti D, Bonifacio M, et al. Impact of comorbidities and body mass index in patients with myelofibrosis treated with ruxolitinib. Ann Hematol. 2019;98(4):889–896. doi: 10.1007/s00277-018-3569-1.
- Patel KP, Newberry KJ, Luthra R, et al. Correlation of mutation profile and response in patients with myelofibrosis treated with ruxolitinib. Blood. 2015;126(6):790–797. doi: 10.1182/blood-2015-03-633404.