Abstract
Aims
Investigate trends in paid lanadelumab costs over time in a population of patients persistent for 18 months, and to understand overall hereditary angioedema (HAE) treatment cost trends, including costs of acute medication/short-term prophylaxis and supportive care. Lastly, we sought to describe the proportion of lanadelumab patients with evidence of down titration via changes in total paid amounts for lanadelumab in a fixed time period.
Methods
Patients were identified in the Merative MarketScan Databases who had ≥1 claim for lanadelumab during 1/1/2018-6/30/2022 (index), a ≤ 60-d gap in days of supply over 18 months, and were enrolled for ≥6 months pre-index and 18 months post-index. Lanadelumab and HAE-specific costs were assessed during follow-up months 0–6, 7–12, and 13–18. Down titration was defined as a ≥ 25% decrease in lanadelumab costs from months 0–6 to months 7–12 or 13–18. Outcomes were compared between time periods using paired t-tests and McNemar’s test.
Results
Fifty-four lanadelumab users were included; 25 (46%) had evidence of down titration. Lanadelumab costs decreased from $316,724 to $269,861 to $246,919 in months 0–6, 7–12, and 13–18, respectively (p < .01); total HAE treatment costs decreased from $377,076 to $329,855 to $286,074 in months 0–6, 7–12, and 13–18, respectively (p < .01).
Limitations
Persistence was determined via days of supply on medication claims; use of the medication was not confirmed. Down titration was based on costs; the lanadelumab regimen could not be assessed. Results may not be generalizable to uninsured patients or those without commercial or Medicare insurance.
Conclusions
Patients on long-term prophylaxis with lanadelumab experienced a significant reduction (24%) in HAE treatment costs over 18 months, driven by lower costs of acute medications and lanadelumab down titration. Down titration among appropriate patients with controlled HAE may lead to substantial savings in healthcare costs.
Introduction
Hereditary angioedema (HAE) is a rare autosomal dominant disorder characterized by unpredictable episodes of swelling in the organs and subcutaneous tissue, with prevalence estimated to be 1:50,000 in the United States (US)Citation1 Diagnosis of HAE is often difficult and delayed due to variability in clinical presentation, and the physical and emotional toll on HAE patients can result in significant reductions in quality of life (QoL)Citation2.
HAE results from deficient or dysfunctional C1 esterase inhibitor (C1-INH) activity, with C1-INH induced swelling believed to be mediated by bradykinin via triggering of increased vascular permeabilityCitation1,Citation3. Treatment for HAE is aimed at treating and avoiding the progression of acute attacks with effective on-demand therapies, as well as attack prevention with appropriate long-term prophylactic (LTP) medicationsCitation1. Recent advances in LTP provide HAE patients more options with improved safety and efficacy, including lanadelumab, which was approved by the Food and Drug Administration (FDA) in August 2018Citation1. In both a randomized controlled trial (RCT) and an open-label extension (OLE) study, the use of lanadelumab has been shown to reduce the frequency of serious HAE attacks by up to 87% compared to baseline or placebo, and to reduce the need for on-demand treatmentCitation4,Citation5. Interventional and observational data have also demonstrated that the benefits of lanadelumab extend to patient-reported outcomes, including health-related QoL improvements and greater treatment satisfaction over timeCitation4–7. Moreover, given the efficacy of two different dosing regimens in the phase III trialCitation4, lanadelumab is the only LTP medication for HAE with a labeled indication for a reduction in dosing frequency (“down titration”) if the patient is well-controlled (e.g. attack free) for more than six monthsCitation8. The labeled down titration for lanadelumab is defined as a reduction in the dosing frequency of the subcutaneous injection from 300 mg every two weeks to 300 mg every four weeks. Despite this, in a recent phase IV study only 16.9% of established users were being treated with lanadelumab 300 mg every four weeks9.
Payers have constrained budgets and seek to manage therapeutic areas where there may be opportunities for cost savings. In addition to facilitating personalized dosing regimens for patients, lanadelumab can provide an opportunity to reduce costs over time through a reduction in acute attacks and a proportion of patients being controlled on a reduced dosing frequency that is 50% of the cost of normal dosing. Real-world evidence on insured patients in the US can inform payers of the true economic expectations of lanadelumab.
The objective of this analysis was to investigate lanadelumab cost trends in a US population of HAE patients persistent for 18 months. We also sought to understand the overall HAE-specific cost trends over 18 months, including costs of acute medication/short-term prophylaxis and supportive care in addition to lanadelumab costs. Lastly, we sought to describe the proportion of lanadelumab patients with evidence of down-titration via changes in total paid amounts for lanadelumab in a fixed time period.
Methods
Study design and data source
This retrospective observational study used administrative healthcare insurance claims in the Merative MarketScanFootnotei Commercial and Medicare Databases between July 1, 2017 and June 30, 2022. The MarketScan Commercial Database contains the inpatient and outpatient medical and outpatient prescription drug experience of employees and their dependents, covered under a variety of employer-sponsored fee-for-service and managed care health plans in the United States. The MarketScan Medicare Database contains the medical and pharmacy experience of retirees with Medicare Supplemental and Medicare Advantage plans paid for by employers, including the employer-paid portion, out-of-pocket patient expenses, and the Medicare-covered portion of the payment.
Study population
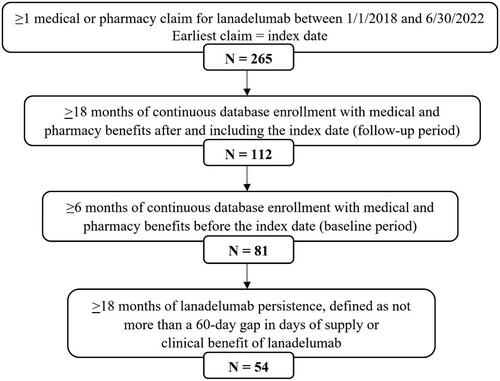
To be included in the study, patients were required to have at least one pharmacy claim with a National Drug Code (NDC) for lanadelumab (47783-0644-01; 47783-0646-01), or at least one medical claim with a Healthcare Common Procedure Coding System (HCPCS) code for lanadelumab administration (J0593), between January 1, 2018 and June 30, 2022; the earliest lanadelumab claim was considered a patient’s index date. At least 18 months of continuous database enrollment with medical and pharmacy benefits after and including the index date (follow-up period) were also required, as well as at least six months of continuous enrollment before the index date (baseline period). Qualifying patients had evidence of at least 18 months of persistence on lanadelumab, defined as having no more than a 60-d gap in days of supply or clinical benefit (for medical claims) of lanadelumab during the 18-month follow-up period. There is currently no validated definition of lanadelumab persistence. Lanadelumab is most commonly dispensed with a 28 d supply, allowing for one dose every two weeks. However, given the labeled indication for down titration to administration every four weeks, it is possible for a 28 d supply to actually cover 56 dCitation8. Therefore, we selected a conservative 60 d allowable gap in days of supply in order to include patients who were dosing every four weeks while also minimizing the inclusion of patients who were not persistent during the study period. Patients with any claims for lanadelumab during the baseline period were excluded from the study.
Demographic characteristics were assessed on the index date (earliest lanadelumab claim) and included age, age category (<18, 18–34, 35–44, 45–54, 55–64, 65+), sex, payer (Commercial, Medicare), plan type (comprehensive/indemnity, exclusive/preferred provider organization [EPO/PPO], consumer-driven/high-deductible health plan [CDHP/HDHP], point-of-service [POS] with or without capitation, health maintenance organization [HMO], other/unknown), and index year (2018–2021).
Outcomes
Given the labeled indication for down titration of lanadelumab after 6 months of attack-free treatment, and to allow comparison of costs before and after down titration, all outcomes were assessed during three intervals within the 18-month follow-up period: months 0–6, months 7–12, and months 13–18 of lanadelumab persistence. Using 6-month time periods also served to smooth out the month-to-month variance in outcomes attributable to irregular timing of lanadelumab dispensing. The primary endpoint of the research was average per-patient lanadelumab costs, compared across the three intervals, hypothesizing a downward trend over time. The secondary endpoint of the research was average per patient HAE-specific costs compared over the three intervals, which included the sum of the costs of acute and short-term prophylaxis (STP) HAE treatments (C1 esterase inhibitors, ecallantide, icatibant), and costs of supportive care treatments (anti-emetics, non-steroidal anti-inflammatory drugs [NSAIDs], opioids) in addition to lanadelumab costs. The number of claims was also collected for each HAE-related treatment, and the quantity dispensed was captured for lanadelumab claims. Outlier acute/STP treatment claims were observed with single paid amounts greater than $200,000 (>95th percentile of the distribution). Using Tukey’s ruleCitation10 to define high-cost outliers was considered and would have set a cut-off of $120,000. However, an examination of the distribution of acute/STP claim costs showed a clear inflection point after $130,000, with no claims between $130,000 and $200,000. We, therefore, opted to impute costs of acute/STP claims greater than $200,000. The cost of these claims were imputed with the median paid amount among claims with the same NDC.
Healthcare resource utilization was also assessed during the three follow-up intervals. The proportion of patients with each type of healthcare encounter and the average number of the following healthcare encounters were assessed: inpatient admissions, emergency room (ER) visits, outpatient office visits, other outpatient visits (e.g. laboratory, radiology, outpatient surgery), and outpatient pharmacy.
The proportion of patients with evidence of lanadelumab down titration was assessed and defined as a decrease in lanadelumab costs of at least 25% between months 0–6 and months 7–12, or between months 0–6 and months 13–18. The definition used costs of lanadelumab over time instead of quantity dispensed because of the unknown reliability of the quantity dispensed variable (i.e. vials dispensed, packages dispensed, mL dispensed). The 25% decrease in costs was chosen as opposed to 50% to allow for patients to be counted as down-titrated if their dose was changed during a time interval as opposed to before or upon entering the next time interval and to account for the timing of when lanadelumab was dispensed (i.e. last day of the 7–12 month interval or the first day of the 13–18 month interval). The 25% definition was calibrated by comparing the lanadelumab costs in patients who were defined as down titrated vs those who did not have evidence of down titration, expecting about a 50% difference in costs between the two groups. Patients with evidence of down titration in months 7–12 were assessed for whether they were maintained on the down-titrated dose or returned to initial dosing in months 13–18.
Statistical analysis
Lanadelumab costs and HAE-specific costs are described by means and standard deviations (SD). Average per-patient lanadelumab and HAE-specific costs of the population were compared between months 0–6 and 7–12, and between months 0–6 and 13–18, using paired t-tests. Healthcare resource utilization and quantities of HAE-specific treatments are also described across the three intervals using means and SD and compared using paired t-tests for continuous variables and McNemar’s test for categorical variables.
Results
Study population
A total of 265 patients with a lanadelumab claim were initially identified, 54 (20%) of whom met all eligibility criteria and were included in the analysis (). Qualifying patients were an average of 42.0 (±15.4) years old on the date of their index lanadelumab claims, 59% were female, and 94% were commercially insured ().
Table 1. Demographic characteristics assessed on the date of the index lanadelumab claim.
Lanadelumab and HAE-specific costs
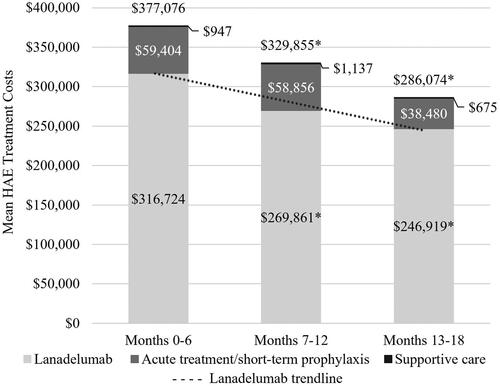
Lanadelumab costs were significantly lower in months 7–12 and 13–18, compared to months 0–6 (), demonstrating a downward trend in costs (0–6 months: $316,724, 7–12 months: $269,861, 13–18 months: $246,919 [p < .01]). Costs of overall HAE-specific treatments were significantly lower in months 7–12 and 13–18, compared to months 0–6 as well (0–6 months: $377,076, 7–12 months: $329,855, 13–18 months: $286,074 [p < .01]). Downward cost trends for acute/STP HAE medications contributed to lower total HAE treatment costs over time, with 35.2% lower acute/STP treatment costs in months 13–18, compared to months 0–6. However, the decrease in acute/STP medication costs was not statistically significant due to the large variance. Consistent with the cost results, the number of claims for lanadelumab and HAE acute/STP treatments reduced over time as well. Supportive care treatment costs were minimal and did not have a significant impact on overall HAE-specific costs.
Healthcare resource utilization
The mean number of outpatient office visits and prescription claims decreased across the three follow-up intervals, though not significantly (). ER utilization decreased from months 0–6 to months 7–12 but was highest in months 13–18. Few patients (<10%) had an inpatient admission during all time intervals. More than half (58–88%) of medical costs were attributable to non-ER/non-office outpatient visits among all patients throughout follow-up (months 0–6: $8,868 ±$42,182; months 7–12: $5,163 ±$16,625; months 13–18: $7,504 ±$35,132).
Table 2. Treatment-related and all-cause healthcare utilization during 18 months of lanadelumab persistence.
Lanadelumab down titration
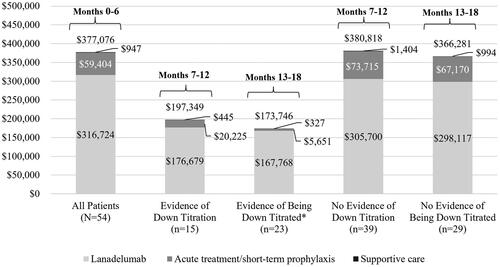
A total of 25 patients (46.3%) had evidence of lanadelumab down titration during follow-up: 15 (27.8%) during months 7–12 and 10 (18.5%) with down titration during months 13–18. Patients with evidence of down titration after 6 months of lanadelumab treatment were slightly younger than those without evidence of down titration after 6 months (40.1 ± 15.0 vs. 42.8 ± 15.7 years) and those without evidence of down titration any time during follow-up (43.0 ± 15.7 years). Baseline attack rates and other characteristics that may differentiate HAE severity were not measured in this analysis and so cannot be compared between patients who did and did not down titrate. Most patients with evidence of down titration during months 7–12 (n = 13; 86.7%) had evidence of remaining down-titrated during months 13–18. Lanadelumab costs in patients with evidence of down titration were 43.7% lower than lanadelumab costs in patients without evidence of down titration in the 13–18 month interval, supporting our definition of down titration (). Additionally, total HAE-specific costs were 53% lower among patients with evidence of being down titrated during months 13–18.
Figure 3. Per patient costs for hereditary angioedema treatments during follow-up months 0–6 among all patients and during months 7–12 and 13–18 among patients with and without evidence of down titration of lanadelumab. *Down titrated in months 7–12 and remained down titrated in months 13–18, or newly down titrated in months 13–18.
Discussion
To our knowledge, this is the first analysis using real-world US claims data to investigate the potential cost reductions for payers and the healthcare system associated with long-term HAE prophylaxis with lanadelumab. In a population of patients persistent on lanadelumab, real-world lanadelumab costs significantly decreased over time, up to 23% over an 18-month period. Long-term prophylaxis with lanadelumab was also associated with a significant decrease in total HAE treatment-related and all-cause costs over 18 months. This decrease was driven by both a reduction in acute treatment/STP costs and down titration of lanadelumab among patients who were assumed to be attack free for at least 6 months.
Nearly half (46%) of patients in this study had evidence of lanadelumab down titration during follow-up, with more than half of those patients down titrating within 12 months of treatment initiation. In contrast, a phase IV observational study of patients on lanadelumab found just 19.2% of prevalent lanadelumab users to be on a down-titrated dosing regimen after an average of 16 months of treatment, and no new lanadelumab users were on a down-titrated regimen after 12 monthsCitation9. Differences in study populations and definitions of down titration may contribute to differences in the estimation of down titration prevalence. Additionally, down titration trials and gradual down titration could not be captured in this analysis and could have an impact on down titration rates.
The clinical presentation of HAE varies widely across the patient population and is influenced by several factors. Some patients may require frequent acute medication use or add-on STP to adequately manage their symptoms, even while on LTP. We observed single claims for acute medication >$200,000 and decided to adjust those that fell in the >95th percentile of the distribution by imputing the median cost of claims for the same NDC. The claims that were adjusted may be legitimate paid costs and require further investigation to understand the management of these patients. However, the claims were substantially higher than other similar claims and skewed the data, making it difficult to interpret the results of the greater population of HAE patients. It is suspected that the patients with substantially high acute medication and STP costs are severe and require adjunct prophylaxis or consistent acute medication dosing to manage their HAE. Additionally, many acute medications for HAE are weight-based, therefore high costs could be attributed to patients with high weights. Regardless, high acute treatment or STP use may not infer sub-optimal effectiveness of LTP considering these patients have continued on lanadelumab for 18 months in parallel to their high acute treatment/STP use. It is expected that some patients are more severe and require more intense treatment, and future research can help understand how to optimally manage these patients. Additionally, these treatment patterns may be used in future analyses to categorize the severity of HAE.
The results of this study have implications for several stakeholders, including patients, providers, and payers. Nearly half of the patients had evidence of down titration during the study, and most of those patients down-titrated after 6 months of treatment and remained on a lower frequency regimen for the remainder of the study. Therefore, it may be inferred that lanadelumab successfully prevented HAE attacks among a substantial proportion of patients. These results are relevant not only for patients, who benefit from HAE attack prevention and should be encouraged to remain persistent with treatment but also for providers who may be hesitant to prescribe lanadelumab based on the cost of the initial dosing regimen. Long-term lanadelumab prophylaxis and eventual down titration contributed to significant reductions in lanadelumab-specific and total HAE treatment costs over time, a finding that should also be of interest to payers looking to recognize savings on covered treatments for HAE and make formulary decisions on which medications provide the greatest value.
Limitations
The results of this study should be interpreted in light of some limitations, many of which are inherent to administrative database studies. First, there is potential for misclassification of lanadelumab down titration, since we did not directly assess changes in the frequency of lanadelumab dosing. However, the criteria of a ≥ 25% cost decrease was tested for validity by comparing the lanadelumab costs between the down-titrated group and non-down-titrated group, expecting the down-titrated group to have ∼50% of the costs compared to the group without evidence of down titration. The down-titrated group had 43.7% lower costs compared to the non-down-titrated group, supporting the down-titration definition. Second, lanadelumab persistence and use of other HAE treatments was identified largely by filled outpatient prescriptions; like with all claims data-based studies, we cannot confirm whether patients actually used medications as prescribed. Third, the study sample was mostly comprised of HAE patients with commercial insurance (N = 51), while only three patients had Medicare Supplemental coverage from an employer; therefore, the generalizability of the results to Medicare recipients may be limited. The results also may not be generalizable to patients with other types of insurance (e.g. Medicaid) or those who are uninsured. Future studies with a more diverse payer distribution are therefore warranted. Additionally, only 30% of the 265 patients with a lanadelumab claim met enrollment criteria for inclusion in the study (N = 81), and only two-thirds of those patients (N = 54) were determined to be persistent on lanadelumab for 18 months. Though this may preclude the generalization of the results to all lanadelumab users, the aim of the study was to describe outcomes among long-term lanadelumab users. The geographic distribution of the MarketScan Databases, which draw more heavily from the south than other US regions, may also impact the generalizability of the study results; regional data are not reported for this sample due to reporting restrictions for small sample sizes. Fourth, due to limitations of claims data we do not have information on the type of HAE a patient has. Finally, although we did not aim to measure attack rates or HAE severity due to data limitations, data on attack rates and severity could have helped interpret the data and understand more about the population.
Conclusions
Long-term prophylaxis with lanadelumab can provide substantial cost-savings to the payer because of the unique ability to down-titrate appropriate patients who remain attack free for six months and reduce costs of acute medication and STP. The flexibility to adjust lanadelumab dosing allows physicians and patients to tailor their treatment to what is most suitable for them.
Transparency
Declaration of financial/other interests
CHS is completing a fellowship sponsored by Takeda. BGS is employed by Takeda. NP and KAE are employed by Merative which received funding from Takeda to conduct this study.
Author contributions
All authors contributed to the design and execution of the study and the writing of the manuscript.
Ethics approval and informed consent
All database records are statistically de-identified and certified to be fully compliant with US patient confidentiality requirements set forth in the Health Insurance Portability and Accountability Act of 1996. Because this study used only de-identified patient records and did not involve the collection, use, or transmittal of individually identifiable data, this study was exempted from Institutional Review Board approval.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Previous presentations
This research was presented in part at the 2023 AMCP annual meeting in San Antonio, TX, USA.
Acknowledgements
Programming services were provided by Caroline Henriques of Merative. These services were paid for by Takeda Pharmaceuticals USA, Inc.
Declaration of funding
This study was funded by Takeda Pharmaceuticals USA, Inc.
Data availability statement
Merative MarketScan Research Databases are available to purchase by Federal, non-profit, academic, pharmaceutical, and other researchers. The use of the data is contingent on completing a data use agreement and purchasing the data needed to support the study. More information about licensing the Merative MarketScan Research Databases is available at https://www.merative.com/real-world-evidence.
Notes
i MarketScan is a registered trademark of Merative
References
- Busse PJ, Christiansen SC, Riedl MA, et al. US HAEA medical advisory board 2020 guidelines for the management of hereditary angioedema. J Allergy Clin Immunol Pract. 2021;9(1):132.e3–150.e3. doi: 10.1016/j.jaip.2020.08.046.
- Lumry WR, Settipane RA. Hereditary angioedema: epidemiology and burden of disease. Allergy Asthma Proc. 2020;41(Suppl 1):S08–S13. doi: 10.2500/aap.2020.41.200050.
- Zeerleder S, Levi M. Hereditary and acquired C1-inhibitor-dependent angioedema: from pathophysiology to treatment. Ann Med. 2016;48(4):256–267. doi: 10.3109/07853890.2016.1162909.
- Banerji A, Riedl MA, Bernstein JA, et al. Effect of lanadelumab compared with placebo on prevention of hereditary angioedema attacks: a randomized clinical trial. JAMA. 2018;320(20):2108–2121. doi: 10.1001/jama.2018.16773.
- Banerji A, Bernstein JA, Johnston DT, et al. Long-term prevention of hereditary angioedema attacks with lanadelumab: the HELP OLE study. Allergy. 2022;77(3):979–990. doi: 10.1111/all.15011.
- Busse P, Zaragoza-Urdaz R, Betschel S, et al. Impact of lanadelumab on patient-reported outcomes in hereditary angioedema in the US and Canada: interim findings from the EMPOWER study. J Allergy Clin Immunol. 2022;149(2):AB166. doi: 10.1016/j.jaci.2021.12.549.
- Wedner HJ, Bernstein J, Betschel S, et al. Effectiveness, safety, and patient-reported outcomes (PROs) in patients with hereditary angioedema (HAE) from the United States and Canada treated with lanadelumab: 24-month data from the EMPOWER study. J Allergy Clin Immunol. 2023;151(2):AB131. doi: 10.1016/j.jaci.2022.12.413.
- Takeda Pharmaceutical Company. Takhzyro prescribing information. 2023. Available from: https://www.shirecontent.com/PI/PDFs/TAKHZYRO_USA_ENG.pdf.
- Goodyear MD, Lumry WR, Anderson J, et al. Treatment patterns among patients with HAE-1/2: interim analysis findings from the US and Canada participants in the EMPOWER study. Poster presented at: American Academy of Allergy, Asthma & Immunology Annual Meeting; 2022; Phoenix, AZ.
- Hoaglin DC, Iglewicz B, Tukey JW. Performance of some resistant rules for outlier labeling. JSTOR. 1986;81(396):991–999. doi: 10.1080/01621459.1986.10478363.