Abstract
Aims
To assess the cost-effectiveness of tezepelumab as add-on maintenance therapy compared with standard of care (SoC) for the treatment of patients with severe asthma in Canada.
Material and Methods
A cost utility analysis was conducted using a Markov cohort model with five health states (“controlled asthma”, “uncontrolled asthma”, “previously controlled asthma with exacerbation”, “previously uncontrolled asthma with exacerbation”, and “death”). Tezepelumab plus SoC was compared to SoC (high-dose inhaled corticosteroids plus long-acting beta agonist) using efficacy estimates derived from the NAVIGATOR (NCT03347279) and SOURCE (NCT03406078) trials. The model included the costs of therapy, administration, resource use for disease management, and adverse events. Utility estimates were calculated using a mixed-effects regression analysis of the NAVIGATOR and SOURCE trials. A Canadian public payer perspective was used with a 50-year time horizon, a 1.5% annual discount rate, and the base case analysis was conducted probabilistically. A key scenario analysis assessed the cost-effectiveness of tezepelumab compared with currently reimbursed biologics informed by an indirect treatment comparison.
Results
The base case analysis suggested that tezepelumab plus SoC was associated with a quality-adjusted life-year (QALY) gain of 1.077 compared with SoC alone at an incremental cost of $207,101 (2022 Canadian dollars), resulting in an incremental cost-utility ratio of $192,357/QALY. The key scenario analysis demonstrated that tezepelumab was dominant against all currently reimbursed biologics, with higher incremental QALYs (ranging from 0.062 to 0.407) and lower incremental costs (ranging from −$6,878 to −$1,974). Additionally, when compared against currently reimbursed biologics in Canada, tezepelumab had the highest probability of being cost-effective across all willingness-to-pay (WTP) thresholds.
Conclusion
Tezepelumab provided additional life years and QALYs at additional cost compared with SoC in Canada. In addition, tezepelumab dominated (i.e. more effective, less costly) the other currently reimbursed biologics.
Introduction
Asthma patients are categorized by severity as having mild, moderate or severe asthmaCitation1. According to the Global Initiative for Asthma (GINA) guidelines and the Canadian Thoracic Society (CTS) guidelines, severe asthma is defined as asthma that requires treatment with high-dose inhaled corticosteroids (ICS) and a second controller medication to prevent it from becoming uncontrolled, or which remains uncontrolled despite this therapyCitation1,Citation2. In 2020, it was estimated that approximately 2.8 million Canadians aged 12 years and older suffered from asthma, accounting for 8.7% of the populationCitation3. While severe asthma accounts for approximately 5%–10% of the asthmatic population, these patients experience the highest burden of disease, accounting for up to 50% of total asthma direct costsCitation2.
Inflammatory phenotypes, which are based on biomarker expression or perceived underlying inflammatory biology, are commonly used to group patients with severe asthma including allergic/atopic (increased levels of immunoglobulin E [IgE]), eosinophilic (elevated peripheral blood eosinophil [EOS] counts and elevated fractional exhaled nitric oxide [FeNO] levels), or non-allergic non-eosinophilic. Around half of patients with severe asthma have multiple overlapping phenotypesCitation4,Citation5. Two studies demonstrated that at the time of testing, 12%–27% of severe asthma patients had all three biomarkers present (i.e. IgE, EOS, and FeNO) and 12%–14% of patients had none of the biomarkers presentCitation4,Citation5. Furthermore, phenotypes may also change over time, demonstrating the limitations of classifying patients based on inflammatory driversCitation6.
Patients with severe asthma, regardless of phenotypic subgroups, experience significant clinical and humanistic burdens caused by frequent exacerbations, impaired lung function, and medication side-effects. Both inhaled corticosteroids (ICS) and oral corticosteroid (OCS) are associated with multiple adverse events (AEs), but OCS AEs are more severe ranging from sleep disturbance to osteoporosis and diabetesCitation7–9. According to the International Severe Asthma Registry, the majority of patients with uncontrolled disease (80%) experienced on average of four exacerbations within 12 monthsCitation4. Furthermore, it has been demonstrated that patients with uncontrolled disease had worse lung function and poorer health-related quality of life (HRQoL)Citation10. It has also been reported that patients with severe asthma require substantial healthcare resource utilization, including approximately three-fold greater hospitalizations and two-fold greater unplanned emergency room (ER) visits compared with patients with non-severe asthmaCitation11.
The current recommended approach outlined by GINA and CTS is to follow a stepwise treatment paradigm focused on increasing ICS dose, with the addition of a second controller (e.g. long-acting beta agonists [LABA] or long-acting muscarinic antagonist [LAMA])Citation1,Citation2. As per GINA and CTS guidelines, add-on biologic treatments are recommended for the management of severe asthma if high-dose ICS in combination with other controller medications (i.e. current standard of care [SoC]) fail to provide adequate control. Maintenance OCS use was also previously recommended, however, the recent GINA guidelines do not recommend the use of maintenance OCS due to the associated AEsCitation1,Citation2. Relatively low use of maintenance OCS has been reported in CanadaCitation12. Short-courses of OCS may be used to prevent ER utilization but a clinical goal of reducing reliance upon these is now more generally accepted.
Patients with severe asthma may still experience exacerbations despite treatment with SoC and are therefore eligible for add-on biologic therapy. Currently available biologic treatments in Canada (omalizumabCitation13, mepolizumabCitation14, reslizumabCitation15, benralizumabCitation16, and dupilumabCitation17) target specific phenotypes (e.g. eosinophilic asthma or allergic asthma). However, approximately 40%–60% of patients with severe asthma continue to experience exacerbations (i.e. ≥1 exacerbation per year) despite biologic therapyCitation18–20. This may be due to the fact that current biologics target downstream inflammatory pathways and are prescribed based on patients’ baseline biomarker testing, which may fluctuate over time. Since 15%–20% of patients with severe asthma are estimated to have non-eosinophilic, non-allergic asthma, none of the current biologics are appropriate for a significant proportion of patientsCitation20. Patients who experience a lack of control with their current biologic or are ineligible for a biologic may resort to OCS use, which is associated with serious long-term AEsCitation1,Citation8,Citation21,Citation22. Therefore, severe asthma patients require additional treatment options that reduce exacerbations and provide effective asthma control across inflammatory phenotypes, regardless of biomarker status.
Tezepelumab is a human IgG2 monoclonal antibody that binds to thymic stromal lymphopoietin (TSLP) with high affinity, blocking TSLP at the top of the inflammatory cascade implicated in the pathophysiology of asthma to broadly reduce the initiation and persistence of downstream inflammatory immune responsesCitation23,Citation24. Tezepelumab represents a new class of biologic, with demonstrated efficacy across phenotypes and irrespective of biomarkersCitation25. In Canada, tezepelumab received Health Canada approval in July 2022Citation26 and received a positive reimbursement recommendation from the Canadian Agency for Drugs and Technology in Health (CADTH) in December 2022Citation27.
The objective of this study was to assess the cost-effectiveness of tezepelumab as an add-on maintenance therapy for the treatment of patients with severe asthma in Canada. The secondary objective was to assess the cost-effectiveness of tezepelumab compared with currently reimbursed biologics for the treatment of patients with severe asthma in Canada.
Methods
Modeling methods
A Markov cohort-state transition model was built in Microsoft Excel to conduct a cost-utility analysis for tezepelumab plus SoC (ICS + LABA) compared with SoC alone for patients with severe asthmaCitation28–30. Outcomes of the cost-utility analysis are expressed as incremental costs per quality adjusted life year (QALY) and incremental costs per life year (LY).
Patient population
The modeled population is based on the NAVIGATOR (NCT03347279)Citation31 and SOURCE (NCT03406078)Citation21 trials, where the majority of patients with severe asthma received high-dose ICS and an additional asthma controller (e.g. LABA, LAMA, etc.) and experienced ≥2 exacerbations in the past 12 months and were deemed to be uncontrolled based upon an Asthma Control Questionnaire (ACQ)-6 score ≥1.5 or experienced ≥1 exacerbations in the past 12 months and were OCS-dependent. The mean baseline age of the modeled population was 50.0 years (standard error [SE]: 0.50), 36.6% (SE: 1.4) of the population were male, and 9.4% (SE: 0.9) were OCS users (prednisone-equivalent mean dose of 11.3 mg [SE: 0.50] per day). The modeled cohort was validated as being generally reflective of Canadian patients with severe asthma, based on feedback from Canadian clinical experts.
The NAVIGATOR (NCT03347279)Citation31 and SOURCE (NCT03406078)Citation21 trials which were conducted with approval in accordance with the principles established in the Declaration of Helsinki and the Internal Conference on Harmonization guidelines for good clinical practice, and all the patients or their guardians provided written informed consent before enrollment into the study.
Cost‑effectiveness model
The developed economic model closely aligns with those previously submitted to CADTH for assessment of biologic treatments for patients with severe asthmaCitation32–35. The Markov model included five health states (): (i) Controlled Asthma: patients with ACQ score <1.5 not currently experiencing an exacerbation; (ii) Uncontrolled Asthma: patients with ACQ score ≥1.5 not currently experiencing an exacerbation; (iii) Previously Controlled Asthma with Exacerbation: patients experiencing an exacerbation, who was in the Controlled Asthma health state prior to experiencing an exacerbation; (iv) Previously Uncontrolled Asthma with Exacerbation: patients experiencing an exacerbation, who was in the Uncontrolled Asthma health state prior to experiencing an exacerbation; (v) Death. Patients may transition between the four “alive” states; however, transitions into the exacerbation states are dependent on the previous health state. All patients entered the model with uncontrolled asthma, aligning with NAVIGATOR and SOURCE trials. In the model, an exacerbation was defined as a worsening of asthma symptoms leading to at least one of the following three events: (1) burst of OCS for at least three consecutive days, (2) an ER visit, or (3) hospitalization. Patients may transition to exacerbation states, depending on their previous asthma state (i.e. controlled or uncontrolled). Furthermore, patients in all health states were at risk of either all-cause mortality or asthma-specific mortality (i.e. exacerbation-related mortality) and could transition into the death health state.
Figure 1. Model structure. Abbreviations. CA, controlled asthma; ER, emergency room; OCS, oral corticosteroid; UA, uncontrolled asthma.
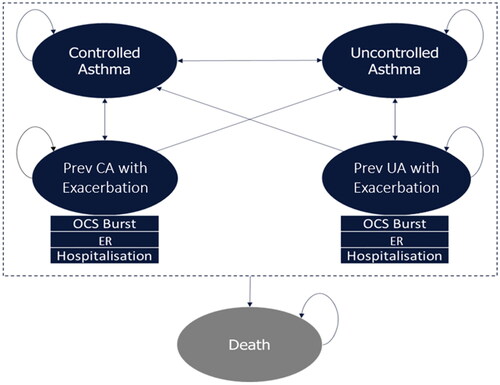
Patients received either tezepelumab or SoC at model entry. In addition, a small proportion of patients (9.4%) in each arm received maintenance OCS (i.e. patients receiving a daily stable maintenance dose of OCS), based on NAVIGATORCitation31 patient characteristics, aligning with the low OCS use in Canada. Patients who entered the model in the tezepelumab arm could transition to SoC alone due to either attrition (i.e., discontinuation due to AEs or lack of efficacy, informed by trial data) or treatment cessation because of non-response at a user-defined assessment week. Patients could also transition from “with maintenance OCS” to “without maintenance OCS” through OCS sparing/discontinuation. Utilities were applied to each health state while drug acquisition and resource use costs were applied per cycle. Patients continued in the model until death occurred or the model time horizon was reached.
In accordance with Canadian guidelines for conducting health economic analysesCitation36, the public payer perspective was used, the time horizon was set to 50 years to reflect a lifetime horizon, and an annual discount rate of 1.5% was applied to both costs and outcomes. The model cycle length was 28-days to align with the tezepelumab treatment cycles and to adequately capture asthma-related events. The response assessment which was defined as any reduction in the rate of exacerbation based on the NAVIGATOR trial or chronic OCS dose from baseline based on the SOURCE trial (with non-response resulting in treatment cessation) was set to 26 weeks to mimic current Canadian clinical practice and reimbursement requirements and was applied to all biologics.
Treatment comparators
To assess the cost-effectiveness of tezepelumab in severe asthma patients regardless of asthma phenotype, tezepelumab plus SoC was compared to SoC alone. Patients received high-dose ICS and LABA as SoC treatment, aligning with GINA and CTS guidelines. Maintenance OCS was also included in SoC treatment for OCS-dependent patients.
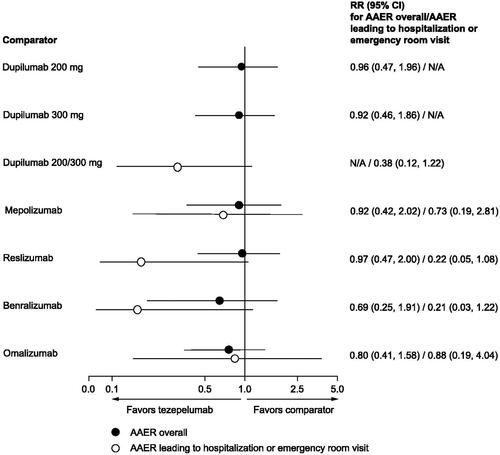
Despite differences in the patient populations for which each biologic is approved, the cost-effectiveness of tezepelumab relative to other currently available biologics was assessed in a scenario analysis. In the absence of head-to-head clinical trial evidence comparing tezepelumab to currently available biologics, a systematic literature review (SLR) and indirect treatment comparison (ITC) were conductedCitation37. Both the SLR and ITC were conducted based on National Institute for Health and Care Excellence (NICE) Decision Support Unit Technical Support DocumentsCitation38. This analysis used an anchored simulated treatment comparison (STC) approach where a regression model was applied to individual patient data (IPD) from NAVIGATORCitation31. The model was then used to simulate the impact of tezepelumab within the trial population of the comparator studyCitation37. The selection of the comparator trial was based on its similarity to NAVIGATOR in terms of patient characteristics, study design, and availability of data for each outcome and comparator. To address any imbalances between the trial populations, demographic and clinical characteristics such as age, sex, body mass index, disease duration, number of exacerbations in the past 12 months, lung function, and treatment-related characteristics (such as OCS users, OCS dose at entry, ICS dose at entry) were adjusted using a regression model. These characteristics were based on input from clinicians and modifiers used in previously published ITCs and were adjusted via regression model to account for imbalances between populationsCitation37. Comparators assessed in the scenario analysis included mepolizumab, benralizumab, omalizumab, and dupilumab. Reslizumab was not considered a comparator in this model because this biologic is not publicly reimbursedCitation39, and has limited use (i.e. <1%) in Canada. Although there were no statistically significant differences observed between the biologics, the STC results show that tezepelumab was favorably and consistently associated with numerically lower annual asthma exacerbation rate (AAER) than the other currently approved biologics ().
Figure 2. Findings from the STC, rate ratios for AAER, tezepelumab versus comparators.
Note: RR reflects the effect of tezepelumab in the comparator study population.
Abbreviations. AAE, annualized asthma exacerbation rate; CI, confidence interval; N/A, not applicable; RR, rate ratio; STC, simulated treatment comparison.
"Tezepelumab compared with other biologics for the treatment of severe asthma, a systematic review and indirect treatment comparison." Menzies-Gow, A., Steenkamp, J., Singh, S., et al., Journal of Medical Economics, copyright © 2022, reprinted by permission of Informa UK Limited, trading as Taylor & Francis Group.
Source: Menzies-Gow et al.Citation37
Clinical inputs
Treatment effect
Clinical efficacy data from the NAVIGATORCitation31 and SOURCECitation40 trials were incorporated into the model to inform transition probabilities (). Exacerbation rate ratios were used to inform transitions from the “day-to-day asthma” state to the “exacerbation” state for tezepelumab in the model. The exacerbation distribution (see Supplementary Material Table A.1) and duration (see Supplementary Material Table A.2) were stratified by exacerbation type (i.e. controlled or uncontrolled), exacerbation outcome (i.e. OCS burst, ER visit, or hospitalization) and by use of maintenance OCS.
Table 1. Transition probabilities (4-week cycle).
A treatment effect of interest is the reduction of maintenance OCS use both in terms of overall dose reduction and OCS discontinuation. To capture this treatment effect, the model initially defined the proportion of patients on maintenance OCS at baseline and applied reductions in the proportion of OCS users. The model assumed that a reduction in maintenance OCS of 90% to 100% was equivalent to OCS discontinuation. Data informing the reduction in maintenance OCS dose with tezepelumab plus SoC and SoC alone was derived from the SOURCE trial (see Supplementary Material Table A.3).
Treatment discontinuation
The model considered two types of treatment discontinuation in patients with and without OCS use: (1) attrition, and (2) treatment cessation due to non-response (see Supplementary Material Table A.4). Attrition was applied per cycle and referred to the proportion of patients discontinuing each intervention based on AEs or non-efficacy related reasons (e.g. withdrawal by patient, protocol deviation, any malignancy, liver function abnormality, lost to follow-up, or COVID-19). The model also included a response assessment at 26 weeks where patients may discontinue due to lack of adequate treatment response, to align with Canadian clinical practice. Patients were considered responders based on OCS dose reduction in SOURCECitation21, and exacerbation rate reduction in NAVIGATORCitation31. Patients were assumed not to discontinue SoC; therefore, treatment stopping parameters were not applied to patients in the SoC arm.
Adverse events
Tezepelumab was well-tolerated across the NAVIGATORCitation31 and SOURCECitation40 trials and there were no statistically significant differences in AEs between tezepelumab and placebo. However, maintenance OCS use is associated with several AEs including type 2 diabetes mellitus, osteoporosis, glaucoma, cataract, myocardial infarction, heart failure, cerebrovascular accident, renal impairment, peptic ulcer, and pneumoniaCitation41. Therefore, these ten AEs associated with maintenance OCS were modeled, with probabilities of each event sourced from a United Kingdom (UK) cohort study commissioned by AstraZeneca using the Optimum Patient Care Research Database and the Clinical Practice Research Datalink databaseCitation41, and validated by Canadian clinical experts (see Supplementary Material Table A.5).
Sources of utility data
Utility values were obtained using a mixed effects regression analysis of NAVIGATORCitation31 and SOURCECitation21 EuroQol 5 Dimension 5 Level (EQ-5D-5L)Citation42 data that incorporated covariates for treatment with tezepelumab, ACQ health state, and exacerbations (). A Canadian-specific value set was usedCitation43. A fixed disutility value was applied for each OCS-related AE event per cycle. Due to a lack of Canadian-specific data, OCS-related AE disutilities were sourced from a NICE submissionCitation44,Citation45 (). Values were applied per cycle (i.e. 28 days; 13 cycles per year).
Table 2. Utility and disutility estimates.
Source of mortality data
The model captured all-cause mortality and exacerbation-related mortality. All-cause mortality forms the baseline mortality rate and was obtained from the most recent life tables available from Statistics CanadaCitation46. All-cause mortality was applied in all health states with the exception of the exacerbation health states, in which only exacerbation-related mortality was applied.
Exacerbation-related mortality was considered a key component to capture. Canadian clinicians noted that patients experiencing exacerbations have a greater risk of mortality than patients who do not experience exacerbations. Canadian clinicians also validated the assumption that a reduction in exacerbations would correspond to reduced exacerbation-related mortality, noting that exacerbations would be the primary cause of asthma-related mortality. Furthermore, published literature has shown an increased risk of death linked with asthma (i.e. 6.2 per 100,000 mortality rate)Citation47.
UK-based estimates obtained from a NICE TA565 submissionCitation45, demonstrating the impact of exacerbations on mortality, with adjustments for exacerbation types aligned to observed data from NAVIGATORCitation31 and SOURCECitation21, were used in the model (). Exacerbation-related mortality was categorized by type of exacerbation (e.g. OCS burst, ER visit, or hospitalization) and age.
Table 3. Exacerbation-specific mortality, per cycle.
Resource use and cost inputs
Drug acquisition & administration
The drug acquisition cost for tezepelumab was obtained from the positive tezepelumab CADTH reimbursement, and the dosing schedule was obtained from the published product monograph ()Citation27. SoC treatment consisting of ICS + LABA and OCS drug acquisition costs were sourced from the Ontario Drug Benefit FormularyCitation48 and dosing schedules were obtained from published product monographsCitation49–53. The weighted-average costs of ICS + LABA and OCS were calculated using the most commonly used ICS + LABA and OCS treatments in Canada, as estimated by Canadian clinical experts (; for detail on the cost and use of each treatment see Supplementary Material Table A.6 and A.7). Drug dispensing fees or markups were not included in the drug acquisition costs. However, administration costs for biologics were included in the base case analysis. It was assumed that 45% of patients would require a healthcare practitioner to administer biologicsCitation54; an estimated 10-minute appointment with a nurse (National Occupation Classification 3102; $39.00/hour)Citation55 resulting in a cost per dose of $2.93. All costs included in the model are in 2022 Canadian dollar values.
Table 4. Cost of treatments (2022 CAD$).
Resource use
Resource use for disease management was based on health state occupancy. Costs were estimated as a function of the expected frequency of resource use in each health state and the unit costs for each type of resource. Resource use frequencies were estimated by three Canadian clinical experts (see Supplementary Material Table A.8). Unit costs were sourced from the Government of Canada Job Bank, 2021Citation56, Ontario Case Costing Initiative, 2021Citation57, and Ontario Physicians Services Schedule of Benefits, 2021Citation58 ().
Table 5. Disease management unit costs (2022 CAD$).
OCS adverse event costs
Canadian-specific databases such as the Ontario Physicians Schedule of BenefitsCitation58 and the Ontario Case Costing ToolCitation57 Canadian costs, as well as the healthcare utilization reported in the UK cohort study, were used to calculate the average annual cost of OCS-related AEs by OCS dose (see Supplementary Material Table A.9).
Analyses
Probabilistic base‑case analysis
A probabilistic analysis was conducted in order to account for the joint uncertainty of the underlying parameter estimates. When possible, the reported SEs from the data sources, or alternatively standard deviations (SDs) or 95% confidence intervals (CIs) used to calculate SE, were used to define parameter uncertainty. When not reported, the SE was estimated as 20% of the default value. The underlying distributions for all variables included in probabilistic analyses are summarized in Supplementary Material Table A.10. Probabilistic analyses were seeded to allow for reproducibility of results and conducted using 1,000 iterations as convergence was observed by 500 runs (e.g. incremental cost-utility rate [ICUR] converged to <5% difference by 500 runs). Notably, sampled health state utility values were capped to ensure that the utility values for non-controlled health states could not exceed the values of the controlled health states. The main outcomes were total discounted costs, LYs, and QALYs with tezepelumab plus SoC compared with SoC. In addition, a cost-effectiveness plane and cost-effectiveness acceptability curves (CEACs) were generated.
Deterministic sensitivity analysis
A series of one-way deterministic sensitivity analyses were conducted to determine the significant drivers of the model and to assess the robustness of the base case. All parameters subject to uncertainty (e.g. transition probabilities, utilities, drug acquisition costs, exacerbation distribution & duration, mortality, resource use, and AEs) were modified within a ± 20% range.
Scenario analysis
Although there is heterogeneity in the indicated patient populations, the cost-effectiveness of tezepelumab relative to other currently reimbursed biologics (omalizumab, mepolizumab, benralizumab, and dupilumab) was assessed in a key scenario analysis. Pairwise comparisons, which have adjusted for differences between the patient populations in the underlying tezepelumab and other biologic trials, were conducted as a key scenario. Relative efficacy estimates of tezepelumab against comparator biologics on AAER, AAER leading to hospitalization, and reduction in maintenance OCS use were informed by pairwise simulated treatment comparisons (STCs) (see Supplementary Material Table A.11 and A.12). The STC results numerically favored tezepelumab against other biologics in all three outcomes. The drug acquisition cost of the currently reimbursed biologics were obtained from the Ontario Exceptional Access Program FormularyCitation59 and dosing schedules were obtained from published product monographs (see Supplementary Material Table A.13)Citation16,Citation32,Citation35,Citation60. Administration costs were also applied to all the comparators (i.e. 10-minute appointment resulting in a cost per dose of $2.93). Due to the vial reconstitution required prior to the administration of omalizumab, a longer administration time was assumed (i.e. 15-minute appointment resulting in a cost per dose of $4.39). Furthermore, when no data were available from the STC (i.e. OCS sparing for omalizumab) comparator biologics were assumed equivalent to tezepelumab. Additional scenario analyses addressing methodological and structural uncertainty (e.g. shorter time horizons, alternative discount rates, and alternative health state utility values [see Supplementary Material Table A.14) were conducted to examine how certain changes to the base case assumptions may influence the ICUR.
Results
Probabilistic base case analysis
The probabilistic discounted results for LYs, QALYs, and costs by treatment and health state are provided in . Over a 50-year time-horizon, tezepelumab plus SoC demonstrated LY and QALY gains of 0.846 and 1.077, respectively, compared with SoC alone. The incremental cost for tezepelumab plus SoC vs SoC alone was $207,101, primarily driven by treatment costs. Compared with SoC alone, tezepelumab plus SoC was more effective and was associated with higher costs, resulting in an ICUR of $192,357/QALY and an incremental cost-effectiveness ratio (ICER) of $244,820 per LY. The deterministic results aligned with the probabilistic results.
Table 6. Summary of comparison of costs, LYs, QALYs (probabilistic base-case; 2022 CAD).
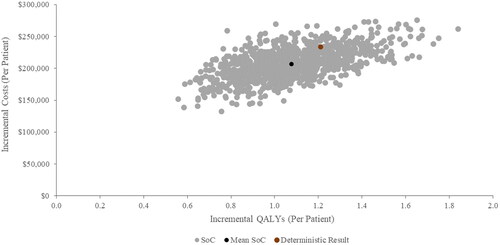
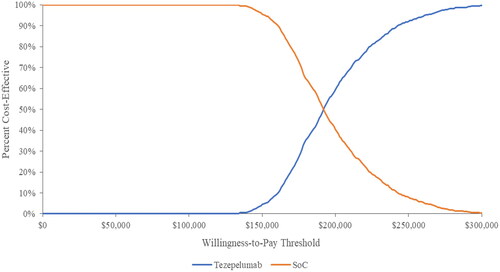
The scatter plot of cost effectiveness results for tezepelumab plus SoC compared with SoC is shown in . In all 1000 iterations, tezepelumab plus SoC yielded more QALYs and higher costs compared to SoC. The CEAC presenting the probability of cost-effectiveness at different willingness to pay (WTP) thresholds is presented in . At WTP thresholds below $193,000/QALYs, SoC had the highest probability of being cost-effective; while tezepelumab plus SoC was most likely to be cost-effective above this threshold.
Deterministic sensitivity analysis
The parameters in the one-way deterministic sensitivity analysis (DSA) that resulted in the largest impact on the ICUR are presented in a tornado diagram with ICURs ranging from $148,478/QALYs to $273,814/QALYs (see Supplementary Material Figure A.1). The parameters that resulted in the largest impact on the overall ICUR included health state utility for controlled asthma, tezepelumab drug acquisition and administration costs, age, transition probabilities (i.e. severe asthma patients moving from uncontrolled asthma health state to the exacerbation health state), and exacerbation mortality risk (OCS burst).
Scenario analysis
The discounted probabilistic key scenario results for tezepelumab vs other available biologics and SoC are summarized in . Compared with benralizumab, dupilumab, mepolizumab and omalizumab, tezepelumab was associated with more QALYs (i.e. incremental QALYs ranging from 0.062 to 0.407) and lower costs (i.e. incremental costs ranging from −$6878 to −$1974) over a 50-year time horizon. Therefore, tezepelumab was dominant against all other biologics.
Table 7. Summary of probabilistic STC scenario analysis results (discounted) for tezepelumab vs. comparators.
The scatter plot for tezepelumab compared with other biologics is shown in Supplementary Material Figure A.2. In the comparison between tezepelumab and the other available biologics, tezepelumab yielded more QALYs (i.e. more effective) and was more costly in >40% of the iterations. The CEAC for the key scenario is presented in supplementary material Figure A.3. Tezepelumab had the highest probability of being cost-effective compared with the currently reimbursed biologics across all WTP thresholds. Additional scenario analyses are summarized in Supplementary Material Table A.15). Overall, most of the additional scenario analyses resulted in similar findings to the base case analysis. The STC results provide an estimate of the relative cost-effectiveness of tezepelumab vs each biologic; however, it would not be appropriate to combine the results of each pairwise comparison.
Discussion
Although severe asthma affects a minority of the population with asthma, it is associated with disproportionate humanistic and economic burden, accounting for 50% of total asthma direct costsCitation2. Currently available biologics in Canada for severe asthma target specific phenotypes, potentially leaving other pathways of airway inflammation wholly unaddressed. Eligibility for current biologics is dependent on biomarker profile; however, studies have demonstrated that approximately half of patients with severe asthma have been shown to change phenotypes in one yearCitation6. Furthermore, up to 49% of severe asthma patients exhibit multiple drivers of inflammationCitation4,Citation61,Citation62. In addition, there are no biologics indicated for patients with severe, non-allergic, non- EOS asthma. Hence, there remains significant unmet need for patients with severe asthma. Tezepelumab has been demonstrated to be an effective treatment that reduces exacerbations and provides effective asthma control across inflammatory phenotypes, regardless of biomarker status, and will help address these unmet needs that remain in severe asthma.
This economic evaluation found that tezepelumab plus SoC provides incremental benefits (i.e. 0.846 LYs and 1.077 QALYs) at additional cost ($207,101) compared with SoC for patients with severe asthma in Canada, with an ICUR of $192,357/QALY over a 50-year time horizon. Furthermore, in the key scenario analysis comparing tezepelumab against other reimbursed biologics, tezepelumab was associated with more QALYs (i.e. incremental QALYs ranging from 0.062 to 0.407) and lower costs (i.e. incremental costs ranging from −$6878 to −$1974) over a 50-year time horizon compared with benralizumab, dupilumab, mepolizumab, and omalizumab. Hence, all other reimbursed biologics were dominated by tezepelumab.
Many published economic analyses of biologics for severe asthma employed similar modeling strategies as used in our model. The majority of published economic models included three health states: day-to-day asthma, exacerbations, and death health statesCitation29,Citation30,Citation63,Citation64. In addition, several published economic models included asthma/exacerbation-related mortalityCitation29,Citation30,Citation63,Citation65. Canadian clinical experts as well as published literature have described the increased risk of mortality associated with exacerbations; therefore, it is important that asthma/exacerbation-related mortality was modeled for the severe asthma populationCitation47,Citation66. However, with appropriate treatment, death from asthma is rare in Canada. Clinical experts have noted that patients who experience exacerbations face a higher risk of death compared to those who do not. Because obtaining clinical trial data to directly demonstrate the varying mortality rates associated with different treatments and types of exacerbations is impractical (i.e. requires a lengthy trial duration [lifetime], and a significant number of patients), it has been indirectly assumed that reducing exacerbations would correspond to a decrease in exacerbation-related mortality. This assumption has been supported by Canadian clinicians who confirmed that exacerbations are the primary cause of asthma-related deaths and reducing these episodes would consequently result in lower mortality rates. Overall, our analysis aligns well with previously published economic models in terms of health states used and the inclusion of exacerbation-related mortality.
In key scenario analyses, pairwise ITCs allowed the comparison of tezepelumab to currently available biologics that target specific phenotypes. An STC was used to inform the efficacy of the other biologics in the key scenario analysis which reduces bias in pairwise ITC results by taking advantage of individual patient data to adjust for effect modifiers and prognostic variables via a regression modelCitation37. Recent ITCs conducted by Ando et al. and Nopsopon et al. compared tezepelumab with benralizumab, mepolizumab, and dupilumabCitation67,Citation68. Most of the studies included in the analyses by Ando et al. and Nopsopon et al. were included in the ITC that informed the current analysis, with the exception of an additional mepolizumab studyCitation69 included by Nopsopon et al. However, the Ando et al. and Nopsopon et al. ITCs included fewer studies overall. Nevertheless, in all three ITCs, tezepelumab was ranked the highest of all included treatments with respect to reduction in AAERCitation67,Citation68. The alignment of results between the three ITCs further supports and validates the relative efficacy of tezepelumab and other biologics.
The model inputs, structure, and assumptions were validated by Canadian clinical experts to ensure they reflect Canadian clinical practice. Extensive scenario testing was performed such as a one-way DSA, a key scenario analysis comparing to currently reimbursed biologics to tezepelumab, and several additional scenario analyses. This allowed us to thoroughly test model uncertainties and the robustness of the base case results.
There were some limitations in our study. Firstly, in the key scenario analysis comparing tezepelumab with other biologics, several inputs were assumed equivalent between tezepelumab and comparators due to a lack of available data. For example, STC estimates were assumed equivalent between tezepelumab and omalizumab for OCS reduction. However, this assumption is unlikely to have a substantial effect on model results as it was not considered a model driver and less than 10% of the population was considered OCS-dependent. Though caution is required in interpreting the results of the key scenario analysis of biologics because differences in the trial populations may have resulted in residual confounding. Secondly, in the absence of robust asthma-related Canadian mortality data, UK asthma-related mortality data was used. This assumption was validated by Canadian clinical experts who confirmed that mortality is similar between UK and Canada. Furthermore, economic models developed for the United States and the Netherlands also utilized the UK asthma-related mortality inputs due to lack of asthma-related mortality data in their regionCitation63,Citation64. Thirdly, the model captures exacerbation-related mortality which is applied only in the exacerbation health states. As all-cause mortality in the exacerbation state was not applied, the probability of death after an exacerbation may be underestimated. However, Canadian clinicians have noted that mortality rates due to asthma are rare in Canada and considered the exacerbation-related mortality in the model appropriate. Lastly, the model incorporated a response assessment, assumed at 26 weeks, to mimic clinical practice and reimbursement requirements. Although response to treatment may be assessed at a later timepoint, scenario analyses have demonstrated that this assumption has minimal effects on the results.
Conclusion
This economic evaluation found that tezepelumab plus SoC provides incremental benefits at additional cost compared with SoC for patients with severe asthma in Canada, with an ICUR of $192,357/QALY over a 50-year time horizon. When compared with other currently available biologics, tezepelumab dominated benralizumab, dupilumab, mepolizumab, and omalizumab, with the highest probability of being cost-effective across all WTP thresholds. Add-on maintenance biologic therapies are strongly recommended for severe asthma patients, who fail to achieve asthma control with high-dose ICS in combination with other asthma controllers. Tezepelumab has been shown to reduce exacerbations across all inflammatory phenotypes, regardless of biomarker status, thereby filling an unmet need in the severe asthma population.
Transparency
Declaration of financial/other relationships
HG, VV, AD, and SS are employees of EVERSANA; AstraZeneca Canada contracted EVERSANA to complete this study. MH and APT are employees of AstraZeneca Canada. AQ and DG are employees of AstraZeneca PLC. APT, AQ, and DG are shareholders of AstraZeneca. IM received honoraria for participating in consultant meetings for AstraZeneca Canada.
Author contributions
MH, AQ, DG, and APT were involved in the conception and design of the study. All authors were involved in the analysis of data and all authors contributed to the interpretation of data. In addition, all authors agree to be accountable for all aspects of the work.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplemental Material
Download MS Word (92.5 KB)Acknowledgements
The authors would like to thank Cal Shephard from AstraZeneca Canada, Mississauga, ON, Canada for his support.
Additional information
Funding
References
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention 2021.
- FitzGerald JM, Lemiere C, Lougheed MD, et al. Recognition and management of severe asthma: a Canadian thoracic society position statement. Can J Respir Crit Care Sleep Med. 2017;1(4):199–221. doi: 10.1080/24745332.2017.1395250.
- Statistics Canada. Table 13-10-0096-01 Health characteristics, annual estimates 2021 October 2021]. Available from: https://www150.statcan.gc.ca/t1/tbl1/en/cv.action?pid=1310009601.
- Denton EP, Tran DB, Canonica TN, et al. Cluster analysis of inflammatory biomarker expression in -the international severe asthma registry. J Allergy Clin Immunol Pract. 2021; (7):2680–2688 e7. doi: 10.1016/j.jaip.2021.02.059.
- Ding B, Chen S, Agrawal A, et al. Distribution of biomarkers in severe asthma and severe uncontrolled asthma. Eur Respiratory Soc. 2021;58:OA4214. doi: 10.1183/13993003.congress-2021.OA4214.
- Kupczyk M, Dahlén B, Sterk P, et al. Stability of phenotypes defined by physiological variables and biomarkers in adults with asthma. Allergy. 2014;69(9):1198–1204. doi: 10.1111/all.12445.
- Xu X, O”Quinn S, Hirsch I, et al. Impact of asthma control status on lung function and patient well-being assessments in patients with severe asthma. A35. SEVERE ASTHMA: American Thoracic Society. 2017;195:A1370–A1370.
- Volmer T, Effenberger T, Trautner C, et al. Consequences of long-term oral corticosteroid therapy and its side-effects in severe asthma in adults: a focused review of the impact data in the literature. Eur Respir J. 2018; 52(4):1800703. doi: 10.1183/13993003.00703-2018.
- Asthma Canada. Asthma Facts and Statistics 2020 November 2021]. Available from: https://asthma.ca/wp-content/uploads/2020/07/Asthma-101.pdf.
- Müllerová H, Cockle SM, Gunsoy NB, et al. Clinical characteristics and burden of illness among adolescent and adult patients with severe asthma by asthma control: the IDEAL study. J Asthma. 2021;58(4):459–470. doi: 10.1080/02770903.2019.1708095.
- Zeiger RS, Schatz M, Dalal AA, et al. Utilization and costs of severe uncontrolled asthma in a managed-care setting. J Allergy Clin Immunol Pract. 2016; 4(1):120–129.e3. doi: 10.1016/j.jaip.2015.08.003.
- Sadatsafavi M, Khakban A, Tavakoli H, et al. Trends in oral corticosteroids use in severe asthma: a 14-year population-based study. Respir Res. 2021;22(1):103. doi: 10.1186/s12931-021-01696-x.
- Novartis Pharmaceuticals Canada. Xolair (omalizumab): Product monograph. Montreal (Canada); 2017. Available from: https://pdf.hres.ca/dpd_pm/00070876.PDF
- GlaxoSmithKline Inc. Nucala (mepolizumab): Product monograph. Mississauga (Canada); 2020. Available from: https://pdf.hres.ca/dpd_pm/00067402.PDF
- Teva Canada Innovation. Cinqair (reslizumab): Product monograph. Montreal (Canada); 2017. Available from: https://pdf.hres.ca/dpd_pm/00038484.PDF
- AstraZeneca Canada. Fasenra (benralizumab): Product monograph. Mississauga (Canada); 2020. Available from: https://pdf.hres.ca/dpd_pm/00054955.PDF
- Sanofi-aventis Canada. Dupixent (dupilumab): Product monograph. Laval (Canada); 2021. Available from: https://pdf.hres.ca/dpd_pm/00071250.PDF
- Reibman J, Tan L, Ambrose C, et al. Clinical and economic burden of severe asthma among US patients treated with biologic therapies. Ann Allergy Asthma Immunol. 2021;127(3):318–325.e2. doi: 10.1016/j.anai.2021.03.015.
- Kavanagh JE, Hearn AP, Dhariwal J, et al. Real-world effectiveness of benralizumab in severe eosinophilic asthma. Chest. 2021;159(2):496–506. doi: 10.1016/j.chest.2020.08.2083.
- Casale T, Molfino NA, Silver J, et al. Real-world effectiveness of mepolizumab in patients with severe asthma and associated comorbidities. Ann Allergy Asthma Immunol. 2021;127(3):354–362. e2. doi: 10.1016/j.anai.2021.05.021.
- Wechsler ME, Colice G, Griffiths JM, et al. SOURCE: a phase 3, multicentre, randomized, double-blind, placebo-controlled, parallel group trial to evaluate the efficacy and safety of tezepelumab in reducing oral corticosteroid use in adults with oral corticosteroid dependent asthma. Respir Res. 2020;21(1):1–10. doi: 10.1186/s12931-020-01503-z.
- Voorham J, Xu X, Price DB, et al. Health care resource utilization and costs associated with incremental systemic corticosteroid exposure in asthma. Allergy. 2019;74(2):273–283. doi: 10.1111/all.13556.
- Gauvreau GM, Sehmi R, Ambrose CS, et al. Thymic stromal lymphopoietin: its role and potential as a therapeutic target in asthma. Expert Opin Ther Targets. 2020;24(8):777–792. doi: 10.1080/14728222.2020.1783242.
- Corren J, Parnes JR, Wang L, et al. Tezepelumab in adults with uncontrolled asthma. N Engl J Med. 2017;377(10):936–946. doi: 10.1056/NEJMoa1704064.
- Menzies-Gow A, Wechsler ME, Brightling CE. Unmet need in severe, uncontrolled asthma: can anti-TSLP therapy with tezepelumab provide a valuable new treatment option? Respir Res. 2020;21(1):268. doi: 10.1186/s12931-020-01505-x.
- AstraZeneca Canada Inc. Tezspire (tezepelumab): Product monograph. Mississauga (Canada); 2022. Available from: https://pdf.hres.ca/dpd_pm/00066831.PDF
- Canadian Agency for Drugs and Technologies in Health. CADTH Reimbursement Recommendation - Tezepelumab (Tezspire). 2022.
- Rind DM, McQueen RB, Herron-Smith S, et al. The effectiveness and value of tezepelumab for severe asthma: a summary from the institute for clinical and economic review”s California technology assessment forum. J Manag Care Spec Pharm. 2022;28(5):577–580. doi: 10.18553/jmcp.2022.28.5.577.
- González-Barcala FJ, Muñoz-Gall X, Mariscal E, et al. Cost-effectiveness analysis of anti–IL-5 therapies of severe eosinophilic asthma in Spain. J Med Econ. 2021;24(1):874–882. doi: 10.1080/13696998.2021.1941065.
- Tohda Y, Matsumoto H, Miyata M, et al. Cost-effectiveness analysis of dupilumab among patients with oral corticosteroid-dependent uncontrolled severe asthma in Japan. J Asthma. 2021;59(11):1–12.
- Menzies-Gow A, Corren J, Bourdin A, et al. Tezepelumab in adults and adolescents with severe, uncontrolled asthma. N Engl J Med. 2021;84(19):1800–1809. doi: 10.1056/NEJMoa2034975.
- Canadian Agency for Drugs and Technologies in Health. CADTH Reimbursement Review Dupilumab (Dupixent). 2021.
- Canadian Agency for Drugs and Technologies in Health. CADTH Pharmacoeconomic Report Benralizumab (Fasenra). 2018.
- Canadian Agency for Drugs and Technologies in Health. CADTH Canadian Drug Expert Committee Recommendation Reslizumab (Cinqair). 2019.
- Canadian Agency for Drugs and Technologies in Health. CADTH Canadian Drug Expert Committee Recommendation Mepolizumab (Nucala). 2016.
- Canadian Agency for Drugs and Technologies in Health. Guidelines for the economic evaluation of health technologies: Canada, 4th edition 2017 October 2021]. Available from: https://www.cadth.ca/guidelines-economic-evaluation-health-technologies-canada-4th-edition.
- Menzies-Gow A, Steenkamp J, Singh S, et al. Tezepelumab compared with other biologics for the treatment of severe asthma: a systematic review and indirect treatment comparison. J Med Econ. 2022;25(1):679–690. doi: 10.1080/13696998.2022.2074195.
- Dias S, Welton NJ, Sutton AJ, et al. A generalised linear modelling framework for pairwise and network meta-analysis of randomised controlled trials. London: National Institute for Health and Care Excellence (NICE) ; 2014.
- Pan-Canadian Pharmaceutical Alliance. Brand Name Drug Negotiations Status. Updated 2022 2022 [February 27, 2022]. Available from: https://www.pcpacanada.ca/negotiations.
- Wechsler ME, Menzies-Gow A, Brightling CE, et al. Evaluation of the oral corticosteroid-sparing effect of tezepelumab in adults with oral corticosteroid-dependent asthma (SOURCE): a randomised, placebo-controlled, phase 3 study. Lancet Respir Med. 2022;10(7):650–660. doi: 10.1016/S2213-2600(21)00537-3.
- Observational and Pragmatic Research Institute Pte Ltd. Oral corticosteroid exposure and chronic disease onset (data on file). 2017.
- EQ-5D. About EQ-5D-5L [December 2022]. Available from: https://euroqol.org/eq-5d-instruments/eq-5d-5l-about/.
- Xie F, Pullenayegum E, Gaebel K, et al. A time trade-off-derived value set of the EQ-5D-5L for Canada. Med Care. 2016;54(1):98–105. doi: 10.1097/MLR.0000000000000447.
- Sullivan PW, Slejko JF, Sculpher MJ, et al. Catalogue of EQ-5D scores for the United Kingdom. Med Decis Making. 2011;31(6):800–804. doi: 10.1177/0272989X11401031.
- National Institute of Care and Excellence. Benralizumab for treating severe eosinophilic asthma. Technology appraisal guidance [TA565]. 2019.
- Statistics Canada. Table 13-10-0114-01 Life expectancy and other elements of the complete life table, three-year estimates, Canada, all provinces except Prince Edward Island 2021 July 2021]. Available from: https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1310011401.
- To T, Simatovic J, Zhu J, et al. Asthma deaths in a large provincial health system. A 10-year population-based study. Ann Am Thorac Soc. 2014;11(8):1210–1217. doi: 10.1513/AnnalsATS.201404-138OC.
- Ontario Drug Benefit [Internet. ]. 2021 cited December 2021]. Available from: https://www.formulary.health.gov.on.ca/formulary/.
- GlaxoSmithKline Inc. Advair Diskus: Product monograph. Mississauga (Canada); 2020. Available from: https://pdf.hres.ca/dpd_pm/00056976.PDF
- Mylan Pharmaceuticals ULC. Wixela Inhub: Product monograph. Etobicoke (Canada); 2020. Available from: https://pdf.hres.ca/dpd_pm/00055254.PDF
- AstraZeneca Canada Inc. Symbicort Turbuhaler: Product monograph. Mississauga (Canada); 2021. Available from: https://pdf.hres.ca/dpd_pm/00059997.PDF
- Organon Canada Inc. Zenhale: Product monograph. Kirkland (Canada); 2021. Available from: https://pdf.hres.ca/dpd_pm/00060636.PDF
- GlaxoSmithKline Inc. Breo Ellipta: Product monograph. Mississauga (Canada); 2019. Available from: https://pdf.hres.ca/dpd_pm/00049111.PDF
- AstraZeneca Data on File. Moderate to Severe Asthma Report Specialist Market. 2021.
- Government of Ontario. Job Bank - Registered Nurse (R.N.) in Canada February 2022]. Available from: https://www.jobbank.gc.ca/marketreport/wages-occupation/993/ca.
- Government of Canada Job Bank. Registered Nurse (R.N.) in Canada 2021 cited 2021 July 2021]. Available from: https://www.jobbank.gc.ca/marketreport/wages-occupation/993/ca.
- Ontario Case Costing Initiative. Costing analysis tool updated 2018 cited 2021 December 2021]. Available from: https://hsimi.ca/occp/occpreports/.
- Ontario Ministry of Health. Ontario schedule of benefits; physician services under the health insurance act. 2021.
- Ontario Ministry of Health. Ontario exceptional access program formulary 2021 cited 2021 September 2021]. Available from: https://www.health.gov.on.ca/en/pro/programs/drugs/odbf/odbf_except_access.aspx.
- Canadian Agency for Drugs and Technologies in Health. Common Drug Review Pharmacoeconomic Review Report (Xolair). 2015.
- Lambrecht BN, Hammad H. The immunology of asthma. Nat Immunol. 2015;16(1):45–56. doi: 10.1038/ni.3049.
- Tran TN, Zeiger RS, Peters SP, et al. Overlap of atopic, eosinophilic, and TH2-high asthma phenotypes in a general population with current asthma. Ann Allergy Asthma Immunol. 2016;116(1):37–42. doi: 10.1016/j.anai.2015.10.027.
- van Nooten F, Stern S, Braunstahl G-J, et al. Cost-effectiveness of omalizumab for uncontrolled allergic asthma in The Netherlands. J Med Econ. 2013;16(3):342–348. doi: 10.3111/13696998.2012.756398.
- Sullivan PW, Li Q, Bilir SP, et al. Cost-effectiveness of omalizumab for the treatment of moderate-to-severe uncontrolled allergic asthma in the United States. Curr Med Res Opin. 2020;36(1):23–32. doi: 10.1080/03007995.2019.1660539.
- Tan LE, Tan WHG, Aziz MIA, et al. Assessing the cost-effectiveness of mepolizumab as add-on therapy to standard of care for severe eosinophilic asthma in Singapore. J Asthma. 2022;59(1):189–199. doi: 10.1080/02770903.2020.1837158.
- Krishnan V, Diette GB, Rand CS, et al. Mortality in patients hospitalized for asthma exacerbations in the United States. Am J Respir Crit Care Med. 2006;174(6):633–638. doi: 10.1164/rccm.200601-007OC.
- Ando K, Fukuda Y, Tanaka A, et al. Comparative efficacy and safety of tezepelumab and other biologics in patients with inadequately controlled asthma according to thresholds of type 2 inflammatory biomarkers: a systematic review and network meta-analysis. Cells. 2022;11(5):819. doi: 10.3390/cells11050819.
- Nopsopon T, Lassiter G, Chen M-L, et al. Comparative efficacy of tezepelumab to mepolizumab, benralizumab, and dupilumab in eosinophilic asthma: a Bayesian network meta-analysis. J Allergy Clin Immunol. 2023;151(3):747–755. doi: 10.1016/j.jaci.2022.11.021.
- Pavord ID, Korn S, Howarth P, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012;380(9842):651–659. doi: 10.1016/S0140-6736(12)60988-X.
- Tam V, Ko Y, Mittmann N, et al. Cost-effectiveness of systemic therapies for metastatic pancreatic cancer. Curr Oncol. 2013;20(2):90–106. doi: 10.3747/co.20.1223.