Abstract
Objectives
To assess and compare health care resource utilization (HCRU) rates of asciminib and bosutinib at the Week 24, Week 48, and Week 96 cutoffs among 3 L + patients with chronic myeloid leukemia in chronic phase (CML-CP) in the randomized ASCEMBL trial.
Methods
Patients in the ASCEMBL trial (Clinicaltrials.gov: NCT03106779) were randomized to receive asciminib 40 mg twice daily (n = 157) or bosutinib 500 mg once daily (n = 76). At each scheduled visit, investigators conducted HCRU assessment on hospitalization, emergency room visit, general practitioner visit, specialist visit and urgent care visit; duration and type of hospitalization for the hospitalized patients; and reasons for HCRU. The number of patients with HCRU, rate of HCRU per patient-year, and length of hospital stay by ward type were compared at Week 24, Week 48, and Week 96 analyses.
Results
Lower proportions of patients receiving asciminib versus bosutinib used any resources including hospitalizations, emergency room visits, general practitioner visits, specialist visits, and urgent care visits (23.6% versus 36.8%, 26.1% versus 39.5%, and 28.6% versus 42.6% at Week 24, Week 48, and Week 96 analyses, respectively). After normalizing for treatment exposure, rates of HCRU for any resource per patient-year were significantly lower for asciminib versus bosutinib: 0.25 (95% CI: 0.18–0.34) versus 0.80 (95% CI: 0.55–1.16) at the Week 24 analysis, 0.20 (95% CI: 0.15–0.27) versus 0.47 (95% CI: 0.32–0.66) at the Week 48 analysis, and 0.17 (95% CI: 0.12–0.22) versus 0.40 (95% CI: 0.27–0.55) at the Week 96 analysis. Among the hospitalized patients, mean length of hospital stay was lower for asciminib than bosutinib for most wards at all three timepoints.
Conclusions
In the ASCEMBL trial, asciminib-treated patients with CML-CP in 3 L + maintained lower resource utilization compared to bosutinib over the long-term.
Introduction
Chronic myeloid leukemia (CML) is a hematological neoplasm that is typically present with the abnormal Philadelphia chromosomeCitation1. Globally, the incidence of CML was estimated at 65,800 cases in 2019, a 54.1% increase in incidence rate compared to 1990Citation2. It accounts for approximately 15% of the newly diagnosed adult leukemia patients in the United States (US)Citation1,Citation3. Patients are diagnosed via blood tests and may report symptoms of bone pain, weakness, fatigue, fever, loss of appetite, weight loss, pain or fullness below the ribs, and night sweatsCitation4.
Although the prognosis of CML diagnosis was poor before 2000, high efficacy of tyrosine kinase inhibitors (TKIs) has significantly improved the survival ratesCitation3,Citation5. CML patients are now reported to have a five-year survival rate of 70% or higherCitation6,Citation7. However, the treatment for third-line or later (3 L+) therapies in patients who fail or are intolerant to a second-line (2 L) TKI is more challenging with minimal efficacy of omacetaxine or rotation of additional second generation TKIs, risks and donor limitations with stem cell transplant, and concerns for safety for some patients with ponatinib.
Asciminib is a first-in-class agent Specifically Targeting the ABL Myristoyl Pocket (STAMP) inhibiting BCR::ABL1 oncoprotein that received US Food and Drug Administration approval in October 2021Citation8, Pharmaceuticals and Medical Devices Agency (Japan) approval in March 2022Citation9, and European Medicines Agency approval in August 2022 for use as a 3 L + therapy among CML in chronic phase (CML-CP) patientsCitation10. Bosutinib and ponatinib were the available treatment options for 3 L + CML-CP patients when the ASCEMBL phase 3 randomized controlled trial was designedCitation11. However, ponatinib had an ongoing trial (OPTIC) which was reassessing its optimal dosingCitation12, and had reported safety concerns in patients with cardiovascular comorbidities which limited its clinical usageCitation13,Citation14. For these reasons, bosutinib was selected as the appropriate reference treatment to compare asciminib against in the ASCEMBL trial. Asciminib demonstrated higher efficacy and better safety and tolerability profile manifested by low discontinuation rate due to adverse events (AEs) compared to bosutinib among 3 L + CML-CP patients in the pivotal ASCEMBL trialCitation14,Citation15.
The economic burden of 3 L + patients is significantly higher compared to those in earlier lines of therapyCitation16,Citation17. A 2015 analysis of IMS PharMetrics Plus Health Plan Claims Database compared the medical and outpatient pharmacy health care resource utilization (HCRU) for 1 L (N = 518), 2 L (N = 180), and 3 L (N = 8) CML patients. Unadjusted average cost per episode (2012 US dollars) in CML patients post-TKI failure who failed 1 L, 2 L, and 3 L therapy was $78,667, $99,624, and $181,029, respectivelyCitation16. Assessing how the efficacy and safety benefits of asciminib may translate into HCRU reduction is an important element of the management decisions for these patients. Trial data from ASCEMBL, a large multi-center trial with 87 study locations around the world, can reflect HCRU pattern for the global CML-CP patient populationCitation18.
A preliminary analysis at the time of the assessment of the primary endpoint for ASCEMBL (i.e. Week 24) suggested a lower HCRU of asciminib compared to bosutinib among patients with 3 L + CML-CPCitation19. It is of interest to decision-makers to assess whether the potential economic benefits of asciminib demonstrated in the Week 24 analysis are still maintained in the long-term follow-up. The objective of this study was to assess and compare, among patients with CML-CP who received asciminib or bosutinib after previously being treated with ≥2 TKIs in the ASCEMBL trial, HCRU rates of asciminib and bosutinib at the Week 24, Week 48, and Week 96 cut-offs.
Methods
This analysis compared the HCRU of asciminib- and bosutinib-treated patients from the ASCEMBL trial. ASCEMBL (Clinicaltrials.gov: NCT03106779) is a phase 3, multi-center, open-label, randomized clinical trial that enrolled adult patients with CML-CP who had previously been treated with ≥2 TKIs. Patients were randomized in a 2:1 ratio to receive asciminib 40 mg twice daily (n = 157) or bosutinib 500 mg once daily (n = 76). The trial protocol was approved by the sites’ institutional review boards and conducted in accordance with the Declaration of Helsinki. Details on trial methodology have been summarized in a previous publication reporting the Week 24 efficacy and safety resultsCitation14.
Measures
Pre-specified measures of HCRU data were collected as an exploratory endpoint within the ASCEMBL trial for hospitalization, emergency room (ER) visit (<24 h), general practitioner visit, specialist visit, and urgent care visit. HCRU was assessed as frequency and duration of hospitalization from baseline up to end of treatment; frequency of ER visits from baseline up to end of treatment; and frequency of additional (unplanned) outpatient office visits for general practitioner, specialist, and urgent care from baseline up to end of treatment. End of treatment was defined as up to 96 weeks after the last patient received the first dose or up to 48 weeks after the last patient randomized to bosutinib had switched to asciminib treatment as permitted per protocol, whichever was longer unless patients had discontinued treatment earlier. For each HCRU, reasons for visit were recorded as related to CML, AE related to CML therapy, or other reason. For hospitalizations, type of ward (hospital unit), length of hospital stays (number of days in ward), and discharge status were also captured.
HCRU assessment
Clinical visits were scheduled at Week 1, followed by visits every two weeks from Week 2 to Week 16, and every four weeks thereafter from Week 20 to Week 96. These planned visits to the trial investigators were not counted towards events of HCRU. HCRU assessments were completed by investigators at each scheduled clinical visit. However, the number of ER visits, general practitioner visits, specialist visits, and urgent care visits were ascertained from the patients. All attempts to collect as much information from the patient as possible were made to minimize selection bias.
Each timepoint of assessment in the main analysis in this paper represents the cut-off date when the last enrolled patient completed the specified weeks in the ASCEMBL trial. For instance, Week 24 represents the cut-off date when the last enrolled patient had been enrolled in the trial for 24 weeks. This can result in a median duration of treatment longer than the corresponding follow-up timepoint of assessment.
A post-hoc sensitivity analysis was conducted in which HCRU was assessed up to Weeks 24/48/96 of treatment exposure for all patients for the three timepoints of assessment. In this sensitivity analysis, all patients had treatment exposure equivalent to or less than the timepoint of analysis.
Statistical analysis
Descriptive analyses were performed, stratified by treatment received (asciminib or bosutinib). For each category and overall, the proportion of patients with any HCRU, the frequency of HCRU, the rate and corresponding 95% confidence intervals (CIs) of HCRU per patient-year on randomized treatment, and length of hospital stay by ward type were summarized using descriptive statistics (mean, standard deviation, median, and range for quantitative variables, and count and percentage for qualitative variables) along with the distribution of reasons for visits (related to CML, AE, or other reason). For length of stay by ward type results, patients who had duration of 0 hospitalization days at the ward they stayed at (i.e. date of admission is the same as date of discharge) were excluded from the descriptive statistics calculations.
Missing data were not imputed for the HCRU analyses. Owing to the exploratory nature of the analyses, no formal statistical analyses were conducted to assess the difference in HCRU rates between asciminib and bosutinib. All analyses were conducted using R version 3.6.1 (R Core Team, Vienna, Austria)Citation20.
Results
Patients
A total of 233 patients were randomly assigned to receive asciminib 40 mg twice a day (n = 157) or bosutinib 500 mg once daily (n = 76) in the ASCEMBL trial (). In the HCRU analyses population, the median age of patients was 52.0 years and 51.5% of the patients were females. Most of the patients (48.1%) had failed on two prior lines of TKI therapies while the rest failed on three or more lines, and the primary reason (63.9%) for discontinuation of last TKI was due to the lack of efficacy. Detailed demographic and clinical characteristics at baseline of the patients in the HCRU analyses are presented in . Of the patients recruited in the asciminib and bosutinib arms, the respective number of cumulative dropouts were 60 (38.2%) and 54 (71.1%) at the Week 24 analysis, 68 (43.3%) and 59 (77.6%) at the Week 48 analysis, and 73 (46.5%) and 61 (80.3%) at the Week 96 analysis.
Figure 1. Patient enrollment. Abbreviations. ATP, adenosine triphosphate; BID, twice a day; CML-CP, chronic myeloid leukemia in chronic phase; QD, once daily; TKI, tyrosine kinase inhibitor.
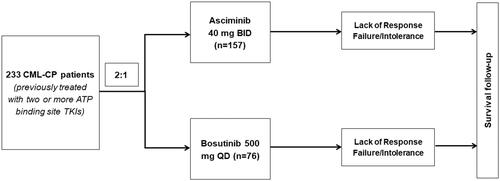
Table 1. Demographic and clinical characteristics of patients at baseline.
HCRU over time
At the Week 24 analysis (data cut-off: 25 May 2020), the median duration of exposure was 43.4 and 29.2 weeks in the asciminib and bosutinib arms, respectively. The median duration of exposure in the asciminib and bosutinib arms, respectively, were 66.9 and 32.6 weeks at the Week 48 analysis (data cut-off: 06 January 2021), and 103.1 and 34.4 weeks at the Week 96 analysis (data cut-off: 06 October 2021).
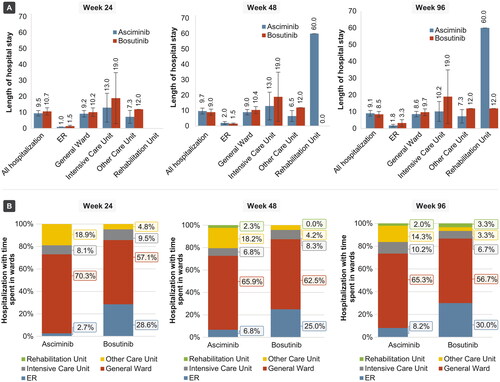
A lower proportion of patients in the asciminib arm compared to the patients in the bosutinib arm used at least one health care resource (hospitalizations, ER visits, general practitioner visits, specialist visits, or urgent care visits) at the Week 24 analysis (23.6% versus 36.8%), at the Week 48 analysis (26.1% versus 39.5%), at the Week 96 analysis (28.6% versus 42.6%) ( and ). With the exception of specialist visits at the Week 96 analysis (14.0% versus 13.2%), compared to the asciminib arm, a greater proportion of the patients in bosutinib arm used each category of specific resources at the Week 24, Week 48, and Week 96 analyses. At all three timepoints, hospitalization was the most common resource used followed by specialist visits for the patients in both treatment arms.
Figure 2. Proportions of patients with HCRU at Week 24, Week 48, and Week 96 analysis, overall and by category. Proportions for specific resources may not add up exactly to the proportion for “any resources” use because some patients used more than one resource. Abbreviations. ER, emergency room; HCRU, health care resource utilization.
Table 2. Frequency of HCRU.
Despite having a lower proportion of patients with any HCRU compared to bosutinib, on average, patients treated with asciminib tended to have a higher overall use of resources, particularly general practitioner and specialist visits (). These differences could be driven by much longer duration of therapy in the asciminib arm due to high rates of early treatment discontinuation of bosutinib, mostly for adverse events. We thus analyzed the use of resources adjusted by time of exposure.
Rate and reasons for HCRU over time
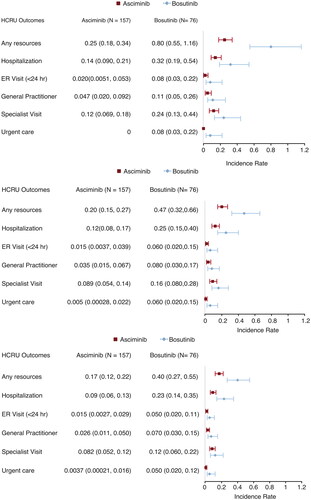
Accounting for differences in treatment exposure period, patients receiving asciminib had significantly lower rates of overall HCRU per patient-year compared to those receiving bosutinib at the Week 24, Week 48, and Week 96 analyses (). Respective rates of HCRU per patient-year for asciminib and bosutinib arms were 0.25 (95% CI: 0.18–0.34) and 0.80 (95% CI: 0.55–1.16) at the Week 24 analysis, 0.20 (95% CI: 0.15–0.27) and 0.47 (95% CI: 0.32–0.66) at the Week 48 analysis, and 0.17 (95% CI: 0.12–0.22) and 0.40 (95% CI: 0.27–0.55) at the Week 96 analysis. Similarly, lower rates of HCRU were observed for each of the specific resources among patients in the asciminib arm compared to those in bosutinib arm; however, the differences in rates were not statistically significant.
Figure 3. Rates of HCRU per patient-year at Week 24, Week 48, and Week 96 analysis. Abbreviations. ER, emergency room; HCRU, health care resource utilization.
Although the overall hospitalization data made no differentiation for severity of hospitalization (i.e. short stay versus long stay), a similar pattern of numerically but not significantly lower rates of HCRU per patient-year was also observed for the asciminib-treated patients in a subgroup analysis of patients with short (<5 days) versus long stays (≥5 days) (Supplementary Data: Apdx_Figure 1).
Overall, most HCRU, particularly hospitalizations and specialist visits, were for reasons other than AEs or CML for both asciminib and bosutinib (Supplementary Data: Apdx_Table 1). Asciminib was associated with less hospitalizations, ER visits, and urgent care visits due to AEs compared to bosutinib. In contrast, general practitioner visits related to CML were more frequent for asciminib compared to bosutinib for all three cut-offs.
HCRU among hospitalized patients
Length of hospital stay was lower with the asciminib group compared to bosutinib in most ward types at the Week 24, Week 48, and Week 96 analyses (, Supplementary Data: Apdx_Table 2). The mean length of stay across all hospitals at the Week 24 analysis was 9.5 days for asciminib and 10.7 days for bosutinib. The mean lengths of stay with asciminib versus bosutinib were 9.7 days versus 9.0 days at the Week 48 analysis, and 9.1 days versus 8.5 days at the Week 96 analysis. Higher overall hospitalization durations for the asciminib arm compared to bosutinib arm at the Week 48 and Week 96 analyses were driven by a stay at the rehabilitation unit (mean 60 days) for one patient that was observed at longer follow-ups. In the asciminib arm, most hospitalizations were spent in general ward (70.3%, 66.7%, and 65.0% of hospitalizations at the Week 24, Week 48, and Week 96 analyses, respectively, with corresponding mean stays of 9.2 days, 9.0 days, and 8.6 days), followed by “other care unit” (18.9%, 18.2%, and 14.3% of hospitalizations at the Week 24, Week 48, and Week 96 analyses, respectively, with mean stays of 7.3 days, 6.5 days, and 7.3 days). In the bosutinib arm, most hospitalizations were spent in general ward (70.6%, 62.5%, and 56.7% of hospitalizations at the Week 24, Week 48, and Week 96 analyses respectively with corresponding mean stays of 10.2 days, 10.4 days, and 9.7 days), followed by ER (35.3%, 25.0%, and 30.0% of hospitalizations at the Week 24, Week 48, and Week 96 analyses with corresponding mean stays of 1.5 days, 1.5 days, and 3.3 days).
Sensitivity analysis
In the sensitivity analysis set with HCRU assessment by Week 24/48/96 of treatment exposure, the median durations of treatment exposure in the asciminib and bosutinib arms were 24.0 weeks each at Week 24, 48.0 weeks and 31.6 weeks at Week 48, and 95.9 weeks and 31.6 weeks at Week 96, respectively. There was minimal difference between results from the base case analysis and the sensitivity analysis (see Supplementary Data: Apdx_Figure 2, Apdx_Table 3, Apdx_Table 4, and Apdx_Table 5). Overall, the sensitivity analysis found that there was no statistically significant difference in HCRU rates between asciminib and the bosutinib arms by Week 24 but there were significant differences in favor of asciminib in “any HCRU” by Weeks 48 and 96 (Supplementary Data: Apdx_Figure 3).
Discussion
This study compared the Week 24, Week 48, and Week 96 HCRU rates among 3 L + patients with CML-CP in the Phase 3 ASCEMBL trial. The results suggest that patients with CML-CP treated with asciminib had lower resource utilization at all time points compared to bosutinib in terms of overall numbers of patients accessing services and length of stay in hospital. Lower rates of HCRU in asciminib compared to bosutinib observed in the Week 24 assessment were maintained in the long-term assessments.
This is the first analysis to estimate and compare long-term HCRU among 3 L + CML-CP patients receiving asciminib and bosutinib. The results are based on the data from a large multi-center trial with 87 study locations around the world and are very likely to reflect HCRU pattern of the global CML-CP patient population. The results clearly demonstrate the superior place in therapy of asciminib over bosutinib. Better HCRU rates, together with a favorable efficacy, safety and tolerability profileCitation14,Citation15, of asciminib versus bosutinib provide valuable evidence for patients, payers and providers in making an informed decision on the treatment choice.
There are some limitations of our analysis. First, very small rates of resource utilization were likely due to the trial’s 2:1 randomization of asciminib: bosutinib contributing to an overall small starting population of patients treated with bosutinib and exacerbated by the fact that patients in the bosutinib arm were increasingly lost to follow-up or switching due to AEs or lack of response. The relatively short duration of follow-up over which HCRU may occurred might also have contributed to a small number of patients experiencing an event (i.e. having any form of HCRU), which makes it difficult to observe any significant differences between treatments. Although more events were observed during longer follow-up, the number of patients using any form of HCRU remained a small proportion of the overall population. As such, the exploratory nature of the HCRU endpoint, the small sample size, and the low number of HCRU events from ASCEMBL prevented any robust conclusions about the economic (cost-offset) impact of asciminib from being made. Second, the small numbers of observations also meant the relative frequency of HCRU and mean length of stay in hospital were heavily influenced by select patients. For example, there was only one instance of hospitalization in patients treated with asciminib that resulted in a very long stay in the rehabilitation unit and consequently increased the average length of stay for overall hospitalizations. In another instance, a patient had an extremely high number of specialist visits that resulted in a much higher average number of specialist visits for asciminib. Third, most of the reasons for patient hospitalizations were due to “other reasons,” rather than for reasons related to CML disease or treatment AE. Absence of detailed information on the reasons for hospitalization, as well as the unavailability of laboratory and imaging investigations as additional resources of interest, limits further insight into the economic impact of asciminib and wider applicability of this data. Collection of accurate laboratory and other resource utilization data, especially for unplanned visits, can be particularly challenging as such visits are likely to be at local facilities and not captured in the trial’s central database. Fourth, there were imbalances in the asciminib arm and the bosutinib arm on the number of prior lines of TKI therapy, and reasons for discontinuation of last TKICitation14. These differences in patient characteristics could have an effect on patients’ duration of exposure to treatment and overall resource use. To account for potential imbalances in the patient characteristics, we report exposure-adjusted HCRU. Fifth, patients in the ASCEMBL trial were recruited from 87 centers in 25 countriesCitation18,Citation21, some of which had few patients enrolled or had patients enrolled only in one intervention arm. This limited our ability to explore any potential differences in resource use between centers in the current analyses. Future research can explore whether local, regional or national differences in healthcare management affects the overall resource use. Finally, although the patterns of HCRU as observed in the trial setting are compelling and reflect actual patient data, they may not reflect what will be seen in routine clinical practice. In the real-world, clinical experience with patient management for a novel therapy like asciminib is still lower compared to an established standard of care like bosutinib.
Conclusions
Asciminib maintained a consistently lower resource utilization compared to bosutinib at the Week 24, Week 48, and Week 96 analyses in patients with CML-CP from the ASCEMBL trial. These findings further support the superiority of asciminib over bosutinib as a 3 L + therapy among CML-CP patients. Future data from real-world usage will be needed to validate these findings.
Transparency
Declaration of financial/other relationships
JEC is a consultant for Novartis, Pfizer, Takeda and Sun Pharma; his institution received research support from BMS, Novartis, Pfizer, Takeda and Sun Pharma. DR received honoraria and/or advisory board payments from Incyte, Novartis and Pfizer. MJM is a consultant for Novartis, BMS, Takeda and Pfizer; his institution received research support from Novartis, BMS, Sun Pharma/SPARC. DT and PW are employees of EVERSANA, Inc who were paid consultants to Novartis. KJ and AY are employees of Novartis and hold stock with Novartis. KS received research funding and advisory board payments from Novartis. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
All authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article. KJ and AY were involved in the conception and design of the study. DT and PW were involved in programming and conducting the analyses. JEC, DR, MJM, and KS were involved in reviewing the data, and all authors participated in interpreting the results. All authors critically reviewed the manuscript for intellectual content and approved the final version of the manuscript. All authors had full access to the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Previous presentation
An abstract of this analysis was presented virtually at ISPOR Europe 2022, Vienna, Austria, 6–9 November 2022.
Supplemental Material
Download MS Word (1,015 KB)Acknowledgements
The authors thank Parash Mani Bhandari (EVERSANA) for medical writing assistance, which was funded by Novartis.
Additional information
Funding
References
- American Cancer Society. Key statistics for chronic myeloid leukemia. Atlanta (GA): American Cancer Society; [cited 2022 Aug 5]. Available from: https://www.cancer.org/cancer/chronic-myeloid-leukemia/about/statistics.html
- Hu Y, Li Q, Hou M, et al. Magnitude and temporal trend of the chronic myeloid leukemia: on the basis of the global burden of disease study 2019. J Clin Oncol Glob Oncol. 2021;7:1429–1441. doi: 10.1200/GO.21.00194.
- Jabbour E, Kantarjian H. Chronic myeloid leukemia: 2020 update on diagnosis, therapy and monitoring. Am J Hematol. 2020;95(6):691–709. doi: 10.1002/ajh.25792.
- Mayo Clinic. Chronic myelogenous leukemia. USA: Mayo Clinic; [cited 2022 Aug 5]. Available from: https://www.mayoclinic.org/diseases-conditions/chronic-myelogenous-leukemia/symptoms-causes/syc-20352417
- American Cancer Society. Cancer facts & figures 2019. Atlanta (GA): American Cancer Society; 2019.
- National Cancer Institute. Cancer stat facts: leukemia—chronic myeloid leukemia (CML). Bethesda (MD): SEER; [cited 2022 Aug 5]. Available from: https://seer.cancer.gov/statfacts/html/cmyl.html
- National Health Service (NHS). Chronic myeloid leukaemia. UK: National Health Service; [cited 2023 Aug 5]. Available from: https://www.nhs.uk/conditions/chronic-myeloid-leukaemia/
- U.S. Food & Drug Administration. FDA approves asciminib for Philadelphia chromosome-positive chronic myeloid leukemia. USA: Food & Drug Administration; [cited 2022 Aug 5]. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-asciminib-philadelphia-chromosome-positive-chronic-myeloid-leukemia
- Pharmaceuticals and Medical Devices Agency. New drugs approved in FY. Japan: Pharmaceuticals and Medical Devices Agency; [cited 2022 Aug 5]. Available from: https://www.pmda.go.jp/files/000246734.pdf
- European Medicines Agency. Scemblix. The Netherlands: European Medicines Agency; [cited 2022 Aug 5]. Available from: https://www.ema.europa.eu/en/medicines/human/summaries-opinion/scemblix
- Baccarani M, Deininger MW, Rosti G, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122(6):872–884. doi: 10.1182/blood-2013-05-501569.
- Cortes JE, Lomaia E, Turkina A, et al. Interim analysis (IA) of OPTIC: a dose-ranging study of three ponatinib (PON) starting doses. J Clin Oncol. 2020;38(15_suppl):7502–7502. doi: 10.1200/JCO.2020.38.15_suppl.7502.
- Molica M, Scalzulli E, Colafigli G, et al. Insights into the optimal use of ponatinib in patients with chronic phase chronic myeloid leukaemia. Ther Adv Hematol. 2019;10:2040620719826444. doi: 10.1177/2040620719826444.
- Réa D, Mauro MJ, Boquimpani C, et al. A phase 3, open-label, randomized study of asciminib, a STAMP inhibitor, vs bosutinib in CML after 2 or more prior TKIs. Blood. 2021;138(21):2031–2041. doi: 10.1182/blood.2020009984.
- Rea D, Mauro MJ, Hochhaus A, et al. Efficacy and safety results from ASCEMBL, a phase 3 study of asciminib versus bosutinib (BOS) in patients (pts) with chronic myeloid leukemia in chronic phase (CML-CP) after ≥2 prior tyrosine kinase inhibitors (TKIs): week 96 update. J Clin Oncol. 2022;40(16_suppl):7004–7004. doi: 10.1200/JCO.2022.40.16_suppl.7004.
- McGarry LJ, Chen YJ, Divino V, et al. Increasing economic burden of tyrosine kinase inhibitor treatment failure by line of therapy in chronic myeloid leukemia. Curr Med Res Opin. 2016;32(2):289–299. doi: 10.1185/03007995.2015.1120189.
- Negi H, Agrawal R, Vieira J, et al. PCN231 humanistic and economic burden in patients with chronic myeloid leukemia - a review of the literature. Value Health. 2021;24:s63. doi: 10.1016/j.jval.2021.04.321.
- ClinicalTrials.gov. Study of efficacy of CML-CP patients treated with ABL001 versus bosutinib, previously treated with 2 or more TKIs. USA: National Institutes of Health; [cited 2022 Aug 5]. Available from: https://clinicaltrials.gov/ct2/show/NCT03106779
- Smith BD, Cortes JE, Rea D, et al. CML-353: health care resource utilization (HCRU) with asciminib and bosutinib among patients with chronic myeloid leukemia in chronic phase (CML-CP) previously treated with ≥2 tyrosine kinase inhibitors (TKIs): results from the multicenter, open-label phase 3 ASCEMBL trial. Clin Lymphoma Myeloma Leuk 2021;21:s334. doi: 10.1016/S2152-2650(21)01781-X.
- R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2022.
- National Institute for Health and Care Excellence. Asciminib for treating chronic myeloid leukaemia after 2 or more tyrosine kinase inhibitors [ID3813]: committee papers. UK: National Institute for Health and Care Excellence; [cited 2023 Jul 3].