Abstract
Background and objectives
The Oncotype DX Breast Recurrence Score test is used to estimate distant recurrence risk of hormone receptor-positive (HR+) and human epidermal growth factor receptor 2-negative (HER2–) early-stage breast cancer and inform decisions on the use of adjuvant chemotherapy. A model-based budget impact analysis compared the Oncotype DX test in combination with clinical–pathological risk against using clinical–pathological risk alone for HR+/HER2– node-negative (N0) and node-positive (N1; 1–3 axillary lymph nodes) early-stage breast cancer patients.
Materials and methods
Test and medical costs associated with treatment of breast cancer were assessed through a US healthcare payer perspective. Distributions of patients by Recurrence Score result and distant recurrence probabilities with chemo-endocrine and endocrine therapy were derived from the TAILORx (N0) and RxPONDER (N1) trials. Changes in budget impact were evaluated over a 5-year horizon for a 1,000,000-member hypothetical health plan.
Results
With the Oncotype DX test, there was an incremental budget impact of $261,067 (per member per month (PMPM): $0.004), in the N0 population, and $56,143 (PMPM: $0.001) in the N1 population over the 5-year period. The largest budget impact reduction in the N0 population was attributed to reduced breast cancer recurrence costs (incremental: −$633,457, PMPM: −$0.011), while chemotherapy sparing reduced costs in the N1 population (incremental: −$94,884, PMPM: −$0.002).
Conclusion
The clinical benefit of using the Oncotype DX test to inform adjuvant chemotherapy decisions has been shown in multiple randomized controlled trials. This analysis demonstrated that while using the Oncotype DX test to inform adjuvant chemotherapy decisions may slightly increase US healthcare costs over an initial 5-year time horizon (driven by a cost increase in the first year with cost savings reflected in remaining 4 years), there is significant scope for cost savings when assessing beyond this period due to avoided downstream costs of distant recurrence and long-term chemotherapy adverse events. PMPM costs also remain low across all populations examined, demonstrating a close-to-neutral budget impact.
Background
Early-stage invasive breast cancer (defined by stage 1, 2, or 3 A) is a heterogeneous, phenotypically diverse disease composed of several molecular subtypes that have distinct prognoses and responses to therapy. Sixty-five percent of women with breast cancer are diagnosed at this stageCitation1, with nearly 30% ultimately developing metastatic lesionsCitation2. Tumors that show estrogen or progesterone-receptor expression are referred to as hormone-receptor-positive (HR+), and tumors that do not display abnormal human epidermal growth factor receptor 2 (HER2) expression are referred to as HER2-negative (HER2–). The majority of all breast cancer tumors are HR+/HER2–Citation3, and this subset of breast cancer, when treated appropriately, has the highest survival rate among the different molecular subtypes of breast cancerCitation4.
The assessment of clinical–pathological factors is used in standard practice and includes tumor grade, size, nodal burden, patient age, and menopausal status. These are used to estimate the risk of recurrence and to select patients for either endocrine or chemo-endocrine therapy as the standard of care adjuvant therapy for HR+/HER2– early-stage breast cancerCitation5–7. Before the availability of genomic tumor profiling, the use of clinical–pathological factors was informed by the 2000 NIH Consensus ConferenceCitation8. A less aggressive approach with endocrine therapy alone is generally used for patients who have a lower risk of recurrence. Chemotherapy followed by endocrine therapy is a more aggressive approach utilized in higher-risk patientsCitation9.
In the last two decades, gene-expression profiling using multigene assays (MGAs) has been used in addition to clinical–pathological factors to improve adjuvant treatment decisions. The Oncotype DX Breast Recurrence Score test (the Oncotype DX test, Exact Sciences, Madison, WI) assesses the expression of 21 genes in tumor tissue. The subsequent Recurrence Score (RS) result from 0 to 100 represents the estimated 10-year risk of future distant recurrence and supports decisions on whether to use adjuvant chemotherapy. The use of the Oncotype DX test in HR+/HER2– early breast cancer is recommended in US clinical practice guidelinesCitation7.
The Oncotype DX test was validated in two prospective phase III randomized controlled trials: TAILORx in node-negative (N0) and RxPONDER in lymph node-positive (N1; 1–3 positive lymph nodes) HR+/HER2– early-stage breast cancerCitation10,Citation11. TAILORx demonstrated that adjuvant endocrine therapy and chemo-endocrine therapy had a similar efficacy for women >50 with node-negative cancer who had an RS result of between 11 and 25. RxPONDER demonstrated that among premenopausal women with one to three positive lymph nodes and an RS result of 25 or lower, those who received chemo-endocrine therapy had longer invasive disease-free survival and distant relapse-free survival than those who received endocrine therapy only. However, postmenopausal women with similar characteristics did not benefit from adjuvant chemotherapy. The Oncotype DX test can be used to guide the choice of endocrine therapy vs. chemo-endocrine therapy, resulting in tailored treatment decisions being made, benefiting both the patient and healthcare system.
Previous budget impact analyses have shown the Oncotype DX test to be cost-saving in the German marketCitation12, but no such analysis has been conducted from a US healthcare perspective. A budget impact analysis for MammaPrint informed by data from the MINDACT study reported a net cost saving from a US healthcare payer perspective, suggesting that the use of MGAs can free up substantial healthcare resourcesCitation13. There is a need to estimate the budget impact of the Oncotype DX test from a US healthcare perspective, considering the new evidence published from the TAILORx and RxPONDER trials, which used an updated RS classification for high-risk patients (RS > 25 instead of RS > 30)Citation10,Citation11.
Methods
Intervention and comparators
A budget-impact model was developed to assess the use of the Oncotype DX test in combination with clinical–pathological risk assessment compared with using clinical–pathological risk assessment alone to guide adjuvant chemotherapy decisions for HR+/HER2– N0 and N1 early-stage breast cancer in the US. The base-case analysis compared testing of all patients eligible for adjuvant chemotherapy with the Oncotype DX test vs. using clinical–pathological risk alone for the whole population.
Study population and perspective
The budget impact analysis was conducted from a mixed Medicare, Medicaid, and commercial payer perspective based on the patient distribution obtained from the US Census BureauCitation14.
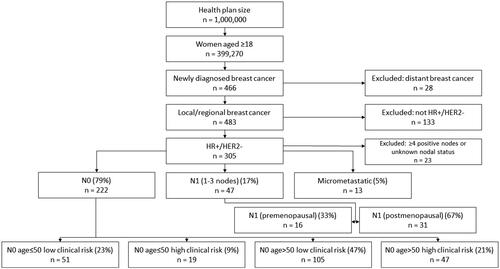
The starting population in the model assumed a hypothetical health plan with 1 million members. The number of patients with early-stage breast cancer eligible for testing with the Oncotype DX test was derived using US Census Bureau population estimatesCitation15 and incidence of local or regional breast cancer from SEERCitation16,Citation17. The average age of patients under each payer perspective (Medicare, Medicaid and commercial) was sourced from US Census Bureau dataCitation18. This was then combined with incidence of breast cancer based on age from the SEER databaseCitation19, to produce payer-perspective-specific prevalence estimates.
The proportion of women with HR+/HER2– early-stage breast cancer by nodal status was obtained from Nelson et al.Citation20 Additional subgroup analyses for incremental budget impact were completed for N0 patients aged ≤50 and >50 in low- and high-clinical-risk groups, premenopausal and postmenopausal N1 patients, patients with micrometastatic (N1mi) tumors, and a combined group of all HR+/HER– patients. Subgroup analyses were guided by the data available on relevant factors available. RxPONDER stratified patients by menopausal status, a well-known determinant for prognosis of distant recurrence and chemotherapy benefit. TAILORx stratified patients by clinical risk, which is also a prognostic factor for distant recurrence, and age, which can be used as a proxy for menopausal status. These analyses are shown in the Online Supplement.
The process of estimating the eligible study population is summarized in . The sources for the data used to inform the flow diagram are shown in .
Table 1. Base-case parameter values, ranges, and distributions.
Table 2. Payer perspective conversion rates.
Model structure
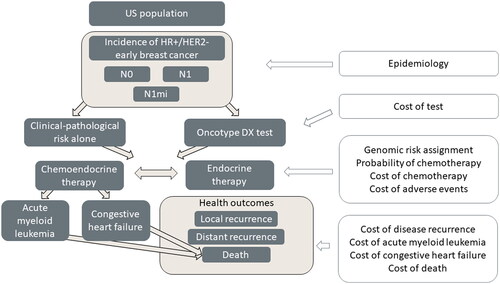
An executable model was built in Microsoft Excel and Visual Basic for Applications. The model split patients into two hypothetical cohorts, one with utilization and one without utilization of the Oncotype DX test. Patient flow was then modeled over five 1-year cycles, tracking the consequences of adjuvant treatment decisions on the cost of treatment over a 5-year time horizon. The model structure is presented in .
Clinical inputs
Upon entering the model, patients are assessed using either the Oncotype DX test or clinical–pathological risk alone and are allocated to adjuvant chemo-endocrine therapy or endocrine therapy alone. It was assumed that the distribution of underlying genomic risk, which is defined by the RS result in the Oncotype DX arm of the model, is identical for both the clinical–pathological risk arm and the Oncotype DX arm in the model. The cost differences between the comparison arms in the model were thus solely driven by differences in chemotherapy assignment. Patients were stratified according to their RS result into three groups defined using cut-points validated in the TAILORx study for N0 (0–10, 11–25, 26–100)Citation10 and RxPONDER for N1 (0–13, 14–25, 26–100)Citation11. Chemotherapy assignment for Oncotype DX tested patients was conditional on the assigned RS subgroup.
The probability of being assigned to adjuvant chemotherapy based on clinical–pathological risk alone or conditional on the RS result was based on advice from breast oncologists in the US (survey methods are described in the Online supplement), with scenario analyses included to test alternative estimates from SEERCitation34,Citation35. A targeted literature review identified analyses informed by the SEER database (based on a cohort of patients diagnosed up to 2016) as the best-available data source for chemotherapy assignment. However, this was deemed inappropriate for the model base case, as it does not currently reflect recent changes in clinical practice that have been brought about by the RxPONDER study, and only refects changes brought about from TAILORx to a limited extent. There is also evidence to suggest that chemotherapy rates are underreported in SEERCitation36. Data from SEER were tested in the scenario analysis, and when used the conclusions in this study were unchanged.
In the clinical–pathological risk alone arm of the model, all patients were assumed to have the same probability of chemotherapy assignment, which was informed by clinical expert opinion. The impact of clinical–pathological factors on chemotherapy assignment was accounted for by stratifying the cohorts according to clinical risk and age for the N0 cohort or menopausal status for the N1 cohort, considering the availability of data from clinical studies.
During a given model cycle, patients who are recurrence-free can remain disease-free, develop distant recurrence, acute myeloid leukemia (AML), or congestive heart failure (CHF) if they are treated with chemotherapy, or die. After developing distant recurrence, AML, or CHF, patients remain in this health state unless they die. The probability of distant recurrence for N0 was obtained from distant recurrence-free interval (DRFI) estimates in the TAILORx trial, in which patients with RS < 11 were assigned endocrine therapy, those with RS 11–25 were randomized to chemo-endocrine therapy or endocrine therapy alone, and all patients with RS > 25 were assigned to chemo-endocrine therapyCitation10. Estimates of chemotherapy benefit for the low- and high-RS groups were obtained from the NSABP B-20 studyCitation23. Distant recurrence estimates for N1 patients with RS ≤ 25 were obtained from the RxPONDER studyCitation26. To inform the RS > 25 group, who were excluded from the RxPONDER study, DRFI estimates for endocrine therapy-assigned patients from TransATACCitation24,Citation37 were combined with chemotherapy benefit estimates from SWOG-8814Citation25. It was assumed that 10.5% of patients who experience a distant recurrence have had a prior local recurrence, to account for its cost impactCitation38. Patients assigned to adjuvant chemotherapy were assumed to have a higher risk of developing AML and CHF, with transition probabilities derived from literatureCitation27,Citation28. Patients in the model could die from breast cancer after distant recurrence, or from AML, CHF, or any other cause. No excess breast-cancer-related mortality was assumed for patients who remain recurrence-free. Disease-related mortality was obtained from the literature, with recurrence-free baseline mortality being taken from national life Tables (32).
Subgroup inputs
The SEER database also informed RS patient distribution and chemotherapy allocation for N1miCitation22. In the absence of N1mi-specific data for some probability of distant recurrence and chemotherapy benefit, data for the N0 population were used based on advice from US clinical experts. Clinical practice recommendations from National Comprehensive Cancer Network (NCCN) suggest that treatment of N1mi patients should be the same as for patients with N1 tumorsCitation7. The results of a scenario analysis to test the impact of using N1 inputs instead are reported in Table S7 in the Online Supplement.
The probability of distant recurrence for premenopausal and postmenopausal subgroups for N1 stratified according to RS result subgroups was derived from DRFI data from RxPONDER reported at the San Antonio Breast Cancer Symposium 2021Citation26, and chemotherapy allocation data were derived from the survey of breast cancer experts.
Probability of distant recurrence and distribution of risk scores in the N0 population subgroups (aged ≤50, or >50 with low or high clinical risk) was sourced from Sparano et al. Citation21. Probability of chemotherapy in this group was sourced through clinical expert elicitation.
Cost inputs
Test costs and the costs of adjuvant endocrine therapy and chemotherapy were applied in the baseline year, with treatment costs for local and distant recurrence, AML, and CHF estimated in years 2–5. Chemotherapy and endocrine therapy regimens were in line with NCCN guidelines to reflect US clinical practiceCitation7. Treatment patterns for adjuvant chemotherapy, endocrine therapy, and associated treatments (e.g. growth factors) were based on a survey of nine breast cancer experts in the US (Supplement Table S6). These were combined with drug unit costs obtained from the Medicare fee schedule (Supplement Table S3) to estimate the total cost per regimen reported. The cost of distant recurrence included drug costs for treatments used in metastatic breast cancer, and assumptions were validated by experts in breast cancer in the US (Table S1 in the Online supplement). Treatment costs are the combination of all nontest costs [recurrence-free disease management, early breast cancer chemo-endocrine therapy including adverse events (AEs), early breast cancer endocrine therapy, local recurrence, distant recurrence, AML, CHF, and terminal care]. The number of patients undergoing chemotherapy and experiencing distant recurrence is also shown. Cost assumptions for short- and long-term consequences of adjuvant chemotherapy were obtained from a previous economic evaluation of the Oncotype DX test in the US.Citation27 Unit costs of short-term adverse events were applied to patients assigned to chemotherapy, weighted according to their frequency reported in clinical trials. A summary of model parameter inputs is reported in .
Costs were presented in 2021 US dollars (USD). Costs published in previous years were adjusted in line with inflationCitation39. Future costs and outcomes were not discounted, given the short time horizon of the analysis, and to align with best budget impact modeling practice in the USCitation40. All costs sourced from a Medicaid perspective have been converted to the Medicare perspective using the Henry J. Kaiser Family Foundation Medicaid-to-Medicare fee indexCitation41. Cost inputs for the commercial perspective were converted using published indicesCitation42. Adjustment was carried out for all costs except the cost of the genomic assays.
Analytical approach
The net budget impact was estimated over the 5-year model horizon as the difference in the aggregate cost between the scenario of using the Oncotype DX test and the scenario where all chemotherapy decisions were based on clinical–pathological risk assessment alone. Additional metrics reported were incremental cost per health plan member per month (PMPM) and per eligible breast cancer patient tested, patient tested per month (PTPM). A probabilistic sensitivity analysis was conducted to characterize the uncertainty from all parameters in the model combined. The probabilistic sensitivity analysis was run with 1,000 iterations with inputs varied in correspondence to input ranges specified in , and the aggregate results were collected (the mean of all iterations). The choice of distributions for the draws was based on the type of input (e.g. beta distribution for transition probabilities, gamma for unit costs). This analysis was presented using Tornado diagrams to identify model parameters which contributed the most toward uncertainty in the model and have the most substantial impact on results. The diagrams display the effect on model results of varying the value of each parameter across a predetermined range, which reflects the confidence interval reported in the original published source or the assumed distribution.
Additional analyses were reported for selected subgroups of the model population, including patients with N1mi tumors, patient subgroups with N1 tumors defined by menopausal status, and N0 patient groups defined by age and clinical risk (shown in the Online Supplement).
Scenario analyses () tested alternative data sources and assumptions in the model. In the N0 population, probability of chemotherapy based on clinical–pathologic risk alone was varied from 15% to 35% and was tested based on the extremities of clinical expert estimation. Probability of chemotherapy based on the Oncotype DX Breast RS test was varied in the intermediate-risk group to demonstrate the sensitivity of this parameter to change. Hazard ratio of chemotherapy benefit in the high-risk group was varied between 0.12 and 0.62 in line with parameter uncertainty limits from the literatureCitation23. In the N1 population, probability of chemotherapy based on clinical–pathological risk alone was varied from 25% to 45% and was tested based on the extremities of clinical expert estimation. Probability of chemotherapy based on the Oncotype DX Breast RS test was varied in the intermediate-risk group for the postmenopausal population to demonstrate the sensitivity of this parameter to change. The hazard ratio of chemotherapy benefit in the high-risk group was varied between 0.35 and 1 in line with parameter uncertainty limits from the literatureCitation25. The cost of the Oncotype DX test was reduced and increased by 10% to demonstrate the impact of varying this parameter. All patients were varied to show 100% of patients reflective of cost and epidemiological inputs of commercial, Medicare, and Medicaid payer perspective. Finally, the cost of chemotherapy (including G-CSF and administration costs) and G-CSF alone was reduced by 50% to demonstrate the sensitivity of these parameters to change.
Results
Base-case budget-impact analysis results
When comparing the current scenario where patients are assessed using clinical–pathological risk alone, against an alternative scenario where patients are assessed using the Oncotype DX test, over the 5-year period, there was an incremental budget impact of $261,067 ($0.004 per member per month, $19.611 per PTPM) in the N0 population (shown in ), and $56,143 ($0.001 per member per month, $19.909 per PTPM) in the N1 population (shown in ). A full breakdown of budget impact over a 5-year period is shown in for N0 patients and for N1 patients. Both show an overall positive (cost-increasing) budget impact for the initial year where patients are tested with the Oncotype DX test; however, this is followed by a negative (cost-saving) budget impact for the subsequent 4 years as patients become less costly to treat. Based on the PMPM, the overall budget impact of inclusion of the Oncotype DX test is relatively budget-neutral.
Table 3. Cost breakdown by category N0 combined population.
Table 4. Cost breakdown by year N0 combined population.
Table 5. Cost breakdown by category N1 combined population.
Table 6. Cost breakdown by year N1 combined population.
The budget impact varied according to subgroups defined by age and clinical risk (for N0) and menopausal status (for N1), reflecting the impact of these prognostic clinical–pathological factors on chemotherapy decisions and the probability of distant recurrence. The budget impact was relatively higher for patients aged >50 with low clinical risk, which was driven by increased chemotherapy allocation in this subgroup compared with the clinical–pathological risk alone comparator. Conversely, the Oncotype DX test was cost-saving for the subset with age >50 and high clinical risk, reflecting reduced chemotherapy use among patients with RS ≤ 25 and reduced cost of treating distant recurrence due to reduced under-treatment of patients with RS > 25. The budget impact was similar across N1 subgroups by menopausal status, with larger cost savings in the postmenopausal subgroup. Full details of subgroup analysis results are shown in Table S7 in the Online Supplement.
Budget impact analysis for all HR+/HER2– patients combined
Analyses were also conducted for a combined group of all HR+/HER2– patients, which included all N0, N1, and N1mi patients. This resulted in an incremental budget impact of $366,816 ($0.006 per member per month, $20.032 per patient tested per month) over a 5-year period, which is shown in . A full breakdown of budget impact over a 5-year period is shown in . This is similar to the N0 and N1 results, where there is a positive (cost-increasing) budget impact for the initial year where patients are tested, followed by a negative (cost-saving) budget impact for the subsequent 4 years as patients become less costly to treat.
Table 7. Cost breakdown by category all HR+/HER2– combined.
Table 8. Cost breakdown by year all HR+/HER2– combined.
Clinical outcomes
Clinical outcomes (no. of patients assigned to chemotherapy, no. of patients with distant recurrence) have been reported in , for the N0 population, N1 population, and the whole HR+/HER2– population combined. In the N0 subgroup, 4 out of 58 patients were allocated from endocrine therapy alone to chemo-endocrine therapy after using the Oncotype DX test. In the N1 subgroup, 5 out of 23 patients were spared chemotherapy. When combined, the number of patients allocated to chemo-endocrine therapy for the full HR+/HER2– was unchanged. Overall, three cases of distant recurrence were prevented with the use of the Oncotype DX test; all were in the N0 subgroup.
Table 9. Clinical outcomes.
Sensitivity analyses
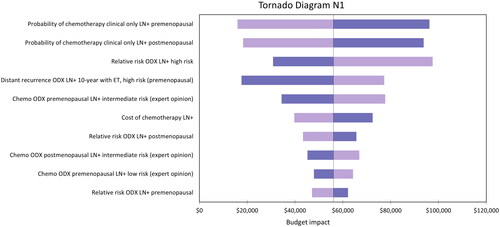
Sensitivity analyses were conducted for the N0 and N1 populations. Probabilistic sensitivity analyses are presented in Tornado diagrams for the N0 population in , and N1 population in . The N0 population base-case model budget impact was particularly sensitive to the hazard ratio of distant recurrence with chemotherapy in the high-risk group (RS 26–100). This was also the case in the N1 population although to a lesser extent. The parameter having the most substantial impact on results in the N1 population is the probability of being allocated chemotherapy based on clinical–pathologic risk alone.
Figure 3. Tornado diagram representing the 10 parameters that had the largest impact on the model results for the N0 population.
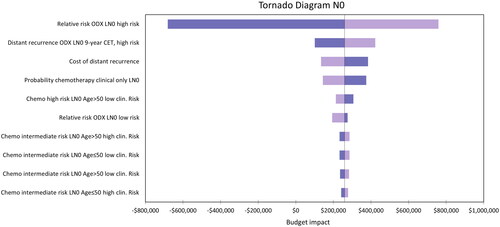
Figure 4. Tornado diagram representing the 10 parameters that had the largest impact on the model results for the N1 population.
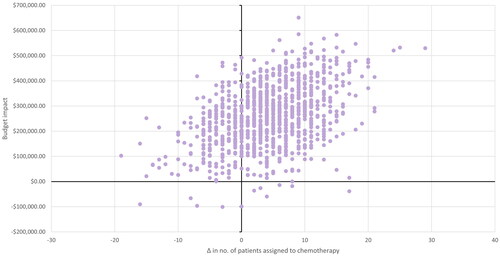
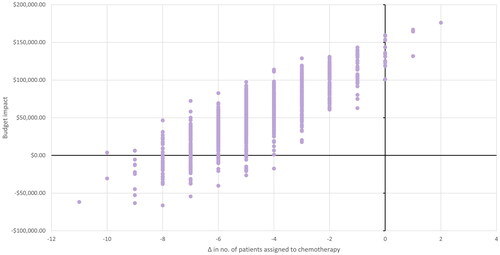
Results from the probabilistic sensitivity analysis are presented in terms of total costs, treatment costs, and number of patients undergoing chemotherapy or experiencing distant recurrence. The analysis results for the N0 population are shown in and , and for the N1 population in and . In the N0 population, an additional four patients are allocated to chemotherapy with Oncotype DX testing, with three being spared from distant recurrence. In the N1 population, five patients are spared from chemotherapy with Oncotype DX testing, although a similar number of patients have distant recurrence in both scenarios. The differences from total costs and treatment costs are broadly aligned with the deterministic analysis. Total budget impact ranged from $29,459 to $491,749 for N0, and from −$22,174 to $128,173 for N1.c
Table 10. Probabilistic sensitivity analysis results N0 combined population.
Table 11. Probabilistic sensitivity analysis results N1 combined population.
Scenario analyses
Scenario analysis results are presented in . The scenario that had the largest impact in both groups was changing the payer perspective solely to Medicare. This was largely due to a higher average age in this population, meaning a higher incidence of breast cancer and a larger number of patients tested with the Oncotype DX test.
The scenario where the hazard ratio of chemotherapy benefit in the high-risk (RS > 25) N0 population was varied to a lower limit of 0.12 resulted in the largest decrease in net budget impact. Conversely, when this value was varied to an upper limit of 0.62, this resulted in the second-largest increase in net budget impact (after the Medicare scenario described above) out of all the scenarios tested. This is the same parameter that had the largest impact on the model in the sensitivity analysis (). The results in the N1 subgroup were sensitive to the probability of treatment with chemotherapy after patients were assessed using clinical–pathologic risk alone. Decreasing this probability to 25% increased the net budget impact to $56,642, and increasing it to 45% resulted in net cost savings (−$49,090).
Table 12. Scenario analysis for N0 and N1 combined populations.
Discussion
Interpretation of findings
The analysis demonstrated that use of the Oncotype DX test when compared with clinical–pathological risk alone to guide adjuvant chemotherapy decisions increases the budget impact in the N0 and N1 populations over the first 5 years in the model. Over a 5-year horizon positive (cost-increasing) budget impact was shown in both populations. However, the initial year where patients are tested had a positive budget impact, was followed by a negative (cost-saving) budget impact for the subsequent 4 years as patients become less costly to treat. Based on the PMPM, the overall budget impact of inclusion of the Oncotype DX test is relatively budget-neutral. However, there is potential for large future healthcare system savings beyond the 5-year time horizon as patients are more appropriately allocated to endocrine or chemotherapy treatment pathways, thereby avoiding the need for costly treatment of distant recurrence through less under-treatment of patients with RS > 25 who would benefit from chemotherapy, and avoiding the risk of chemotherapy-induced AML for patients with RS ≤ 25 who may otherwise be over-treated with chemotherapyCitation43,Citation44. It has also been shown that payer perspective has a large impact on costs. When evaluated from a Medicare payer perspective, costs increased significantly, driven by increased breast cancer prevalence due to a higher average patient age. While a short time horizon may be of interest to payers, future studies should consider a longer time horizon to fully elucidate the value of the Oncotype DX test as demonstrated by studies in the literature. Estimated use of chemotherapy in the overall N0 patient group increased, while there was a reduction in the number of patients given chemotherapy in the N1 population. A substantial reduction in the cost of breast cancer recurrence (both local and distant) was shown in both patient groups. Budget-impact results differed across patient subgroups for N0, reflecting an increase in chemotherapy use for patients with low clinical risk (as defined in TAILORx) and chemotherapy sparing for women classified as having high clinical risk. The only subgroup to report an increase to local and distant recurrence costs was the N1 premenopausal group, but this was subject to significant uncertainty in the underlying data informing the probability of chemotherapy assignment, as described in the section below. Both N1 premenopausal and N1 postmenopausal reported a decrease in early cancer chemo-endocrine therapy cost.
Model uncertainty
The hazard ratio of distant recurrence with chemotherapy in the RS > 25 group in the N0 population, and the probability of being allocated to chemotherapy based on clinical–pathologic risk alone in the N1 population, had the largest impact on the model results. The probabilistic results did not vary substantially from the deterministic results. The analysis results were particularly sensitive to parameters informing chemotherapy use with and without the Oncotype DX test based on clinical expert opinion, and to parameters used to estimate rates of distant recurrence.
The use of tests and treatment decisions for premenopausal N1 patients varies across the US, possibly due to uncertainty in evidence from RXPONDERCitation11, which was used to inform this study. The evidence from RXPONDERCitation11 was published recently, and clinical practice may still be changing in response to these new findings. This uncertainty is reflected in NCCN guidelinesCitation7, which recommend chemotherapy to be considered in this subgroup. This raises uncertainty around the relevance of examining an N1 combined population (including premenopausal and postmenopausal subgroups), considering differences in clinical practice and chemotherapy benefit for the two groups. Disaggregated N1 premenopausal and postmenopausal subgroups were reported separately in the Online supplement to fully explore these differences.
Study limitations
Where possible, the model used clinical parameters obtained from studies conducted in the US. In the absence of US data (or data from multinational randomized controlled trials), the model used inputs from other countries. In particular, this relates to estimates derived from the most recent appraisal of tumor profiling tests in early breast cancer in the UKCitation24 and the TransATAC studyCitation37. The NICE appraisal and economic evaluation is an authoritative and highly detailed source, albeit the use of input values from a UK source may reduce the representativeness of the current model results to US clinical practice.
Chemotherapy allocation in the model was informed using clinical expert opinion, in the absence of appropriate published studies that captured recent changes in clinical practice in response to evidence emerging from the TAILORx and RxPONDER studies. The uncertainty linked to these inputs was characterized using both one-way and probabilistic sensitivity analyses.
Recent studies have examined differences in outcomes according to race and ethnicity in the TAILORx trialCitation45 and RxPONDER trialCitation46. Both studies reported no statistically significant differences in RS distribution among race subgroups. Black patients were reported to have worse outcomes measured using invasive disease-free survival and distant recurrence, although no statistically significant differences in chemotherapy benefit. The Oncotype DX test is prognostic of breast cancer outcomes and able to predict chemotherapy benefit in patients of difference racial and ethnic backgrounds. Race and ethnicity could be considered in future economic evaluations but could be complicated by the multifactorial relationship between race, ethnicity, and social determinants of health with patient treatment and outcomes.
Study strengths
To the best of the authors’ knowledge, this is the first US-based budget-impact analysis of the Oncotype DX test in breast cancer that included the costs of distant recurrence and long-term AEs as consequences of adjuvant chemotherapy decisions. This allowed the current study to estimate the full impact of Oncotype DX test adjuvant chemotherapy decisions on healthcare costs in the US over a 5-year period. A key limitation of previous economic evaluations of the 21-gene assay identified in the Wang et al. systematic reviewCitation27 was the failure to include clinical–pathological factors, and other information that could influence chemotherapy decisions, such as patient age. This study addresses this limitation by including a subgroup analysis for N0 patients that incorporates both age and clinical risk, which were demonstrated to be important factors influencing chemotherapy allocation and outcomes. For the N1 population, the model considers menopausal status as a risk factor in line with data reported in the RxPONDER study. The present analysis incorporates chemotherapy allocation estimates reflecting clinical practice in the US and distant recurrence outcomes informed using the latest evidence from clinical studies, which addresses other limitations identified in the Wang et al. review.
Comparison with published evidence
The impact of using the Oncotype DX test to guide adjuvant chemotherapy decisions in HR+/HER2– early-stage breast cancer on local and national healthcare budgets has been assessed in several countries, although the authors did not identify any studies conducted from a US healthcare payer perspective. It was therefore crucial from a policy standpoint to produce an analysis from a US healthcare payer perspective, to quantify the impact of using the Oncotype DX test to inform treatment decisions.
Lux et al. estimated the budget impact of the Oncotype DX test and three other MGAs (MammaPrint, EndoPredict and Prosigna) from a German healthcare payer and societal perspectiveCitation12. The study compared the net budget impact of each test against using clinical–pathological risk assessment alone. The Oncotype DX test was found to be the only test associated with cost savings from a healthcare payer perspective over a 1-year time horizon. The other three tests were associated with increased costs from both a healthcare payer and societal perspective.
The budget impact of the Oncotype DX test for a cohort of 146 women with node-negative early-stage breast cancer in the UK concluded that the use of the test resulted in a net increase of £220,326 to the healthcare payerCitation47. Conversely, another study of 201 N0 and N1 patients in the UK estimated a net cost saving to the National Health Service for both patient subgroupsCitation48. However, both UK-based studies only considered the direct cost of the assay and adjuvant chemotherapy, and did not consider other cost consequences of chemotherapy decisions (treatment of local and distant recurrence, short-term and long-term chemotherapy AEs).
Hornberger et al. conducted a real-world analysis of 952 women with early-stage breast cancer and found an average saving of $1,160 per patient tested with the Oncotype DX test compared with clinical–pathologic risk alone, when data were examined over a lifetime horizon of 40 yearsCitation49.
Hochheiser et al. performed a meta-analysis of 12,202 N0 or N1 women using data from public databases and found that relative to no testing, Prosigna and the Oncotype DX test resulted in lower use of chemotherapy, while EPClin and MammaPrint resulted in higher chemotherapy allocation. The study also found that only testing with the Oncotype DX test resulted in no risk of increasing the number of distant recurrencesCitation50. These results support this analysis, which found that the Oncotype DX test resulted in chemotherapy-related savings from lower use of chemotherapy), and a lower cost related to lower incidence of distant recurrence.
Dinan et al. examined 30,058 patients aged between 66 and 75 years diagnosed with and without use of the Oncotype DX test. It was found that 1 year after diagnosis, testing with the Oncotype DX test was associated with lower overall and chemotherapy-related costs for patients with high-risk diseaseCitation51. This is consistent with the findings in this study, as cost savings were demonstrated in the N0 Age > 50 high-clinical-risk subgroup.
Recommendations for future research and practice
The analysis presented in this article showed that the use of the Oncotype DX test to guide chemotherapy decisions was associated with direct healthcare cost savings. This was both through chemotherapy sparing for patients who are unlikely to benefit from chemotherapy based on the most recent evidence (from prospective randomized phase III trials) and through reduction in under-treatment of patients with high RS results, which reduced the need for costly treatments for distant recurrence. The impact on the latter was conservative, given the short horizon of the analysis. When deciding on the use of the Oncotype DX test, decision-makers therefore need to consider the future healthcare cost savings from reduced under-treatment, as well as the direct effect of chemotherapy sparing on budgets.
The cost estimates produced in this model were sensitive to assumptions for the change in chemotherapy allocation resulting from using the Oncotype DX test, which were obtained from clinical expert opinion. A decision-impact study looking at pre- and post-Oncotype DX test decisions in a cohort of early-stage breast cancer patients across the relevant nodal and menopausal subgroups is needed to fully validate these assumptions.
Future studies could also look at the aggregate cost impact on the patient population in terms of out-of-pocket treatment costs and impact on productivity. Inclusion of additional stratification factors that can be used in the assessment of clinical–pathological riskCitation52,Citation53 could also be considered in future work.
Transparency
Declaration of financial/other relationships
VB: employee of Putnam PHMR, who have received funding from Exact Sciences, the manufacturer of the Oncotype DX test.
EL: employee of Putnam PHMR, who have received funding from Exact Sciences. Gebra Cuyun Carter: employee and stockholder of Exact Sciences.
RL has no conflicts of interest to declare.
CR: employee and stockholder of Exact Sciences.
SC: employee and stockholder of Exact Sciences.
YA: served as paid consultant for Exact Sciences.
JF: served as paid consultant for Exact Sciences.
Reviewer disclosures
Peer reviewers on this manuscript have received an honorarium from JME for their review work but have no other relevant financial relationships to disclose.
Supplemental Material
Download MS Word (77.4 KB)Acknowledgements
None stated
Additional information
Funding
References
- Cancer.net. Breast Cancer: statistics [Internet]. 2022. https://www.cancer.net/cancer-types/breast-cancer/statistics#:∼:text=Sixty-five
- Redig AJ, Mcallister SS. Breast cancer as a systemic disease: a view of metastasis. J Intern Med. 2013;274(2):113–126. doi:10.1111/joim.12084.
- Blows FM, Driver KE, Schmidt MK, et al. Subtyping of breast cancer by immunohistochemistry to investigate a relationship between subtype and short and long term survival: a collaborative analysis of data for 10,159 cases from 12 studies. PloS Med. 2010;7(5):e1000279. doi:10.1371/journal.pmed.1000279.
- Howlader N, Cronin KA, Kurian AW, et al. Differences in breast cancer survival by molecular subtypes in the United States. Cancer Epidemiol Biomarkers Prev. 2018;27(6):619–626. doi:10.1158/1055-9965.EPI-17-0627.
- Biganzoli L, Wildiers H, Oakman C, et al. Management of elderly patients with breast cancer: updated recommendations of the international society of geriatric oncology (SIOG) and european society of breast cancer specialists (EUSOMA) [internet]. Lancet Oncol. 2012;13:e148–60. doi:10.1016/S1470-2045(11)70383-7.
- National Institute for Health and Care Excellence. Early and locally advanced breast cancer: diagnosis and management. 2018.
- National Comprehensive Cancer Network. NCCN Guidelines: breast Cancer, version 4.2022. 2022.
- National Institutes of Health Consensus Development Panel. National institutes of health consensus development conference statement: adjuvant therapy for breast cancer, november 1–3, 2000. JNCI J Natl Cancer Inst. 2001;93(13):979–989.
- Ellis P, Barrett-Lee P, Johnson L, et al. Sequential docetaxel as adjuvant chemotherapy for early breast cancer (TACT): an open-label, phase III, randomised controlled trial. Lancet. 2009;373(9676):1681–1692. doi:10.1016/S0140-6736(09)60740-6.
- Sparano JA, Gray RJ, Makower DF, et al. Adjuvant chemotherapy guided by a 21-Gene expression assay in breast cancer. N Engl J Med. 2018;379(2):111–121. doi:10.1056/NEJMoa1804710.
- Kalinsky K, Barlow WE, Gralow JR, et al. 21-Gene assay to inform chemotherapy benefit in Node-Positive breast cancer. N Engl J Med. 2021;385(25):2336–2347.
- Lux MP, Nabieva N, Hildebrandt T, et al. Budget impact analysis of gene expression tests to aid therapy decisions for breast cancer patients in Germany. Breast. 2018;37:89–98. doi:10.1016/j.breast.2017.11.002.
- Retèl V, Joore M, van ‘t Veer L, et al. Abstract P4-12-01: mammaPrint is cost-effective compared to clinical risk assessment in early stage breast cancer. Cancer Res [Internet]. 2018;78(4_Supplement):P4-12-01–P4-12-01. doi:10.1158/1538-7445.SABCS17-P4-12-01.
- US Census Bureau. Current Population Survey, 2019 and 2021 Annual Social and Economic Supplement. 2020. https://www.census.gov/programs-surveys/cps/techdocs/cpsmar21.pdf
- U.S. Census Bureau. Population by age and sex [Internet]. 2019 https://data.census.gov/cedsci/
- National Cancer Institute. Female Breast Cancer—Cancer Stat Facts [Internet]. 2022. https://seer.cancer.gov/statfacts/html/breast.html
- DeSantis CE, Ma J, Gaudet MM, et al. Breast cancer statistics, 2019. CA Cancer J Clin. 2019 Nov 1;69(6):438–451. doi:10.3322/caac.21583.
- U.S. Census Bureau. American Community Survey 5-Year Estimates Subject Tables [Internet]. 2022. https://data.census.gov/cedsci/.
- National Cancer Institute. Cancer Stat Facts: female Breast Cancer [Internet]. 2022. https://seer.cancer.gov/statfacts/html/breast.html
- Nelson DR, Brown J, Morikawa A, et al. Breast cancer-specific mortality in early breast cancer as defined by high-risk clinical and pathologic characteristics. PloS One. 2022;17(2):e0264637. doi:10.1371/journal.pone.0264637.
- Sparano JA, Gray RJ, Ravdin PM, et al. Clinical and genomic risk to guide the use of adjuvant therapy for breast cancer. N Engl J Med. 2019;380(25):2395–2405. doi:10.1056/NEJMoa1904819.
- Roberts MC, Miller DP, Shak S, et al. Breast cancer-specific survival in patients with lymph node-positive hormone receptor-positive invasive breast cancer and oncotype DX recurrence score results in the SEER database. Breast Cancer Res Treat. 2017;163(2):303–310. doi:10.1007/s10549-017-4162-3.
- Geyer CE, Tang G, Mamounas EP, et al. 21-Gene assay as predictor of chemotherapy benefit in HER2-negative breast cancer. NPJ Breast Cancer. 2018;4(1):37. doi:10.1038/s41523-018-0090-6.
- National Institute for Health and Care Excellence. Tumour profiling tests to guide adjuvant chemotherapy decisions in early breast cancer. 2018.
- Albain KS, Barlow WE, Shak S, et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol. 2010;11(1):55–65. doi:10.1016/S1470-2045(09)70314-6.
- Kalinsky K. Predicting chemotherapy benefit in Node-Positive breast cancer with the 21-gene test: the oncotype DX breast recurrence score® test. In San Antonio Breast Cancer Conference, San Antonio, TA; 2021.
- Wang SY, Chen T, Dang W, et al. Incorporating tumor characteristics to maximize 21-Gene assay utility: a Cost-Effectiveness analysis. J Natl Compr Canc Netw. 2019;17(1):39–46. doi:10.6004/jnccn.2018.7077.
- Petrelli F, Borgonovo K, Cabiddu M, et al. Mortality, leukemic risk, and cardiovascular toxicity of adjuvant anthracycline and taxane chemotherapy in breast cancer: a meta-analysis. Breast Cancer Res Treat. 2012;135(2):335–346. doi:10.1007/s10549-012-2121-6.
- National Institute for Health and Care Excellence. Liposomal cytarabine–daunorubicin for untreated acute myeloid leukaemia. 2018.
- Kunst NR, Alarid-Escudero F, Paltiel AD, et al. A value of information analysis of research on the 21-Gene Assay for Breast Cancer Management. Value Health. 2019;22(1):1102–1110. doi:10.1016/j.jval.2019.05.004.
- Kurosky SK, Mitra D, Zanotti G, et al. Treatment patterns and outcomes of patients with metastatic ER+/HER-2- breast cancer: a multicountry retrospective medical record review. Clin Breast Cancer. 2018;18(4):e529–e538. doi:10.1016/j.clbc.2017.10.008
- National Institute for Health and Care Excellence. Abemaciclib with an aromatase inhibitor for previously untreated, hormone receptor-positive, HER2-negative, locally advanced or metastatic breast cancer. 2019.
- Johnston S, Martin M, Di Leo A, et al. MONARCH 3 final PFS: a randomized study of abemaciclib as initial therapy for advanced breast cancer. NPJ Breast Cancer. 2019;5:5. doi:10.1038/s41523-018-0097-z.
- Choi IS, Jung J, Kim BH, et al. The 21-Gene recurrence score assay and prediction of chemotherapy benefit: a propensity Score-Matched analysis of the SEER database. Cancers (Basel) [Internet]. 2020 Jul 8;12(7):1829. doi:10.3390/cancers12071829.
- Petkov VI, Miller DP, Howlader N, et al. Breast-cancer-specific mortality in patients treated based on the 21-gene assay: a SEER population-based study. NPJ Breast Cancer. 2016;2(1):16017. doi:10.1038/npjbcancer.2016.17.
- Noone AM, Lund JL, Mariotto A, et al. Comparison of SEER treatment data with medicare claims. Med Care. 2016;54(9):e55–64–e64.
- Sestak I, Buus R, Cuzick J, et al. Comparison of the performance of 6 prognostic signatures for estrogen receptor–positive breast cancer a secondary analysis of a randomized clinical trial. JAMA Oncol. 2018;4(4):545–553. doi:10.1001/jamaoncol.2017.5524.
- Arias E, Xu J. United States life tables. Natl Vital Stat Rep. 2020;69(12):1–45.
- OECD. Consumer price indices (CPIs) - Complete database [Internet]. 2021. https://stats.oecd.org/index.aspx?DataSetCode=PRICES_CPI.
- ICER. A Guide to ICER’s Methods for Health Technology Assessment. 2020;
- Henry J. Kaiser family foundation. Medicaid-to-Medicare Fee Index [Internet]. 2021. https://www.kff.org/medicaid/state-indicator/medicaid-to-medicare-fee-index/?currentTimeframe=0&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D
- MedPAC. Medicare Payment Advisory Commission (MedPAC). Report to the Congress: medicare payment policy. 2019.
- Berdunov V, Millen S, Paramore A, et al. Cost-effectiveness analysis of the oncotype DX breast recurrence score test in node-positive early breast cancer. J Med Econ. 2022;25(1):591–604. doi:10.1080/13696998.2022.2066399.
- Berdunov V, Carter GC, Laws E, et al. HSR22-129: the cost of treatment of HR+/HER2– Early breast cancer and implications for the Cost-Effectiveness of multigene assays in the US. J Natl Compr Cancer Netw. 2022;20(3.5):HSR22-129. doi:10.6004/jnccn.2021.7241.
- Albain KS, Gray RJ, Makower DF, et al. Race, ethnicity, and clinical outcomes in hormone Receptor-Positive, HER2-Negative, Node-Negative breast cancer in the randomized TAILORx trial. J Natl Cancer Inst. 2021;113(4):390–399. doi:10.1093/jnci/djaa148.
- Abdou Y, Barlow WE, Gralow JR, et al. Abstract GS1-01: race and clinical outcomes in the RxPONDER trial (SWOG S1007). Cancer Res. 2023;83(5_Supplement):GS1-01–GS1-01. doi:10.1158/1538-7445.SABCS22-GS1-01.
- Holt S, Bertelli G, Humphreys I, et al. A decision impact, decision conflict and economic assessment of routine oncotype DX testing of 146 women with node-negative or pNImi, ER-positive breast cancer in the U.K. Br J Cancer. 2013;108(11):2250–2258. doi:10.1038/bjc.2013.207.
- Loncaster J, Armstrong A, Howell S, et al. Impact of oncotype DX breast recurrence score testing on adjuvant chemotherapy use in early breast cancer: real world experience in greater Manchester, UK. Eur J Surg Oncol. 2017;43(5):931–937. doi:10.1016/j.ejso.2016.12.010.
- Hornberger J, Chien R, Krebs K, et al. US insurance program’s experience with a multigene assay for early-stage breast cancer. J Oncol Pract. 2011;7(3 SUPPL):e38s–e45s.
- Hochheiser L, Hornberger J, Turner M, et al. Multi-gene assays: effect on chemotherapy use, toxicity and cost in estrogen receptor-positive early stage breast cancer. J Comp Eff Res. 2019;8(5):289–304. doi:10.2217/cer-2018-0137.
- Dinan MA, Wilson LE, Reed SD. Chemotherapy costs and 21-Gene recurrence score genomic testing among medicare beneficiaries with Early-Stage breast cancer, 2005 to 2011. J Natl Compr Canc Netw. 2019;17(3):245–254. doi:10.6004/jnccn.2018.7097.
- Bhargava R, Esposito NN, O′Connor SM, et al. Magee EquationsTM and response to neoadjuvant chemotherapy in ER+/HER2-negative breast cancer: a multi-institutional study. Mod Pathol. 2021;34(1):77–84. doi:10.1038/s41379-020-0620-2.
- Sparano JA, Crager MR, Tang G, et al. Development and validation of a tool integrating the 21-Gene recurrence score and Clinical-Pathological features to individualize prognosis and prediction of chemotherapy benefit in early breast cancer. J Clin Oncol. 2021;39(6):557–564. doi:10.1200/JCO.20.03007.