Abstract
Background: Commonly used methods of comparison (e.g. network meta-analyses) require common comparator(s) across trials, such as placebo in placebo-controlled trials. Recent literature indicates that route of administration differences across placebo arms of clinical trials in pain disorders may contribute to differences in placebo effect.
Methods: We conducted a meta-regression on placebo data from pivotal clinical trials of anti-calcitonin gene-related peptide (anti-CGRP) monoclonal antibodies for migraine prevention to quantify the impact of route of administration, migraine type (episodic/chronic), and number of prior treatment failures on placebo reduction in monthly migraine days (MMDs) across weeks 1–12 of treatment. A systematic literature review of Embase, MEDLINE, the Cochrane Library, and grey literature conducted in June 2021 identified 14 relevant, randomized placebo-controlled trials for analysis.
Results: After testing models with different covariates, a meta-regression was fitted to the extracted placebo data with the covariates of route of administration, migraine type, and proportion of patients with ≥2 prior preventive treatment failures. An intravenous route of administration for the placebo arm was a predictor for higher MMD reduction. Predictors of lower MMD reduction were migraine type (episodic migraine) and a higher proportion of patients having ≥2 failed preventive treatments.
Conclusions: The efficacy of intravenous anti-CGRP monoclonal antibodies are likely underestimated, and differences in the route of administration of placebo may necessitate use of alternative methods that do not assume the presence of a common comparator when comparing anti-CGRP monoclonal antibodies in migraine prevention. Further research into the contextual effects of the placebo effect is warranted.
Background
Migraine, a common disorder affecting more than 1 billion people around the globe, places a substantial humanistic and socio-economic burden on affected individuals, their families, and societyCitation1,Citation2. Migraine frequency is a predictor of lost productivity in the workplace and householdCitation3. For many years, the only available pharmacologic management included analgesics or nonsteroidal anti-inflammatory drugs for mild attacks, and triptans for more severe attacksCitation4. More recently, the development of drugs that are able to prevent migraine by targeting the trigeminal sensory neuropeptide calcitonin gene-related peptide (CGRP) or its receptor have caused a seismic shift in migraine managementCitation5.
To date, four anti-CGRP monoclonal antibodies have been approved in the US and elsewhere for migraine prevention (erenumab, fremanezumab, galcanezumab, and eptinezumab), along with two small-molecule inhibitors (gepants)Citation6. However, there are currently no head-to-head trials of the anti-CGRP monoclonal antibodies, resulting in a proliferation of indirect treatment comparisons (ITCs), such as network meta-analyses, to assess relative treatment effectivenessCitation7–11. At first glance, ITCs would appear to be straightforward, since all the anti-CGRP pivotal trials used the reduction in monthly migraine days (MMDs) as the primary endpoint and all were placebo-controlled. However, there is a great deal of evidence to suggest that simple subtraction of placebo response from active treatment response may result in highly inaccurate estimates of therapeutic efficacyCitation12. There are several contextual aspects to placebo administration that can greatly impact patient responses to medication in clinical trials of migraine, including patient expectationCitation13–15, route of administrationCitation16,Citation17, and physician demeanorCitation18.
While the use of randomized, controlled trials aims to overcome bias related to perceptions of drug efficacy, by blinding the investigators and participants to the treatment received, the issue of route of administration is less easily addressed. This is of particular interest when comparing the anti-CGRPs, since three of the available products are administered subcutaneously (SC) and one (eptinezumab) is administered intravenously (IV). A key assumption is that the common comparator (i.e. placebo) is essentially congruent across trials. However, the results of a recent meta-analysis of treatment administration for chronic migraine clearly illustrated that SC and IV placebo produce different responsesCitation17 and therefore cannot be assumed to be directly interchangeable across studies. As a result, the ‘true’ effect of a treatment, as measured by its efficacy relative to placebo response, might be underestimated in clinical trials if the placebo contextual effect (such as IV administration) is strong.
The objective of this analysis is to understand whether, and to what extent, the route of administration should be considered when conducting an ITC between anti-CGRP monoclonal antibodies for the preventive treatment of migraine by assessing how the placebo effect varied in trials using SC or IV administration. Given that the pivotal anti-CGRP studies also varied in terms of migraine type (episodic vs chronic) and number of prior treatment failures, we also conducted meta-regressions to control for these factors.
Methods
Data sources
Data for this analysis were extracted from a systematic literature review (see and Methods, Supplementary File 1). In brief, electronic databases including Embase, MEDLINE, and the Cochrane Library were searched for randomized, controlled trials of migraine-preventive treatments from inception to June 2021. Additional grey literature searching encompassed 2020–2021 headache/migraine congresses, the ClinicalTrials.gov website, and several Health Technology Assessment websites. Only clinical studies in humans, published in English language, were selected. While the literature review was not fully comprehensive, it was judged to be sufficiently complete for the purposes of informing the current analysis. In addition, data on file from the DELIVER trial (which was not yet published at the time of conducting the analysis) was used to supplement the data identified by the SLR.
Figure 1. PRISMA diagram for the literature review.
*Number of studies included from the original SLR does not align with the original SLR report because pooled studies excluded in the original SLR were re-reviewed, and those reporting novel data in prior treatment failure subgroups have now been included in the SLR.
PRISMA, Preferred reporting items for systematic reviews and meta-analyses; TLR, Targeted literature review
We included data from published, placebo-controlled trials for anti-CGRPs conducted in patients with either episodic or chronic migraine. Where available, phase 3 or phase 4 randomized, controlled trials with a double-blind period of at least 12 weeks’ duration were identified. If such studies were unavailable for any anti-CGRP, data from phase 2 randomized, controlled studies were used instead. For this analysis, only data from the placebo arm of each included trial were evaluated. For a few phase 3, anti-CGRP therapeutic trials (ARISE, PROMISE-1, and PROMISE-2), the percentage of patients who had failed ≥2 prior preventive migraine treatments could not be identified in the literature; thus assumptions were made. The phase 3 ARISE study of erenumab 70 mg vs placebo in patients with episodic migraine reported only the percentage of patients who had failed 1 or more prior treatment (n = 115/291, 39.5%)Citation19; however, as this was comparable with that reported in another phase 3 study of erenumab in episodic migraine (STRIVE; n = 127/391, 39.8%), it was assumed that the percentage of patients who had failed ≥2 treatments in ARISE was the same as the percentage in STRIVE. For the phase 3 studies of eptinezumab (PROMISE-1 in episodic migraineCitation20; PROMISE-2 in chronic migraineCitation21) the percentages of patients with ≥2 treatment failures were not captured in the case report form; thus, it was assumed that there were no patients with ≥2 treatment failures in either study. The potential implications of this assumption are presented in the Discussion section.
Meta-regression
A meta-regression was conducted for placebo response, with the percentage of patients with ≥2 treatment failures as a covariate for both episodic and chronic migraine for SC trials. This step was intended to assess whether the percentage of patients with ≥2 treatment failures was significant and whether the coefficient estimate was similar between two types of migraine across several SC trials. A similar assessment was not feasible for IV trials, as there was only one for episodic migraine and one for chronic migraine. Consequently, we conducted a meta-regression including placebo data from all the anti-CGRP (SC and IV) clinical trials identified in the systematic literature review to understand the impact on placebo MMD response of the following variables: type of migraine (episodic or chronic), percentage of patients with ≥2 prior treatment failures in each trial, and the route of administration for treatment (i.e. SC or IV). To assess whether the route of administration impacted MMD reduction in the placebo arms differently in episodic or chronic migraine, an interaction term between the route of administration and type of migraine was introduced into the meta-regression. Finally, we assessed whether the IV effect differed between episodic and chronic migraine.
Sensitivity analyses to increase number of data points
Some of the trials identified in the literature review only included patients who had failed ≥2 prior therapies before enrollment. Several other trials included both treatment-naïve and treatment-experienced patients. Some trial sponsors published subgroup analyses specific to those patients who had failed ≥2 prior treatments. Therefore, we conducted sensitivity analyses in which these subgroup data were included alongside the overall phase 3 study population. In other words, each trial could contribute to two data points in the sensitivity analyses. Having two data points per trial has critical statistical limitations, due to double-counting of some patients (namely, patients who had ≥2 prior treatment failures); however, this approach was used to assess the robustness of the results by increasing the number of data points in the analysis. To address this methodological limitation, the mean and standard error (SE) for patients with 0 or 1 failed prior therapies were computed by the authors (see Methods, Supplementary File 1). The meta-regression was then rerun and compared with the aforementioned sensitivity analysis (i.e. with potentially two data points per trial: placebo effect for patients with 0 or 1 prior failures, and placebo effect for patients with ≥2 prior failures).
Statistical analyses
The endpoint used for the analyses was the reduction in MMDs. We used the published data for each clinical study to extract or calculate the change from baseline in MMDs over weeks 1–12. If the average change from baseline from weeks 1–12 was not reported in the publication, the mean changes from baseline to weeks 4, 8, and 12 were calculated, and the SE of the change from baseline to week 12 was used, since SE values were comparable across all timepoints.
For the random-effects meta-regressions, we used the rma function from the ‘metafor’ package in RCitation22,Citation23. A DerSimonian-Laird estimator was usedCitation24. To estimate the impact of route of administration on placebo response, the route of administration was included as a dummy variable, in which the value was set as 1 if placebo administration was IV (namely for PROMISE-1, PROMISE-2, and DELIVER) and as 0 if placebo administration was via a different route of administration (i.e. SC), as shown in . Coefficient estimates, SE, and 95% confidence intervals (CI) were reported. For P-values, a threshold of 0.05 was used to determine if changes in the predictor were significantly associated with changes in the response. Importantly, given that the number of trials utilizing IV administration was small, P-values were interpreted with caution.
Table 1. Placebo response in clinical trials of anti-CGRPs.
Results
Clinical studies
A total of 18 articles detailing 14 studies were identified from the systematic literature review for inclusion in the meta-regression. Of these, 7 studies reported data from patients with episodic migraine onlyCitation19,Citation20,Citation25–29, 4 from patients with chronic migraine onlyCitation21,Citation30–32, and 3 from populations including both episodic and chronic migraineCitation33–35. An additional 4 articles reported specific subgroup data for patients with ≥2 prior treatment failuresCitation36–39. Details of the studies, including patient numbers, administration method, and mean (SE) change from baseline in MMDs in weeks 1–12 are shown in . As expected, only the eptinezumab studies (PROMISE-1Citation20, PROMISE-2Citation21, and DELIVERCitation35) were associated with IV administration.
Effect of SC placebo on MMDs according to type of migraine and prior treatment failures
In episodic migraine, the meta-regression showed a relationship (p < 0.001) between the percentage of patients with ≥2 treatment failures and the reduction in MMDs from baseline with placebo: placebo-treated patients who failed 2 or more therapies had a lower MMD reduction (1.78 MMDs), compared with patients who did not previously fail any therapies and who had almost no placebo response (-0.26 MMDs) ( and ).
Figure 2. Relationship between MMDs reduction and subcutaneous treatment failures for (A) episodic migraine and (B) chronic migraine.
MMDs, Monthly migraine days.
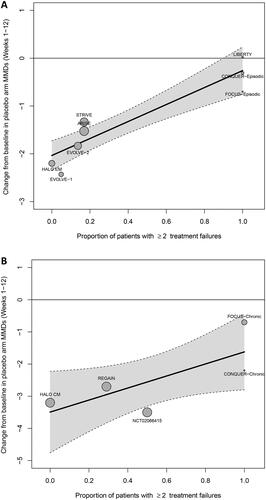
Table 2. Relationship between MMDs reduction and subcutaneous treatment failures (3592 total patients, EM and CM trials).
Furthermore, there was a relationship between the percentage of patients with ≥2 treatment failures and the reduction in MMDs from baseline with placebo in chronic migraine, although there was a greater level of uncertainty (wider CIs and p = 0.057; and ). This may reflect the smaller number of trials and data points available for chronic migraine compared with episodic migraine. However, the coefficient estimates of the impact of the proportion of patients with ≥2 treatment failures were comparable between chronic migraine (1.88) and episodic migraine (1.77).
In the sensitivity analyses, which included the data from the publications of phase 3 trials reporting subgroup analyses of patients with ≥2 treatment failures, the point estimate for episodic migraine was unaffected (see , Supplementary 1) and the CIs were narrowed (see , Supplementary 1). Adding the subgroup data for chronic migraine slightly modified the coefficient estimate of prior treatment failure (from 1.87 to 1.81), which became closer to that of episodic migraine, and the P-value was less than 0.05; however, the 95% CI of the impact of prior treatment failure remained wider in chronic migraine than in episodic migraine.
Incremental IV placebo effect
The results of the meta-regression of the placebo effect with route of administration, type of migraine, and percentage of patients with ≥2 treatment failures are shown in . The MMD reduction with IV placebo was 1.34 days lower (95% CI −1.86, −0.81; p < 0.001) than with SC placebo. When the interaction term between administration and migraine type was added, the P-value just missed the 5% threshold (p = 0.071). Directionally, the data indicate that an IV route of administration generated a higher incremental placebo response in chronic migraine (1.96 MMDs) than in episodic migraine (1.01 MMDs). The IV effect was numerically lower in patients with ≥2 prior treatment failures (0.47 MMDs) than in those with <2 prior treatment failures, but the P-value was 0.44. After splitting the trial results into <2 and ≥2 treatment failures, the IV effect remained similar (1.37 MMDs; p < 0.001) (see , Supplementary File 1 and , Supplementary File 1).
Table 3. Placebo reduction in MMDs with administration route, migraine type, and percentage of patients as covariates.
Discussion
The results of this analysis show that the placebo response in migraine clinical studies may vary according to the route of administration, as well as between the type of migraine being investigated (episodic vs chronic), and according to the patient history of prior treatment failures. Importantly, even after accounting for migraine type and clinical history, patients who received IV placebo had a greater response (-1.34 MMDs) than those who received SC placebo. Therefore, the efficacy of eptinezumab, which is administered via the IV route, may be underestimated in comparisons that assume a common placebo effect, supporting the results of another meta-analysis that evaluated several indirect treatment comparison methodologies between anti-CGRPs that varied in their assumptions of placeboCitation11. Notably, our study likely underestimates the IV placebo effect, which may be even greater, given the conservative estimates made regarding the number of patients with ≥2 prior treatment failures in PROMISE-1 and PROMISE-2 (i.e. if the true percentage of patients with ≥2 prior treatment failures was greater than 0, then the IV coefficient would increase in the meta-regression). Overall, the finding that intravenous administration was associated with larger placebo response is consistent with previous meta-analysesCitation17,Citation40.
Additional sources of placebo effect included the severity of the migraine (i.e. episodic vs chronic migraine) and the number of prior treatment failures. Our results indicate that within the IV administration setting for eptinezumab, the placebo response differs between migraine types, with a higher incremental placebo response in chronic (2.0 MMDs) than in episodic migraine (1.0 MMD) patients; although, the P-value for the interaction variable was above 0.05 (p = 0.07), possibly due to the very low number of trials with IV placebo administration or a ceiling effect due to the definition of episodic migraine. This is in alignment with another systematic review and meta-analysis which found differences of 1.5 MMDs in studies of episodic migraine and 2.23 MMDs in studies of chronic migraine, which also found that 67% of interventional benefit was due to contextual effects (including placebo effect) rather than direct effect of anti-CGRP action; however, the analysis did not account for administration (IV vs SC) Citation18. We also found that the IV effect was lessened for patients with ≥2 prior treatment failures (0.47 fewer MMDs gained vs treatment-naïve patients), but with p > 0.05. This is in alignment with previous research finding that the proportion contextual effect (i.e. ratio of mean MMD change in control group to experimental group) was lower in anti-CGRP studies in episodic migraine including patients with ≥3 prior treatment failuresCitation18. Interestingly, the relationship between percentage of treatment failure and geographic location was high (correlation between percentage of 2+ treatment failures and percentage of North American patients was −0.91 in EM). Therefore, we cannot rule out that patient continent of origin, rather than percentage of ≥2 prior treatment failures, was the driver of different placebo responses.
In the absence of head-to-head trials, ITCs are necessary to compare the treatment effect between two products for a disease or condition. ITCs are typically contingent on a common comparator between studies, which is most often placebo in clinical trials. Though placebo and treatment responses can be affected by several contextual factors (e.g. number of previous treatment failures, presence of medication-overuse headache, funding of drug used, administration personnel/location), only some are captured in clinical trials and real-world evidenceCitation41. When factors affecting placebo response are not controlled for in ITCs, the conclusions drawn may underestimate the total efficacy of some treatments and can impact regulatory and reimbursement decisions. For the current landscape of anti-CGRP monoclonal antibodies, clinical guidelines do not distinguish between the four available anti-CGRPsCitation42–44, and the analysis here suggests that factors such as mode of administration, migraine type, and prior preventive treatment failures should be taken into account. These factors may also affect cost-effectiveness comparisons which are reliant on comparative efficacy estimatesCitation45–47.
Further research is needed to definitively determine the causes underlying the greater placebo response associated with IV administration, but one explanation is that the increased healthcare contact at the point of administration may contribute to the observed higher placebo effect in IV trials. The number of clinic visits, the time taken to administer the IV infusion (30–60 min), and the extended period of post-infusion monitoring (2–4 h) in the PROMISE trialsCitation20,Citation21 all allowed the opportunity for greater interaction with clinicians and nurses, which may impact the subsequent treatment response. Prior studies of patients with other medical conditions have indicated that among individuals who prefer IV treatment administration, the reasons cited include the presence of healthcare professionals and the feeling of greater procedural safetyCitation48,Citation49, as well as a belief that IV treatment may be associated with greater efficacy than SCCitation49.
Limitations
There were several limitations associated with our analysis, based on both the available data and the methodology used for the meta-regression. The meta-regression was used to account for heterogeneity in trials due to differences in patient populations, but data on certain populations were limited. The publication of subgroup analyses of patients who had failed ≥2 therapies also complicated the analysis, and we elected to include these alongside their ‘parent’ phase 3 data in the meta-regression that included type of migraine (episodic or chronic), percentage of patients with ≥2 prior treatment failures in each trial, and the route of administration for treatment (i.e. SC or IV) as covariates. , Supplementary File 1 attempts to address this limitation but having access to the subgroup data for 0 or 1 treatment failures would have been preferable. Finally, we only included anti-CGRP monoclonal antibodies in this analysis, and it remains to be determined what impact oral, intranasal, or intramuscular routes of administration might have on placebo responses to other pharmacologic treatments, such as gepantsCitation50 or onabotulinumtoxinACitation51.
Conclusions
To conclude, in this meta-regression, we found evidence to suggest that the efficacy of eptinezumab, an IV anti-CGRP monoclonal antibody, may be underestimated in ITCs that assume a common placebo effect. The contextual placebo effects inherent in current clinical trial design require further scrutiny, and alternative comparison methods (other than standard Bayesian network meta-analysis) may need to be implemented to ensure accurate and unbiased treatment comparisons.
Transparency
Declaration of financial/other relationships
SAR owns stock or stock options in Novartis. SAR and XYL are employees of H. Lundbeck A/S, Copenhagen, Denmark.
Author contributions
SAR and XYL designed the study, reviewed the literature, analyzed the data, interpreted the results, critically revised the manuscript, and approved the final text for publication.
Availability of data and materials
All data generated or analyzed during this study are included in this published article (and its supplemental digital content).
Reviewer disclosures
A reviewer on this manuscript has disclosed that they are the principle investigator on all 4 anti-CGRP monoclonal antibody phase-3 trials. The rest of the peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Ethics approval and consent to participate
Not applicable.
Previous presentations
Some data in this manuscript were submitted as an abstract to the European Headache Federation Annual Congress (EHF 2022); Vienna, Austria: 9–11 December 2022
Supplemental Material
Download MS Word (121.3 KB)Acknowledgements
The authors thank Sally-Anne Mitchell, PhD, and Jessica Weaver, PhD, of The Medicine Group, LLC (New Hope, PA, USA) for providing medical writing support, which was funded by H. Lundbeck A/S and in accordance with Good Publication Practice guidelines.
Additional information
Funding
References
- Stovner LJ, Hagen K, Linde M, et al. The global prevalence of headache: an update, with analysis of the influences of methodological factors on prevalence estimates. J Headache Pain. 2022;23(1):34. doi: 10.1186/S10194-022-01402-2.
- Ashina M, Katsarava Z, Do TP, et al. Migraine: epidemiology and systems of care. Lancet. 2021;397(10283):1485–1495. doi: 10.1016/S0140-6736(20)32160-7.
- Husøy A, Katsarava Z, Steiner TJ. The relationship between headache-attributed disability and lost productivity: 3 attack frequency is the dominating variable. J Headache Pain. 2023;24(1):7. doi: 10.1186/s10194-023-01546-9.
- Ferrari MD, Goadsby PJ, Burstein R, et al. Migraine. Nat Rev Dis Primers. 2022;8(1):2. doi: 10.1038/s41572-021-00328-4.
- Edvinsson L, Haanes KA, Warfvinge K, et al. CGRP as the target of new migraine therapies—successful translation from bench to clinic. Nat Rev Neurol. 2018;14(6):338–350. doi: 10.1038/s41582-018-0003-1.
- Chiang CC, Schwedt TJ. Calcitonin gene-related peptide (CGRP)-targeted therapies as preventive and acute treatments for migraine—the monoclonal antibodies and gepants. Progress in Brain Research. Vol 255. Elsevier B.V.; 2020:143–170. doi: 10.1016/bs.pbr.2020.06.019.
- Masoud AT, Hasan MT, Sayed A, et al. Efficacy of calcitonin gene-related peptide (CGRP) receptor blockers in reducing the number of monthly migraine headache days (MHDs): a network meta-analysis of randomized controlled trials. J Neurol Sci. 2021;427:117505. doi: 10.1016/j.jns.2021.117505.
- Fernández-Bravo-Rodrigo J, Pascual-Morena C, Flor-García A, et al. The safety and efficacy of calcitonin gene-related peptide (CGRP) monoclonal antibodies for the preventive treatment of migraine: a protocol for multiple-treatment systematic review and meta-analysis. Int J Environ Res Public Health. 2022;19(3):1753. doi: 10.3390/ijerph19031753.
- Chen YY, Ye XQ, Tang TC, et al. Calcitonin gene-related peptide monoclonal antibodies versus botulinum neurotoxin a in the preventive treatment of chronic migraine: an adjusted indirect treatment comparison meta-analysis. Front Pharmacol. 2021;12(671845):671845. doi: 10.3389/fphar.2021.671845.
- Alasad YW, Asha MZ. Monoclonal antibodies as a preventive therapy for migraine: a meta-analysis. Clin Neurol Neurosurg. 2020;195:105900. doi: 10.1016/j.clineuro.2020.105900.
- Fawsitt CG, Thom H, Regnier SA, et al. Comparison of indirect treatment methods in migraine prevention to address differences in mode of administration. J Comp Eff Res. 2023;12(7):e230021. doi: 10.57264/cer-2023-0021.
- Lund K, Vase L, Petersen GL, et al. Randomised controlled trials may underestimate drug effects: balanced placebo trial design. PLOS One. 2014;9(1):e84104. doi: 10.1371/journal.pone.0084104.
- Kam-Hansen S, Jakubowski M, Kelley JM, et al. Altered placebo and drug labeling changes the outcome of episodic migraine attacks. Sci Transl Med. 2014;6(218):218ra5. doi: 10.1126/scitranslmed.3006175.
- Vase L, Wartolowska K. Pain, placebo, and test of treatment efficacy: a narrative review. Br J Anaesth. 2019;123(2):e254–e262. doi: 10.1016/j.bja.2019.01.040.
- Schmidt K, Berding T, Kleine-Borgmann J, et al. The beneficial effect of positive treatment expectations on pharmacological migraine prophylaxis. Pain. 2022;163(2):e319–e327. doi: 10.1097/j.pain.0000000000002341.
- Macedo A, Farré M, Baños JE. A meta-analysis of the placebo response in acute migraine and how this response may be influenced by some of the characteristics of clinical trials. Eur J Clin Pharmacol. 2006;62(3):161–172. doi: 10.1007/s00228-005-0088-5.
- Swerts DB, Benedetti F, Peres MFP. Different routes of administration in chronic migraine prevention lead to different placebo responses: a meta-analysis. Pain. 2022;163(3):415–424. doi: 10.1097/j.pain.0000000000002365.
- Forbes RB, McCarron M, Cardwell CR. Efficacy and contextual (placebo) effects of CGRP antibodies for migraine: systematic review and meta-analysis. Headache. 2020;60(8):1542–1557. doi: 10.1111/head.13907.
- Dodick DW, Ashina M, Brandes JL, et al. ARISE: a phase 3 randomized trial of erenumab for episodic migraine. Cephalalgia. 2018;38(6):1026–1037. doi: 10.1177/0333102418759786.
- Ashina M, Saper J, Cady R, et al. Eptinezumab in episodic migraine: a randomized, double-blind, placebo-controlled study (PROMISE-1). Cephalalgia. 2020;40(3):241–254. doi: 10.1177/0333102420905132.
- Lipton RB, Goadsby PJ, Smith J, et al. Efficacy and safety of eptinezumab in patients with chronic migraine: PROMISE-2. Neurology. 2020;94(13):e1365–e1377. doi: 10.1212/WNL.0000000000009169.
- Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Soft. 2010;36(3):1–48. Accessed April 13, 2022 doi: 10.18637/jss.v036.i03.
- R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing. Published 2022. Accessed April 13, 2022. https://www.r-project.org/.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2.
- Stauffer VL, Dodick DW, Zhang Q, et al. Evaluation of galcanezumab for the prevention of episodic migraine: the EVOLVE-1 randomized clinical trial. JAMA Neurol. 2018;75(9):1080–1088. doi: 10.1001/jamaneurol.2018.1212.
- Skljarevski V, Matharu M, Millen BA, et al. Efficacy and safety of galcanezumab for the prevention of episodic migraine: results of the EVOLVE-2 phase 3 randomized controlled clinical trial. Cephalalgia. 2018;38(8):1442–1454. doi: 10.1177/0333102418779543.
- Reuter U, Goadsby PJ, Lanteri-Minet M, et al. Efficacy and tolerability of erenumab in patients with episodic migraine in whom two-to-four previous preventive treatments were unsuccessful: a randomised, double-blind, placebo-controlled, phase 3b study. Lancet. 2018;392(10161):2280–2287. doi: 10.1016/s0140-6736(18)32534-0.
- Goadsby PJ, Reuter U, Hallström Y, et al. A controlled trial of erenumab for episodic migraine. N Engl J Med. 2017;377(22):2123–2132. doi: 10.1056/NEJMoa1705848.
- Dodick DW, Silberstein SD, Bigal ME, et al. Effect of fremanezumab compared with placebo for prevention of episodic migraine: a randomized clinical trial. JAMA. 2018;319(19):1999–2008. doi: 10.1001/jama.2018.4853.
- Silberstein SD, Dodick DW, Bigal ME, et al. Fremanezumab for the preventive treatment of chronic migraine. N Engl J Med. 2017;377(22):2113–2122. doi: 10.1056/NEJMoa1709038.
- Tepper S, Ashina M, Reuter U, et al. Safety and efficacy of erenumab for preventive treatment of chronic migraine: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol. 2017;16(6):425–434. doi: 10.1016/s1474-4422(17)30083-2.
- Detke HC, Goadsby PJ, Wang S, et al. Galcanezumab in chronic migraine: the randomized, double-blind, placebo-controlled REGAIN study. Neurology. 2018;91(24):e2211–e2221. doi: 10.1212/wnl.0000000000006640.
- Ferrari MD, Diener HC, Ning X, et al. Fremanezumab versus placebo for migraine prevention in patients with documented failure to up to four migraine preventive medication classes (FOCUS): a randomised, double-blind, placebo-controlled, phase 3b trial. Lancet. 2019;394(10203):1030–1040. doi: 10.1016/S0140-6736(19)31946-4.
- Mulleners WM, Kim BK, Láinez MJA, et al. Safety and efficacy of galcanezumab in patients for whom previous migraine preventive medication from two to four categories had failed (CONQUER): a multicentre, randomised, double-blind, placebo-controlled, phase 3b trial. Lancet Neurol. 2020;19(10):814–825. doi: 10.1016/S1474-4422(20)30279-9.
- Ashina M, Lanteri-Minet M, Pozo-Rosich P, et al. Safety and efficacy of eptinezumab for migraine prevention in patients with two-to-four previous preventive treatment failures (DELIVER): a multi-arm, randomised, double-blind, placebo-controlled, phase 3b trial. Lancet Neurol. 2022;21(7):597–607. doi: 10.1016/S1474-4422(22)00185-5.
- Ashina M, Tepper S, Brandes JL, et al. Efficacy and safety of erenumab (AMG334) in chronic migraine patients with prior preventive treatment failure: a subgroup analysis of a randomized, double-blind, placebo-controlled study. Cephalalgia. 2018;38(10):1611–1621. doi: 10.1177/0333102418788347.
- Ruff DD, Ford JH, Tockhorn-Heidenreich A, et al. Efficacy of galcanezumab in patients with chronic migraine and a history of preventive treatment failure. Cephalalgia. 2019;39(8):931–944. doi: 10.1177/0333102419847957.
- Ruff DD, Ford JH, Tockhorn‐Heidenreich A, et al. Efficacy of galcanezumab in patients with episodic migraine and a history of preventive treatment failure: results from two global randomized clinical trials. Eur J Neurol. 2020;27(4):609–618. doi: 10.1111/ene.14114.
- Goadsby PJ, Paemeleire K, Broessner G, et al. Efficacy and safety of erenumab (AMG334) in episodic migraine patients with prior preventive treatment failure: a subgroup analysis of a randomized, double-blind, placebo-controlled study. Cephalalgia. 2019;39(7):817–826. doi: 10.1177/0333102419835459.
- Tepper SJ, Cirillo J, Kim E, et al. The temporal trend of placebo response in migraine prevention from 1990 to 2021: a systematic literature review and meta-analysis with regression. J Headache Pain. 2023;24(1):54. doi: 10.1186/s10194-023-01587-0.
- Vandenbussche N, Pisarek K, Paemeleire K. Methodological considerations on real-world evidence studies of monoclonal antibodies against the CGRP-pathway for migraine: a systematic review. J Headache Pain. 2023;24(1):75. doi: 10.1186/s10194-023-01611-3.
- Ailani J, Burch RC, Robbins MS, . The American headache society consensus statement: update on integrating new migraine treatments into clinical practice. Headache. 2021;61(7):1021–1039. doi: 10.1111/head.14153.
- Sacco S, Amin FM, Ashina M, et al. European headache federation guideline on the use of monoclonal antibodies targeting the calcitonin gene related peptide pathway for migraine prevention – 2022 update. J Headache Pain. 2022;23(1):67. doi: 10.1186/s10194-022-01431-x.
- Mitsikostas DD, Alexoudi A, Arvaniti C, et al. Hellenic headache society recommendations for the use of monoclonal antibodies targeting the calcitonin gene-related peptide pathway for the prevention of migraine and cluster headache—2023 update. SN Compr Clin Med. 2023;5(1):118. doi: 10.1007/s42399-023-01452-w.
- Khanal S, Underwood M, Naghdi S, et al. A systematic review of economic evaluations of pharmacological treatments for adults with chronic migraine. J Headache Pain. 2022;23(1):122. doi: 10.1186/s10194-022-01492-y.
- National Institute for Health and Care Excellence. NICE health technology evaluations: the manual. process and methods [PMG36]; 2022. Accessed July 23, 2023. https://www.nice.org.uk/process/pmg36/chapter/economic-evaluation#modelling-methods.
- CADTH. Guidelines for the economic evaluation of health technologies. Ottawa (Canada): CADTH; 2017.
- Overton PM, Shalet N, Somers F, et al. Patient preferences for subcutaneous versus intravenous administration of treatment for chronic immune system disorders: a systematic review. Patient Prefer Adherence. 2021;15:811–834. doi: 10.2147/PPA.S303279.
- Santus P, Ferrando M, Baiardini I, et al. Patients beliefs on intravenous and subcutaneous routes of administration of biologics for severe asthma treatment: a cross-sectional observational survey study. World Allergy Organ J. 2019;12(4):100030. doi: 10.1016/j.waojou.2019.100030.
- Moreno-Ajona D, Villar-Martínez MD, Goadsby PJ. New generation gepants: migraine acute and preventive medications. J Clin Med. 2022;11(6):1656. doi: 10.3390/jcm11061656.
- Blumenfeld A, Silberstein SD, Dodick DW, et al. Method of injection of onabotulinumtoxina for chronic migraine: a safe, well-tolerated, and effective treatment paradigm based on the PREEMPT clinical program. Headache. 2010;50(9):1406–1418. doi: 10.1111/j.1526-4610.2010.01766.x.

