 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Aim
The POLARIX trial showed that Pola + R-CHP (polatuzumab vedotin plus rituximab, cyclophosphamide, doxorubicin, and prednisolone) prolongs progression-free survival (PFS) in patients with previously untreated diffuse large B-cell lymphoma (DLBCL) compared with R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone), the conventional standard of care, with a similar safety profile. However, Pola + R-CHP has not been evaluated from the viewpoint of health economics in Japan. This study evaluates the cost-effectiveness of Pola + R-CHP for previously untreated DLBCL from a Japanese public healthcare payer’s perspective.
Methods
A partitioned survival analysis model was constructed to estimate lifetime costs and effectiveness of Pola + R-CHP and R-CHOP in previously untreated DLBCL who had an International Prognostic Index score (IPI) score of ≥2. A parametric survival model was applied to data analyzed in the POLARIX trial to estimate the lifetime overall survival (OS) and PFS for each treatment. The parameters required for the model were based on the results of a literature search and expert opinion.
Results
The incremental cost-effectiveness ratio (ICER) of Pola + R-CHP vs. R-CHOP was JPY2,710,238 per quality-adjusted life year (QALY), less than the ICER of JPY7.5 million per QALY that is considered to be cost-effective based on the threshold of the Japanese cost-effectiveness evaluation system. One-way sensitivity analysis showed that the parameters influencing the results of the analysis were median PFS and the total cost per regimen of salvage chemotherapy, patient weight, and patient age. Probabilistic sensitivity analysis showed that the probability of Pola + R-CHP having superior cost-effectiveness was 99.2% when the reference value was JPY7.5 million. The results of scenario analysis suggested that prolongation of PFS was an important factor in the evaluation of cost-effectiveness in previously untreated DLBCL with or without prolongation of OS.
Conclusions
This study suggests that Pola + R-CHP is a cost-effective treatment for previously untreated DLBCL in Japan under the public health insurance system.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common non-Hodgkin lymphoma subtype, representing ∼30–40% of all newly diagnosed non-Hodgkin lymphoma patients in JapanCitation1.
Systemic chemotherapy is recommended for patients diagnosed with DLBCL. R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone) has been the standard of care for DLBCL recommended in Japanese and international guidelines for ∼20 years. However, in ∼30–40% of patients with DLBCL who receive first-line treatment with R-CHOP, the disease will eventually relapse or become refractory to treatment, resulting in a lower quality of life (QoL) and poor prognosis. Although various regimens have been developed for more effective curative treatment, including multi-agent combination therapy and molecularly targeted therapy, none of these regimens have surpassed the efficacy of R-CHOPCitation2–4.
Polatuzumab vedotin is an antibody–drug conjugate that combines a monoclonal antibody targeting CD79b—a cell-surface antigen expressed exclusively on all mature B cells except plasma cells—with the cytotoxic agent monomethyl auristatin E. Polatuzumab vedotin has been approved and covered by the public health insurance system for the treatment of relapsed or refractory DLBCL in Japan since 2021Citation5. A phase III, multi-institutional, randomized, double-blind, placebo-controlled study (POLARIX: GO39942 trial) compared the efficacy and safety of Pola + R-CHP (polatuzumab vedotin plus rituximab, cyclophosphamide, doxorubicin, and prednisolone) combination therapy vs. R-CHOP therapy for previously untreated CD20-positive DLBCL with International Prognostic Index (IPI) score of ≥2 That study demonstrated the superiority of Pola + R-CHP with respect to the primary endpoint of progression-free survival (PFS) (hazard ratio for progression, relapse, or death = 0.73 [95%CI: 0.57–0.95; p = 0.02]), but the overall survival (OS) did not differ significantlyCitation6 (hazard ratio for death = 0.94 [95%CI: 0.65–1.37; p = 0.75]). In August 2022, based on data from the POLARIX trial and other studies, Pola + R-CHP was approved for the treatment of previously untreated DLBCL in Japan.
The Minds Manual for Guideline Development 2020 ver. 3.0, published by the Japan Council for Quality Health CareCitation7, suggests that clinical practice guidelines require a health economic evaluation, including a cost-effectiveness analysis, whenever the recommendations of the guidelines are likely to be affected by the cost or cost-effectiveness of medical treatment.
Polatuzumab vedotin for relapsed or refractory DLBCL was assessed under Japan’s health technology assessment system, and it is reported to be a cost-effective treatment. However, the cost-effectiveness of the combination therapy of Pola + R-CHP for previously untreated DLBCL has not yet been assessed in Japan. Because Pola + R-CHP combination therapy has become a new standard of care for previously untreated DLBCL, it is important to evaluate its cost-effectiveness in consideration of the most efficient allocation of medical resources.
This study aims to evaluate the cost-effectiveness of Pola + R-CHP combination therapy vs. R-CHOP therapy in previously untreated patients with DLBCL in Japan.
Methods
Target population and comparator
The POLARIX trial is the only randomized controlled trial to directly compare Pola + R-CHP combination therapy with R-CHOP therapy. The target population of the current analysis was the population of previously untreated patients with DLBCL in the POLARIX trial who had an IPI score of ≥2 and who had received either Pola + R-CHP combination therapy or the comparator, R-CHOP therapyCitation6. Each therapy had a 21-day cycle, and each therapy was administered for the maximum number of cycles in the POLARIX trial.
Model structure
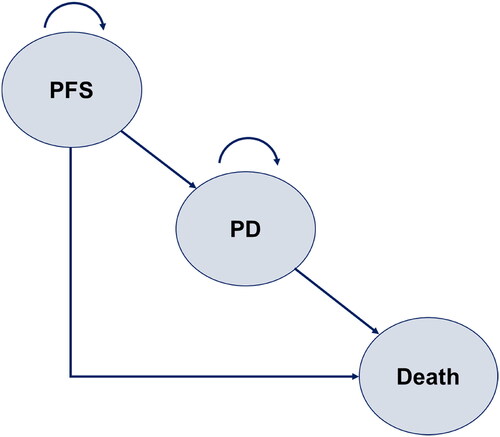
A partitioned survival analysis (PartSA) model was constructed with three health states: PFS, progressive disease (PD), and death (). A PartSA model is a method for estimating long-term costs and effectiveness of treatment by predicting changes over time in the proportion of patients with each health status, based on multiple survival curves. The current analysis incorporated OS and PFS survival curves; the proportion of patients in the PD state was calculated from the difference between the OS survival curve and the PFS survival curveCitation8. A 1-week analysis cycle and a lifetime time horizon were used. The analysis was from the perspective of Japanese public healthcare payer’sCitation9.
Figure 1. Structure of the PartSA model. A partitioned survival analysis (PartSA) model was constructed with three health states: PFS, progressive disease (PD), and death. The model incorporated OS and PFS survival curves, and the proportion of patients in the PD state was calculated from the difference between the OS survival curve and the PFS survival curve. Abbreviations. PD, progressive disease; PFS, progression-free survival.
Assumptions
It has been reported that among patients with DLBCL, those who maintain a longer PFS ultimately have a longer OSCitation10,Citation11. In this study, we set the standardized mortality ratio (SMR) for patients who maintained PFS for 24 months after starting first-line therapy to 1.22 (95%CI = 1.09–1.37), and for those who relapsed within 24 months, the SMR was set to be 2.42 (95%CI = 2.30–2.55)Citation12. SMRs were weighted based on the proportions of patients in the states of PFS and PD at 24 months from baseline, and long-term OS and PFS curves were estimated considering excess deaths from DLBCL. The Abridged Life Table for Japan 2021 was used for mortality in the general populationCitation13.
In both Pola + R-CHP combination therapy and R-CHOP therapy, it was assumed that salvage chemotherapy was continued until death after the transition to PD because PartSA model is unable to consider treatment sequence and the number of treatment lines in PD state. It was also assumed that a certain proportion of patients would receive autologous stem cell transplantation (ASCT) and/or chimeric antigen receptor T-cell (CAR-T) therapy in addition to salvage chemotherapy. It was assumed that all patients received end-of-life care immediately before death.
Data source
The model is constructed using data from the POLARIX trial, whose large sample size and small bias make it sufficient as the sole data source. However, because clinical background information on Japanese patients could not be determined solely from the POLARIX trial data, some parameters for the model were taken from the literature insteadCitation14–16 ().
Table 1. Model inputs.
To predict long-term OS and PFS, parametric survival functions were fitted to the latest cutoff data from the POLARIX trial (as of June 2022). The POLARIX PFS data was extended with R-CHOP arm in GOYA trial after 42 months, assuming equal treatment effect to estimate more accurately the long-term remissionCitation17. The goodness-of-fit of each parametric function was evaluated from the fitting statistics of the Akaike information criterion and the Bayesian information criterion and visual consistencyCitation18. Figure S2 showed log-cumulative hazard plots. As the lines are not parallel to each other, we assumed that the proportional hazard assumption does not hold in both PFS and OS.
Peripheral neuropathy and adverse events (AEs) that differed by 4% or more between the two groups (nausea, diarrhea, febrile neutropenia) were included in the analysis, based on expert opinion regarding the clinical importance of AEs in DLBCL treatmentCitation6.
Because the utility values of patients with DLBCL measured in Japan have not been reported, we used the utility values estimated from those collected in the GOYA trial, which compared the efficacy and safety of G-CHOP (obinutuzumab-CHOP) vs. R-CHOP in patients with previously untreated DLBCLCitation17. The utility values collected in the GOYA trial by the EuroQol 5-dimensional 3-level questionnaire (EQ-5D-3L) were adjusted by propensity score weighting based on patient backgrounds from the POLARIX trialCitation19. The utility values under the states of PFS and PD were estimated by adjusting for patient background using a linear mixed-effect model for repeated measures. Propensity scores were calculated using age, sex, Eastern Cooperative Oncology Group (ECOG) Performance Status, bone marrow involvement, IPI score, bulky disease, disease stage, lactate dehydrogenase, and cell-of-origin subtype, based on the method of Morschhauser et al.Citation20. The disutility for each AE was extrapolated from estimates obtained from a literature searchCitation21,Citation22. Based on expert opinion, the duration of disutility due to AEs was assumed to be 14 days for all AEs.
To estimate drug costs and administrative costs, the National Health Insurance (NHI) pricing and the Medical Fee Point for 2022 were usedCitation23,Citation24. Drug costs were calculated using the prices of brand-name drugs and based on the number of vials used considering drug wastage. Drug doses were calculated based on body weight and body surface area, and the number of vials needed was calculated by the combination of vials that would minimize drug wastage. Administrative costs included costs of outpatient visits, hospitalization, laboratory tests, physiological function tests, and imaging tests. The consumption of medical resources required for each treatment and the frequency of medical interventions were estimated based on the results of a questionnaire survey completed by clinical experts of the authors (K.Y. and S.F.) independently. The percentage of patients receiving ASCT after relapse was set to be 25%Citation2. No data were available on the proportion of patients who undergo CAR-T therapy; therefore, based on expert opinion, we assumed that 1 in 16 (6.3%) of the target population will receive CAR-T therapy.
The cost per week of salvage chemotherapy was calculated from the average cost per regimen of salvage chemotherapy, including the follow-up period and median PFS. The cost per regimen of salvage chemotherapy was calculated as the average of four regimens (CHASE-R [cyclophosphamide, cytosine arabinoside, etoposide, dexamethasone, rituximab], R-DeVIC [rituximab, etoposide, dexamethasone, ifosfamide, carboplatin], R-GCP [rituximab, gemcitabine, cisplatin, dexamethasone], and BR [rituximab, bendamustine]) considered to be commonly used in Japanese clinical practice, according to expert opinion. The follow-up cost was assumed to be the same as that of first-line therapy. The median PFS with salvage chemotherapy was assumed to be 5.2 monthsCitation5,Citation25. End-of-life care costs per patient were extracted from the data of a Japanese retrospective observational studyCitation26.
Ethical matters
The study protocol was reviewed and approved by a third-party ethics committee (MINS Research Ethics Committee, Tokyo, Japan). The study was conducted in compliance with the Japanese Ethical Guidelines for Medical and Biological Research Involving Human Subjects and was registered in the Japan Registry of Clinical Trials (jRCT 1031230035).
Data analyses
The incremental cost-effectiveness ratio (ICER) was used as the cost-effectiveness measure in this analysis. Based on the Japanese system of cost-effectiveness evaluationCitation27, Pola + R-CHP combination therapy was defined to be cost-effective compared to R-CHOP therapy if the ICER was 7.5 million Japanese yen (JPY) or less per quality-adjusted life year (QALY). Both cost and effectiveness were discounted at 2% per yearCitation9.
Sensitivity analyses were performed to test the uncertainty and robustness of the model. First, a one-way sensitivity analysis was performed to test the impact of each parameter set in the model on the ICER. Second, each parameter was replaced by a probability distribution, and a probabilistic sensitivity analysis was repeated 1,000 times.
Finally, scenario analyses were performed using each of the following five scenarios: (1) OS of Pola + R-CHP is equivalent to that of R-CHOP, (2) SMR is adjusted by percentages of patients in the states of PFS and PD at 5 years; (3) the utility values of the POLARIX trial are used; (4) SMR in patients who progressed within 24 months is 5, and (5) generic pricing is used. Microsoft Excel 365 and Visual Basic for Applications were used to perform the base-case analysis and all sensitivity analyses.
Results
Base-case analysis
For patients who received Pola + R-CHP combination therapy, the expected cost from the start of first-line treatment until the end of end-of-life care was JPY34,917,885 (USD268,599 [USD1 = JPY130]) and the expected effectiveness was 11.25 QALYs. The expected cost for patients who received R-CHOP therapy was JPY33,889,314 (USD260,687) and the expected effectiveness was 10.87 QALYs. The incremental cost of Pola + R-CHP combination therapy over R-CHOP therapy was estimated to be JPY1,028,571 (USD7,912) and the incremental effectiveness was 0.38 QALYs. Therefore, the ICER for Pola + R-CHP combination therapy compared to R-CHOP therapy was JPY2,710,238 per QALY (USD20,848) (). shows the results of the OS and PFS estimates.
Figure 2. Survival curves of base-case analysis. Parametric survival functions were fitted to the latest cutoff data of the POLARIX trial (as of June 2022) to predict long-term OS and PFS. Abbreviations. OS, overall survival; PFS, progression-free survival.
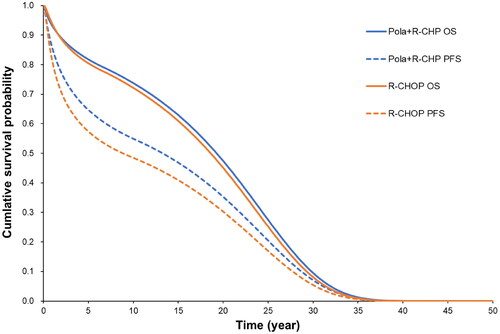
Table 2. Result of base-case analysis.
Sensitivity analysis
Because several results in the one-way sensitivity analysis were “dominant” (reduced cost and increased effectiveness), the incremental net monetary benefit (INMB) was used as the evaluation indicator, and a tornado diagram was the output (). A positive value for INMB meant that the ICER was less than JPY7.5 million (USD57,692) per QALY:
Figure 3. Tornado diagram. The central vertical line in this tornado diagram represents the base-case value. An orange bar shows the result when the parameter uses an upper value, and a blue bar shows the result when the parameter uses a lower value. Abbreviations. BSA, body surface area; CAR-T, chimeric antigen receptor T-cell; INMB, incremental net monetary benefit; JPY, Japanese yen; PD, progressive disease; SMR, standardized mortality ratio.
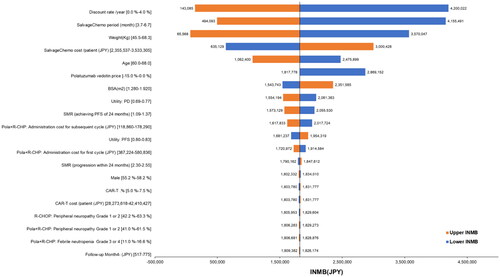
The INMB ranged from JPY143,085 (USD1,101) to JPY4,200,022 (USD32,308). Parameters influencing the results of the analysis were median PFS and the total cost per regimen of salvage chemotherapy, patient weight, patient age, and polatuzumab vedotin price. The upper and lower INMB limits for all parameters were greater than JPY0 (ICER of JPY7.5 million or less per QALY) ().
and show the results of the probabilistic sensitivity analysis. When the ICER reference value was JPY7.5 million (USD57,692) per QALY, the probability of Pola + R-CHP combination therapy being cost-effective was 99.2%.
Figure 4. Probabilistic sensitivity analysis results are plotted on a cost-effectiveness plane. The blue dot shows the ICER in the base-case analysis. The orange dots show the ICERs in 1,000 iterations of the probabilistic sensitivity analysis. The probability of Pola + R-CHP combination therapy being cost-effective was 99.2% (ICER < JPY7.5 million per QALY). Abbreviations. JPY, Japanese yen; PSA, probabilistic sensitivity analysis; QALY, quality-adjusted life year.
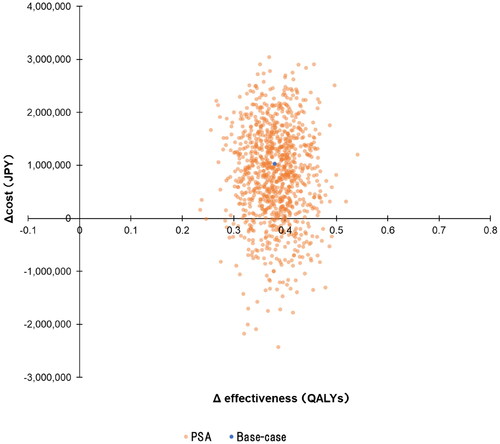
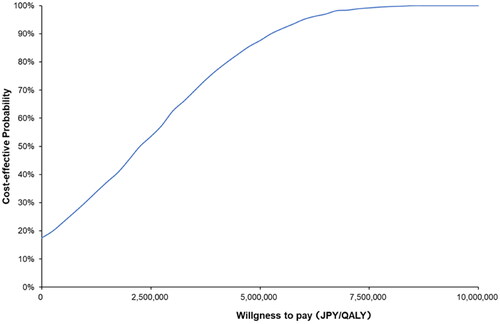
Figure 5. Cost-effectiveness–acceptability curve. The blue line shows the probability of Pola + R-CHP combination therapy being cost-effective at the ICER value on the x-axis. The probability of Pola + R-CHP combination therapy being cost-effective was 99.2% when the reference value was JPY7.5 million per QALY. Abbreviations. JPY, Japanese yen; QALY, quality-adjusted life year.
The scenario analyses showed Pola + R-CHP combination therapy to be cost-effective compared to R-CHOP therapy because when the OS of Pola + R-CHP combination therapy was made equivalent to that of R-CHOP therapy (Scenario 1), the incremental cost was negative. In the other scenarios, the ICERs were not notably different from the results of the base-case analysis ().
Table 3. Results of scenario analyses.
Discussion
This study is the first to analyze and evaluate whether Pola + R-CHP combination therapy in previously untreated patients with DLBCL is cost-effective within the context of the Japanese public health insurance system. Although the cost during first-line treatment is greater with Pola + R-CHP combination therapy than with R-CHOP therapy, our results indicate that compared to R-CHOP therapy, Pola + R-CHP combination therapy notably reduced the cost of salvage chemotherapy after progression, owing to the improvement in PFS. Similarly, the assumption of longer PFS with good health status and slight prolongation of OS led to an incremental QALY gain of 0.38. Based on these results, the ICER for Pola + R-CHP combination therapy was calculated to be JPY2,710,238 (USD20,848) per QALY when R-CHOP therapy was used as a comparator.
The reference value used in the evaluation of the cost-effectiveness of anticancer agents under the Japanese public health insurance system is JPY7.5 million (USD57,692) per QALYCitation27. The ICER for Pola + R-CHP combination therapy was below JPY7.5 million (USD57,692) per QALY in all sensitivity analyses in this study, suggesting that Pola + R-CHP combination therapy is cost-effective as a treatment strategy for previously untreated patients with DLBCL in Japan.
In the United Kingdom (UK) as well, the cost-effectiveness of Pola + R-CHP combination therapy for previously untreated DLBCL as evaluated by the National Institute for Health and Care Excellence (NICE) was also found to be favorableCitation28, which is consistent with the findings of the present study. Another similarity between that study and the present study is that the cost of salvage chemotherapy in post-relapse treatment was noted to be an important parameter influencing the evaluation of the cost-effectiveness of Pola + R-CHP combination therapyCitation28.
The PFS for salvage chemotherapy is one of the most important parameters for estimating cost after disease progression in this analysis, because the efficacy of salvage chemotherapy for relapsed patients may vary widely, depending on patient status. The median PFS of two clinical studies for ASCT ineligible relapsed or refractory DLBCL patients who received BR therapy was 3.7 months (95% CI, 2.1–4.5) and 6.7 months (95% CI, 3.6–13.7), respectivelyCitation5,Citation25. Recently, Terui et al.Citation29 reported that the median PFS for ASCT ineligible Japanese patients received BR therapy was 5.2 months (95% CI 3.6–not evaluable).
We examined the uncertainty of these differences in PFS by using a one-way sensitivity analysis. Even when the median PFS for patients who received salvage chemotherapy was conservatively set at 6.7 months, the ICER was JPY6,198,088 (USD47,678) per QALY, which was lower than the ICER reference value of JPY7.5 million (USD57,692) per QALY. In addition, the total cost per regimen of salvage chemotherapy was calculated from the average of four regimens that are considered common in Japanese clinical practice, according to expert opinion. Although the cost of salvage chemotherapy varies by regimen, we consider that this uncertainty is sufficiently accounted for by the ±20% range of the one-way sensitivity analysis.
A previous United States (US) study by Kambhampati et al.Citation30 reported an ICER for Pola + R-CHP combination therapy of USD84,308 per QALY compared to R-CHOP therapy, and the authors concluded that Pola + R-CHP combination therapy was cost-effective. That study used a state-transition model, unlike the PartSA model used in this study, and assumed that maintaining PFS for 5 years reduces the risk of recurrence and that PFS at 5 years is an important parameter influencing cost-effectivenessCitation30. Those assumptions appear to have clinical relevance, and several studies have suggested that patients with DLBCL who maintain PFS for a longer period of time have improved long-term survivalCitation10,Citation11.
Although the base-case scenario in our study uses SMR based on PFS at 24 months, we also conducted a mortality risk-adjusted analysis based on PFS at 5 years, as was done in the study by Kambhampati et al.Citation30. Although the ICER in that scenario analysis was slightly higher than in the base-case analysis, the specific duration of PFS that is used to predict long-term mortality risk seems to have little impact on the outcome of the analysis.
When the OS for the Pola + R-CHP combination therapy arm was assumed to be equivalent to that for the R-CHOP therapy arm, both the incremental cost and the incremental QALY were reduced compared with the base-case analysis results. This result may be due to the reduction in area under the curve between OS and PFS and expected life year in only Pola + R-CHP, resulting in fewer QALYs gained during PD and a corresponding reduction in the cost of post-relapse treatment. The results of scenario analysis suggested that prolongation of PFS was an important factor in the evaluation of cost-effectiveness in previously untreated DLBCL with or without prolongation of OS.
The estimated utility values for PFS and PD in patients with DLBCL from the POLARIX trial were 0.87 and 0.85, respectivelyCitation6. In contrast, the estimated utility values for PFS and PD in previously untreated patients with DLBCL measured by Putri et al. using EQ-5D-5L were 0.74 and 0.48, respectivelyCitation31. The difference between estimated utility values for PFS and PD from the POLARIX trial was very small, suggesting that the utility for PD may have been overestimated. Therefore, we used data from the GOYA trial, which had a similar patient population and definition of PD.
From a clinical standpoint, using the adjusted utility values from the GOYA trial as the base-case analysis values was preferable. Nonetheless, an analysis using the utility values from the POLARIX trial in terms of concordance with survival outcomes was tested for uncertainty as one of the scenario analyses. Under this scenario, the ICER was estimated to be approximately JPY2,902,727 (USD22,329) per QALY. Therefore, the impact of this uncertainty on the conclusions of this study is considered to be limited.
The primary limitation of this study is the handling of post-relapse therapy. The cost of salvage chemotherapy was estimated based on the average of four regimens (CHASE-R, R-DeVIC, R-GDP, and BR). Another important factor to consider in assessing cost-effectiveness is CAR-T therapy and ASCT administered after salvage chemotherapy, because, although they are costly, they may have an impact on the prognosis. However, it is difficult to incorporate the benefit of these treatments into the long-term OS/PFS curves: although the percentages of such patients are expected to increase in the future, currently only a limited number of institutes in Japan can provide these therapies, especially CAR-T, and the percentages of patients receiving these types of therapy vary considerably by individual institute.
ASCT has been reported to be performed in ∼1 in 4 patients with DLBCL, which is reasonable given the current situation of clinical practice in JapanCitation2. With respect to CAR-T treatment, we made an assumption regarding the percentage of patients receiving this therapy, based on expert opinion. It is unclear how the difference in treatment groups affects the rate of receiving subsequent therapies, such as CAR-T and ASCT. Prolongation of PFS is expected to reduce the expense of costly treatments, such as ASCT after disease progression. We expect that in the future, there will be no difference between Pola + R-CHP combination therapy and R-CHOP therapy in terms of ASCT or CAR-T therapy intervention rates. In the additional scenarios regarding the intervention rates, the incremental costs were slightly changed from the results of the base-case analysis (Table S2).
A second limitation is the method of converting utility estimates. Ideally, for cost-effectiveness analysis of Japanese patients, utility values would be converted from a Japanese value set; however, owing to a lack of supporting evidence for the Japanese subgroup or comparable data of Japanese DLBCL, we had to use values estimated from those measured in the international GOYA trial. The original utility in the GOYA trial was undisclosed data. Therefore, we could not convert using the Japanese value setCitation32. Overcoming this limitation will require further research into the QoL of Japanese patients with DLBCL.
A third limitation is the lack of age data for Japanese patients with untreated DLBCL. We set the median patient age to 64 years in the base-case analysis, based on the following references and expert opinion. One study referencing a Japanese claims database (the Medical Data Vision database) has reported the median age of patients with relapsed/refractory DLBCL to be 68 yearsCitation16; however, the target population in that study is different from the target population of our study. The JCOG0601 trial reported the median age of Japanese patients with first-episode DLBCL to be 62 yearsCitation14. However, the upper age limit for patients in that trial, as in all clinical trials, is set at 80 years; similar to POLARIX, this does not necessarily reflect the real-world clinical situation. According to a real-world study conducted at nine institutions in eight countries, the median age of untreated DLBCL was 64 yearsCitation15. However, this is overseas data, and it is unclear whether it directly reflects the situation in Japan.
We examined the effect of age on ICER by a one-way sensitivity analysis. This analysis showed that the ICER is lower in younger patients. This result may reflect the longer-term efficacy of Pola + R-CHP combination therapy in younger patients than in older patients. Even when the age at diagnosis was assumed to be 68 years, the ICER was still less than JPY7.5 million (USD57,692) per QALY.
Lastly, no data were available for SMR in patients whose disease progressed within 24 months. Therefore, we opted for the SMR value of 2.42 from the diagnosis that had been published in an earlier study including patients with DLBCL who maintained PFS until 24 months and those whose disease progressedCitation12. Myers et al.Citation33 reported that the SMR for relapsed DLBCL after ASCT was 3.4. We expected that the SMR for DLBCL with a poor prognosis would be >3.4. However, we consider that the influence of this parameter is also limited because, in the scenario analysis in which the SMR was assumed to be 5, the ICER was also less than JPY7.5 million (USD57,692) per QALY.
Despite these limitations, the sensitivity analyses suggested that the results of this analysis are highly robust. In addition, in accordance with the analysis guidelines, the base-case analysis assumes the use of brand-name drugs; however, even when brand-name drugs were replaced by generic drugs, the ICER did not exceed JPY7.5 million (USD57,692) per QALY. The results of this study were also consistent with previous studies conducted in the US and the UKCitation28,Citation30. This study shows that Pola + R-CHP combination therapy may not only increase QALY by improving PFS but may also notably reduce treatment costs after relapse.
Conclusions
The findings of this study suggest that Pola + R-CHP combination therapy is a cost-effective treatment for previously untreated DLBCL in Japan under the public health insurance system, with notable cost savings after progression, attributed to improved PFS. Long-term PFS with primary treatment is one key factor influencing healthcare economics in DLBCL. Continued exploration of cost-effectiveness assessments is required in light of anticipated future changes in the treatment landscape.
Transparency
Declaration of funding
The study was funded by Chugai Pharmaceutical Co., Ltd.
Declaration of financial/other relationships
TM, SO, NI, and TO are employees of Chugai Pharmaceutical Co., Ltd.
SS and TM are employees of CRECON Medical Assessment Inc. and are paid consultants for Chugai Pharmaceutical Co., Ltd.
HS received research funding from Towa Pharmaceutical Co., Ltd.
KY reports grants and personal fees from AbbVie, received during the conduct of this study; grants and personal fees from AbbVie, grants and personal fees from Astra-Zeneca, grants and personal fees from Bristol-Myers Squibb/Celgene, grants and personal fees from Chugai, grants and personal fees from Eisai, grants from IQVIA/Genmab, grants and personal fees from Meiji Seika Pharma, grants and personal fees from Nippon Shinyaku, grants and personal fees from Novartis, grants from Ono Pharmaceutical, grants and personal fees from Otsuka Pharmaceutical, grants from Solasia Pharma, personal fees from SymBio, grants and personal fees from Takeda, grants from Yakult, grants and personal fees from Zenyaku, personal fees from HUYA/IQVIA Services Japan, personal fees from Janssen, personal fees from Kyowa Kirin, personal fees from Micron/Daiichi-Sankyo, personal fees from MSD, personal fees from Sanofi, grants from Incyte, personal fees from Nippon Kayaku, and personal fees from Astellas, received outside the period of the submitted work.
SF has no relevant financial or other relationships to disclose.
Author contributions
All authors contributed to the conception and design of the study and interpretation of the data.
SS and TM conducted the data analysis.
All authors contributed to drafting the paper or revising it critically for intellectual content, and all have given final approval of the version to be published.
Acknowledgements
We thank Mathilde Roussel, Rodrigo Ho, and Aino Launonen of F. Hoffmann–La Roche for their roles in designing the study and MIMS Pte Ltd for English-language editing.
Reviewer disclosures
All reviewers of this manuscript have received an honorarium from JME for their review work. A reviewer of this manuscript has disclosed that they have received Gilead consultancy fees. The other reviewers have no other relevant financial relationships to disclose.
Previous presentations
None.
Supplemental Material
Download MS Word (222.2 KB)References
- Lymphoma Study Group of Japanese Pathologists. The World Health Organization classification of malignant lymphomas in Japan: incidence of recently recognized entities. Pathol Int. 2000;50(9):696–702. doi: 10.1046/j.1440-1827.2000.01108.x.
- Friedberg JW. Relapsed/refractory diffuse large B-cell lymphoma. Hematology Am Soc Hematol Educ Program. 2011;2011(1):498–505. doi: 10.1182/asheducation-2011.1.498.
- Japanese Society of Hematology. Zōketsukishuyō shinryō gaidorain [Practical guidelines for hematological malignancies]; 2018 [cited 2023 Mar 28]. Available from: http://www.jshem.or.jp/gui-hemali/table.html
- Iftikhar R, Mir MA, Moosajee M, et al. Diagnosis and management of diffuse large B-cell lymphoma: Society of Medical Oncology, Pakistan Society of Hematology, and Pakistan Society of Clinical Oncology joint clinical practice guideline. J Clin Oncol Glob Oncol. 2021;7:1647–1658. doi: 10.1200/GO.21.00320.
- Ngu H, Takiar R, Phillips T, et al. Polatuzumab vedotin in relapsed or refractory diffuse large B-cell lymphoma. Am Soc Clin Oncol Educ Book. 2022;42(2):1–14. doi: 10.1200/JCO.19.00172.
- Tilly H, Morschhauser F, Sehn LH, et al. Polatuzumab vedotin in previously untreated diffuse large B-cell lymphoma. N Engl J Med. 2022;386(4):351–363. doi: 10.1056/NEJMoa2115304.
- Minds Manual Developing Committee, editor. Minds manual for guideline development 2020, ver. 3.0. Tokyo: Japan Council for Quality Health Care; 2021.
- NICE Decision Support Unit. TSD 19: Partitioned survival analysis as a decision modelling tool [cited 2023 Mar 29]. Available from: https://www.sheffield.ac.uk/nice-dsu/tsds/partitioned-survival-analysis
- Center for Outcomes Research and Economic Evaluation for Health, National Institute of Public Health (C2H). Guideline for preparing cost-effectiveness evaluation to the Central Social Insurance Medical Council [cited 2023 Mar 29]. Available from: https://c2h.niph.go.jp/tools/guideline/guideline_en.pdf
- Jakobsen LH, Bøgsted M, Brown PN, et al. Minimal loss of lifetime for patients with diffuse large B-cell lymphoma in remission and event free 24 months after treatment: a Danish population-based study. J Clin Oncol. 2017;35(7):778–784. doi: 10.1200/JCO.2016.70.0765.
- Shi Q, Schmitz N, Ou FS, et al. Progression-free survival as a surrogate end point for overall survival in first-line diffuse large B-cell lymphoma: an individual patient-level analysis of multiple randomized trials (SEAL). J Clin Oncol. 2018;36(25):2593–2602. doi: 10.1200/JCO.2018.77.9124.
- Maurer MJ, Habermann TM, Shi Q, et al. Progression-free survival at 24 months (PFS24) and subsequent outcome for patients with diffuse large B-cell lymphoma (DLBCL) enrolled on randomized clinical trials. Ann Oncol. 2018;29(8):1822–1827. doi: 10.1093/annonc/mdy203.
- Ministry of Health, Labour and Welfare. Abridged Life Table for Japan; 2021 [cited 2023 Mar 29]. Available from: https://www.mhlw.go.jp/toukei/saikin/hw/life/life21/index.html
- Ohmachi K, Kinoshita T, Tobinai K, et al. A randomized phase 2/3 study of R-CHOP vs CHOP combined with dose-dense rituximab for DLBCL: the JCOG0601 trial. Blood Adv. 2021;5(4):984–993. doi: 10.1182/bloodadvances.2020002567.
- Armitage JO, Weisenburger DD. New approach to classifying non-Hodgkin’s lymphomas: clinical features of the major histologic subtypes. Non-Hodgkin’s lymphoma classification project. J Clin Oncol. 1998;16(8):2780–2795. doi: 10.1200/JCO.1998.16.8.2780.
- Tsutsué S, Makita S, Yi J, et al. Economic burden in treated Japanese patients with relapsed/refractory large B-cell lymphoma. Future Oncol. 2021;17(33):4511–4525. doi: 10.2217/fon-2021-0400.
- Sehn LH, Martelli M, Trněný M, et al. A randomized, open-label, phase III study of obinutuzumab or rituximab plus CHOP in patients with previously untreated diffuse large B-cell lymphoma: final analysis of GOYA. J Hematol Oncol. 2020;13(1):71. doi: 10.1186/s13045-020-00900-7.
- NICE Decision Support Unit. TSD 14: Survival analysis for economic evaluations alongside clinical trials – extrapolation with patient-level data [updated 2013 Mar; cited 2023 Mar 29]. Available from: https://www.sheffield.ac.uk/media/34225/download?attachment
- Kane LT, Fang T, Galetta MS, et al. Propensity score matching: a statistical method. Clin Spine Surg. 2020;33(3):120–122. doi: 10.1097/BSD.0000000000000932.
- Morschhauser F, Feugier P, Flinn IW, et al. A phase 2 study of venetoclax plus R-CHOP as first-line treatment for patients with diffuse large B-cell lymphoma [published correction appears in. Blood. 2021;137(13):1844]. Blood. 2021;137(5):600–609. doi: 10.1182/blood.2020006578.
- Swinburn P, Shingler S, Acaster S, et al. Health utilities in relation to treatment response and adverse events in relapsed/refractory Hodgkin lymphoma and systemic anaplastic large cell lymphoma. Leuk Lymphoma. 2015;56(6):1839–1845. doi: 10.3109/10428194.2014.970542.
- Nafees B, Lloyd AJ, Dewilde S, et al. Health state utilities in non-small cell lung cancer: an international study. Asia Pac J Clin Oncol. 2017;13(5):e195–e203. doi: 10.1111/ajco.12477.
- Yakka kounou hayami hyou 2022 [Japanese drug tariffs 2022]. Tokyo: Igakutushinsya Co., Ltd.; 2022.
- Shinryou tensuu hayami hyou 2022-nen 4-gatsu zohoban [Medical treatment fee point April 2022]. Tokyo: Igakutushinsya Co., Ltd.; 2022.
- Ohmachi K, Niitsu N, Uchida T, et al. Multicenter phase II study of bendamustine plus rituximab in patients with relapsed or refractory diffuse large B-cell lymphoma. J Clin Oncol. 2013;31(17):2103–2109. doi: 10.1200/JCO.2012.46.5203.
- Awano N, Izumo T, Inomata M, et al. Medical costs of Japanese lung cancer patients during end-of-life care. Jpn J Clin Oncol. 2021;51(5):769–777. doi: 10.1093/jjco/hyaa259.
- Hasegawa M, Komoto S, Shiroiwa T, et al. Formal implementation of cost-effectiveness evaluations in Japan: a unique health technology assessment system. Value Health. 2020;23(1):43–51. doi: 10.1016/j.jval.2019.10.005.
- National Institute for Health and Care Excellence. Polatuzumab vedotin in combination for untreated diffuse large B-cell lymphoma [cited 2023 Mar 29]. Available from https://www.nice.org.uk/guidance/gid-ta10785/documents/html-content-5
- Terui Y, Rai S, Izutsu K, et al. A phase 2 study of polatuzumab vedotin + bendamustine + rituximab in relapsed/refractory diffuse large B-cell lymphoma. Cancer Sci. 2021;112(7):2845–2854. doi: 10.1111/cas.14937.
- Kambhampati S, Saumoy M, Schneider Y, et al. Cost-effectiveness of polatuzumab vedotin combined with chemoimmunotherapy in untreated diffuse large B-cell lymphoma. Blood. 2022;140(25):2697–2708. doi: 10.1182/blood.2022016624.
- Putri S, Setiawan E, Saldi SRF, et al. Adding rituximab to chemotherapy for diffuse large B-cell lymphoma patients in Indonesia: a cost utility and budget impact analysis. BMC Health Serv Res. 2022;22(1):553. doi: 10.1186/s12913-022-07956-w.
- Shiroiwa T, Noto S, Fukuda T. Japanese population norms of EQ-5D-5L and health utilities index mark 3: disutility catalog by disease and symptom in community settings. Value Health. 2021;24(8):1193–1202. doi: 10.1016/j.jval.2021.03.010.
- Myers RM, Hill BT, Shaw BE, et al. Long-term outcomes among 2-year survivors of autologous hematopoietic cell transplantation for Hodgkin and diffuse large B-cell lymphoma. Cancer. 2018;124(4):816–825. doi: 10.1002/cncr.31114.
