Abstract
Aims
We evaluated the pharmacoeconomic value of polatuzumab vedotin plus rituximab, cyclophosphamide, doxorubicin, and prednisone (Pola-R-CHP) in previously untreated diffuse large B-cell lymphoma (DLBCL) versus rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP).
Materials and methods
A 3-state partitioned survival model was used to estimate life years (LYs), quality-adjusted LYs (QALYs), and cost impacts of Pola-R-CHP versus R-CHOP. Analyses utilized mixture-cure survival modelling, assessed a lifetime horizon, discounted all outcomes at 3% per year, and examined both payer and societal perspectives. Progression-free survival, overall survival (OS), drug utilization, treatment duration, adverse reactions, and subsequent treatment inputs were based on data from the POLARIX study (NCT03274492). Costs included drug acquisition/administration, adverse reaction management, routine care, subsequent treatments, end-of-life care, and work productivity.
Results
Incremental cost-effectiveness ratios of Pola-R-CHP versus R-CHOP were $70,719/QALY gained and $88,855/QALY gained from societal and payer perspectives, respectively. The $32,824 higher total cost of Pola-R-CHP versus R-CHOP was largely due to higher drug costs ($122,525 vs $27,694), with cost offsets including subsequent treatment (–$52,765), routine care (–$1,781), end-of-life care (–$383), and work productivity (–$8,418). Pola-R-CHP resulted in an increase of 0.47 LYs and 0.46 QALYs versus R-CHOP. Pola-R-CHP was cost-effective in 60.9% and 58.0% of simulations at a willingness-to-pay threshold of $150,000/QALY gained from societal and payer perspectives, respectively.
Limitations
There was uncertainty around the OS extrapolation in the model, and costs were derived from different sources. Recommended prophylactic medications were not included; prophylactic use of granulocyte colony-stimulating factor for all patients was assumed to be equal across treatment arms in POLARIX. Work productivity loss was estimated from a general population and was not specific to patients with DLBCL.
Conclusion
Pola-R-CHP was projected to be cost-effective versus R-CHOP in previously untreated DLBCL, suggesting that Pola-R-CHP represents good value relative to R-CHOP in this setting.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common non-Hodgkin lymphoma subtypeCitation1. In the United States (US), the prevalence of DLBCL is expected to increase by 11% between 2020 and 2025Citation2, which will place a substantial economic burden on the healthcare system. Frontline treatment with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP), has been the standard of care for previously untreated DLBCL, yet approximately 30–40% of patients with advanced-stage disease are refractory to treatment or experience relapse after an initial response and become eligible for second-line and subsequent therapy (2 L+)Citation3. Outcomes in such patients remain generally dismalCitation4. Although emerging, novel therapies such as chimeric antigen receptor T-cell therapy (CAR-T)Citation5–7, tafasitamab and lenalidomideCitation8, bispecific antibodiesCitation9–12, and polatuzumab vedotinCitation13 have the potential to improve outcomes for patients with relapsed or refractory DLBCL, development of novel regimens that are superior to R-CHOP in preventing the occurrence of relapse events is a high priority.
Polatuzumab vedotin, an antibody–drug conjugate that targets CD79b, was evaluated in combination with rituximab, cyclophosphamide, doxorubicin, and prednisone (Pola-R-CHP) as a frontline treatment for DLBCL compared with R-CHOP in the Phase III randomized, double-blind POLARIX study (NCT03274492)Citation14. After a median follow-up of 28.2 months, Pola-R-CHP significantly improved progression-free survival (PFS) relative to R-CHOP (hazard ratio [HR], 0.73; 95% confidence interval [CI]: 0.57–0.95; p = .02), with no difference in overall survival (OS) between the treatment arms (HR, 0.94; 95% CI: 0.65–1.37; p = .75)Citation14. The safety profile of the two regimens was similarCitation14.
With the addition of polatuzumab vedotin to standard-of-care therapy, a better understanding of the value of polatuzumab vedotin in the frontline setting is required, especially with respect to whether the additional efficacy benefits are worth the extra cost and whether moving polatuzumab vedotin into the frontline setting is likely to impact downstream treatment and related costs. Thus, the objective of this analysis was to evaluate the cost-effectiveness of Pola-R-CHP compared with R-CHOP in adults with previously untreated DLBCL in the US, based on data from the POLARIX study.
Methods
Model structure and overview
A partitioned survival model, which has been used previously in cost-effectiveness analysis (CEA) models of DLBCLCitation15–17, was developed to estimate the life years, quality-adjusted life years (QALY), and cost impacts of frontline Pola-R-CHP compared with R-CHOP (). The partitioned survival model used time-to-event data to model the proportion of patients in one of the following three states over time: alive and progression-free, alive with relapse or progression of DLBCL, or dead.
Figure 1. The 3-state (PFS, PD, death) partitioned survival model used in the analysis. Abbreviations. OS, overall survival; PD, progressive disease; PFS, progression-free survival. Figure adapted with permission from Pratz et al. 2022 (doi: 10.1007/s40273-022-01145-7). Copyright © 2022, Springer Nature. This material does not come under the journal’s Open Access licence and is protected.
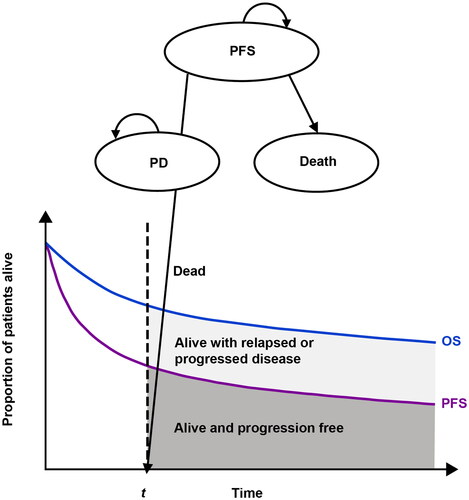
The target population of the CEA model consisted of adults with previously untreated DLBCL based on the POLARIX study (NCT03274492); full methodology, including study design and drug treatment administration, has been previously describedCitation14. Mixture-cure survival modelling was used to extrapolate PFS and OS beyond the study periodCitation18,Citation19. Estimated PFS and OS, drug utilization, treatment duration, adverse reactions, and subsequent treatment inputs were predicted based on data from POLARIX. Both societal and payer perspectives were examined as recommended by the Second Panel on Cost-Effectiveness in Health and MedicineCitation20; the societal perspective included indirect costs (e.g. costs associated with work productivity loss), whereas the payer perspective excluded costs associated with lost work productivity. Patient data were modeled monthly over a lifetime horizon of 60 years to reflect the younger age range observed in the POLARIX study (i.e. 19–80 years)Citation14. All outcomes (costs and health benefits) were discounted at 3% per year.
The primary outcome of interest was the incremental cost-effectiveness ratio (ICER), defined as the difference in total costs divided by the difference in QALYs between Pola-R-CHP and R-CHOP. It is generally thought that an ICER less than $150,000/QALY represents an acceptable value for oncology drugs in the USCitation21; therefore, this threshold was used in our analysis. The model was built using MicrosoftFootnotei Excel 2016Citation22, and was conducted in accordance with the recommendations from the Second Panel on Cost-effectiveness in Health and MedicineCitation20. provides an overview of the model inputs and data sources, which are described in further detail below.
Table 1. Overview of model inputs.
Key cost inputs of the model
The model included costs for drug acquisition and administration, adverse reactions, routine care both during the progression-free and disease-progression (PD) states, and end-of-life costs. The costs of 2 L + therapy were based on new anti-lymphoma therapies used after frontline treatment in the POLARIX study. Indirect costs related to work productivity loss were also estimated. Where applicable, adjustments were made to original costs based on the medical care component of the Consumer Price Index inflated to 2023 US dollarsCitation33.
Drug acquisition costs
Drug acquisition costs were based on unit costs from the Centers for Medicare and Medicaid Services (CMS) average sales price (ASP) from April 2023Citation23, with ASP calculated by dividing the payment values by 1.06. Since polatuzumab vedotin administration is weight-based (1.8 mg/kg; 140-mg and 30-mg vials), costs were calculated based on a mean body weight of 75.92 kg from the POLARIX studyCitation14, and a unit ASP of $111.43/mg. The dosing of rituximab was based on a mean body surface area of 1.86 m2 from POLARIX. Drug costs associated with wastage (e.g. no vial sharing was permitted) were applied in the model. The expected drug acquisition costs per patient were calculated using POLARIX time-to-off-treatment Kaplan–Meier curves (mean in months) for each drug to inform the average drug cost per treatment in weekly cycles. The average number of cycles was 5.2 for polatuzumab vedotin. A maximum of 6 cycles of rituximab was implemented in both the Pola-R-CHP and R-CHOP treatment arms based on the polatuzumab vedotin product insertCitation32. The model used a weighted average of rituximab (24%) and rituximab biosimilars (TRUXIMAFootnoteiii, 27%; RUXIENCEFootnoteiv, 45%; and RIABNIFootnotev, 4%) usage in the market to obtain an overall rituximab cost estimateCitation34.
Drug administration costs
Drug administration costs (first hour: $132.16; subsequent hours: $28.47) were based on the CMS Physician Fee ScheduleCitation24. Length of administration for each drug treatment was obtained from package inserts. Total drug administration costs for each treatment regimen are provided in .
Routine care costs
Routine care costs were derived from a retrospective study analyzing healthcare costs among patients with DLBCL during each line of therapy using IBM MarketscanFootnotevi Commercial and Medicare supplemental claim databasesCitation25. The weekly cost of nontreatment-related office visits, laboratory tests, and radiology tests were summed to define routine care costs; these costs during treatment in the progression-free state were estimated at $986.76/week based on frontline routine care costs using claims dataCitation25. Routine care costs during the PD state were estimated as a one-time cost of $21,949.04 based on a weighted average of costs in 2 L + treatments.
Adverse reaction costs
Grade 3–4 adverse reactions with an incidence of ≥5% in either the Pola-R-CHP or R-CHOP treatment arms from the POLARIX study, including selected Grade 3–4 laboratory abnormalities associated with cytopenias, were considered based on the polatuzumab vedotin product insertCitation32. Grade 3–4 adverse reactions were assumed to be severe and require treatment in an inpatient setting. The costs associated with these adverse reactions were obtained from the Agency for Healthcare Research and Quality’s 2017 Healthcare Cost and Utilization Project National Inpatient database for commercial plansCitation31. A weighted average cost for each adverse reaction was obtained based on ICD-10 codes.
Work productivity costs
Work productivity costs were based on the assumption that patients in the progression-free state missed a single day of work during each intravenous administration. Work productivity was calculated using a human capital approach, where the value of a patient’s productivity was based on cost inputs from the Bureau of Labor Statistics, and assumed a retirement age of 65 years, an average of 1794 h worked/year, an average hourly rate of $33.09, and an employment rate of 62.5%Citation30. Work productivity costs during the PD state were based on the assumption that patients were unable to work.
Costs associated with disease progression (2 L + treatment)
Costs in patients with PD after frontline Pola-R-CHP or R-CHOP included those associated with subsequent treatments from the POLARIX study (treatment categories formed for 2 L + therapy), including CAR-T. The mean number of 2 L + treatments in the POLARIX study was 2.03 and 2.28 for the Pola-R-CHP and R-CHOP treatment arms, respectively. In POLARIX, lower utilization of CAR-T (7.32% vs 9.50%) and stem cell transplantation (SCT; 13.82% vs 17.32%) was observed with Pola-R-CHP versus R-CHOP, with treatment regimen costs for 2 L + therapy based principally on published claims data (where available) or secondarily on the ASP and drug acquisition costs ()Citation23,Citation35,Citation36. The cost of CAR-T of $501,994.57 was based on an average from three US claims databases and the Consumer Price Index adjusted to 2023. As the estimate for CAR-T was based on claims data of patients with continuous enrollment from ≥6 months before through to >3 months after CAR-T infusion until death, end of continuous enrollment, or end of the study period, whichever occurred firstCitation35, we assumed that it included costs of apheresis, bridging therapy, lymphodepletion chemotherapy, CAR-T acquisition and administration, and hospitalization due to a number of reasons, including cytokine release syndrome, neurotoxicity, and immune effector cell-associated neurotoxicity syndrome. The estimate for SCT ($351,913.22) was based on 1-year all-cause costs from a 2019 claims study of patients who received SCT after progression with frontline R-CHOPCitation36.
Table 2. Subsequent treatment after frontline therapy in POLARIX.
End-of-life costs
End-of-life costs, i.e. hospice and inpatient careCitation26, were estimated to be $26,876.53 and were applied during the last month of life in the model as was done previouslyCitation15.
Key clinical inputs of the model
In line with recommendationsCitation37, and as used in previous studiesCitation18,Citation19, a mixture-cure model was chosen for long-term extrapolation of POLARIX PFS and OS data, with the long-term remission fraction (cure fraction) used to inform the OS extrapolation (OS informed by PFS). A mixture-cure model is a statistical approach suitable for long-term extrapolation of PFS and OS data in curative settings, and has been shown to better predict long-term PFS in frontline DLBCL than the standard parametric modelCitation19. The model uses a likelihood-based approach to simultaneously estimate (1) the proportion of patients who are free from disease (long-term remission patients) and are assigned the background mortality rate of an average healthy person with no excess mortality applied, and (2) the proportion of patients who progress and die of the disease (non-long-term remission patients). Clinical data from the GOYA study (NCT01287741) were also used to validate the long-term extrapolations for PFS in a POLARIX-like populationCitation38. The GOYA study was chosen since it included a frontline R-CHOP treatment arm in patients with DLBCL. The study was completed with a median follow-up of 47.4 months (maximum follow-up of 78.2 months)Citation38 versus 28.2 months (maximum follow-up of 43.4 months) in POLARIXCitation14, and full individual patient data were available. Age-specific probability of all-cause mortality was taken from the 2020 Center for Disease Control and Prevention US life tablesCitation39. No excess mortality was considered, which is aligned with a previous publicationCitation40. In a competing risk model analysis that used death due to DLBCL as a competing event to death from other causes, it was shown that the excess mortality seen in patients with DLBCL could be explained by early and late relapses. Based on this approach, the survival of patients with DLBCL was similar to that of the general populationCitation40. Treatment effects of Pola-R-CHP and R-CHOP were assumed to persist throughout the entire time horizon.
Health utilities
With values ranging from 0 (death) to 1 (perfect health), health utilities are measurements of quality of life and multipliers to life year estimates for the calculation of QALYsCitation41. Health utilities from both treatment arms of the GOYA study were implemented in the model given the long-term follow-up and quality of life assessments conducted after disease progression in the study, and there were no differences in health utilities by treatment arm; these were an accepted source for DLBCL utilities by the National Institute of Health and Care Excellence (NICE) review of Pola-R-CHP versus R-CHOP in frontline DLBCLCitation42. EuroQol-5D (EQ-5D) data were not collected after disease progression or after the study treatment period in the POLARIX study. Patients from the GOYA study were matched to those in the POLARIX study using inverse propensity score weighting. In addition to the baseline 3 L utility score, the following clinical characteristics were used to estimate the propensity score: age, sex, Eastern Cooperative Oncology Group performance status (≥2 vs <2), bone marrow involvement, International Prognostic Index score (high vs not high), bulky disease (>7.5 cm, as specified in the GOYA studyCitation38) disease stage (IV vs I–III), lactate dehydrogenase (low/normal vs high), and cell of origin subtype (activated B-cell, germinal center B-cell); selection of these clinical characteristics was based on Morschhauser et al.Citation43.
The utilities for PFS and PD health states were estimated using the least-squares mean method. Health utilities during the progression-free and PD states (0.82 and 0.74, respectively) were based on GOYA EQ-5D-3L results in patients matched to those in the POLARIX study, with US tariffs applied as previously describedCitation44. Health utilities for the general population (i.e. a mean EQ-5D score of 0.86 in patients aged 65–74 years and 0.84 in those aged 75–89 yearsCitation45) were applied for patients who were progression free after Year 3; therefore, patients who were progression free at 3 years and beyond were assumed to have health utilities equal to those in the general population.
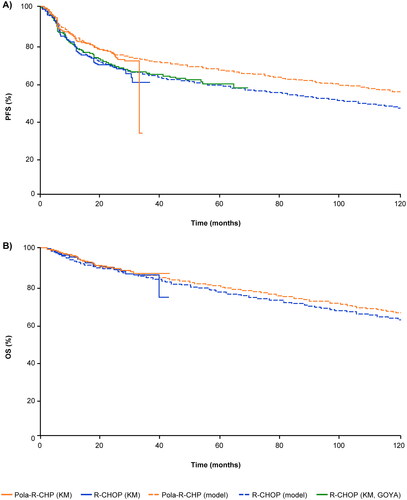
Modeled PFS and OS
Estimated PFS and OS are shown in , respectively. The patient-matched PFS curve for R-CHOP from the GOYA study showed good alignment with the R-CHOP arm from POLARIX (), validating the use of a mixture-cure modeling approach that was applied to both the Pola-R-CHP and R-CHOP data from POLARIX. Based on data from POLARIX, long-term remission fractions of 75% (95% CI: 70–79) for Pola-R-CHP and 64% (95% CI: 56–71) for R-CHOP were estimated using a generalized gamma distribution.
Figure 2. Actual and modeled A) progression-free survival, and B) overall survival based on data from the POLARIX clinical study. Data were modeled using a mixture-cure model. Abbreviations. KM, Kaplan–Meier; OS, overall survival; PFS, progression-free survival; Pola-R-CHP, polatuzumab vedotin plus rituximab, cyclophosphamide, doxorubicin, and prednisone; R-CHOP, rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone.
Additional analyses
One-way and probabilistic sensitivity analyses were performed to assess the impact of uncertainty (±20% of parameter estimates; a conservative range used in cost-effectiveness studies) on cost per QALY-gained outcomes. The one-way sensitivity analysis evaluated the impact of individual variables on the ICER, while the probabilistic sensitivity analysis (using 1,000 Monte Carlo simulations) evaluated cost-effectiveness acceptability from both societal and payer perspectives.
A scenario analysis was conducted to assess the future impact of increased second-line CAR-T utilization following relapse or progression subsequent to completing frontline therapy due to the recent approval of CAR-T (axicabtagene ciloleucel) by the US Food and Drug Administration in the 2 L + settingCitation46. We examined the impact on outcomes of an approximately 2-fold increase in CAR-T use in both treatment arms (given that the population treated at 2 L is larger than the population treated at 3 L and beyond). The analysis assumed increases from the base case in the use of CAR-T from 7.32% to 14.63% and from 9.50% to 18.90% after Pola-R-CHP and R-CHOP, respectively. Additional scenario analyses were conducted on health utilities assigned to the general population at Years 2 and 5 for patients who were progression free.
Data sharing statement
For eligible studies, qualified researchers may request access to individual patient-level clinical data through the clinical study data request platform. At the time of writing this request, the platform is Vivli (https://vivli.org/ourmember/roche/). For up-to-date details on Roche’s Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, available at: https://go.roche.com/data_sharing. Anonymized records for individual patients across more than one data source external to Roche cannot, and should not, be linked owing to a potential increase in the risk of patient reidentification.
Results
Incremental net costs and ICER
Differences in incremental costs between Pola-R-CHP and R-CHOP are shown in . From a societal perspective, the total cost of Pola-R-CHP was $32,824 higher than R-CHOP, mostly driven by greater drug costs ($122,525 vs $27,694). Drug cost offsets for Pola-R-CHP included costs for subsequent lines of therapy (−$52,765), routine care (−$1,781), end-of-life care (−$383), and work productivity (−$8,418). Costs associated with adverse reaction management were $1,258 higher with Pola-R-CHP than with R-CHOP ($13,884 and $12,626, respectively).
Figure 3. Incremental net costs of Pola-R-CHP and R-CHOP. Differences in net costs between Pola-R-CHP and R-CHOP indicated adjacent to each bar. Abbreviations. Pola-R-CHP, polatuzumab vedotin plus rituximab, cyclophosphamide, doxorubicin, and prednisone; R-CHOP, rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone; USD, United States dollars.
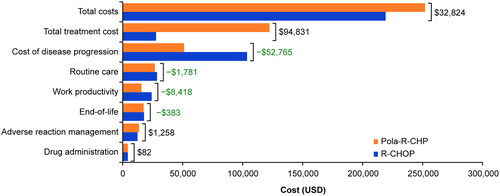
The ICER of Pola-R-CHP versus R-CHOP was $70,719/QALY gained from a societal perspective and $88,855/QALY gained from a payer perspective (which differs from the societal perspective as it does not include costs associated with lost work productivity; ). Pola-R-CHP resulted in an increase of 0.47 life years and 0.46 QALYs compared with R-CHOP ().
Table 3. Cost-effectiveness summary.
Additional analyses
Results of the one-way sensitivity analysis showed that Pola-R-CHP was cost-effective across individual variables, with ICERs <$150,000/QALY (Supplemental Figure 1). The model was most sensitive to subsequent treatment costs for Pola-R-CHP and R-CHOP, the discount rate for treatment efficacy, and adverse reaction management costs. Probabilistic sensitivity analysis results demonstrated that Pola-R-CHP was cost-effective in 60.9% of cases across 1,000 simulations at a willingness-to-pay threshold of $150,000/QALY gained from a societal perspective and 58.0% of simulations from a payer perspective (Supplemental Figure 2).
In the future CAR-T use scenario analysis, the incremental total cost of Pola-R-CHP was $16,883 higher than that of R-CHOP when the use of second-line CAR-T increased, and the ICER was $36,373/QALY gained from the societal perspective. From the payer perspective, the incremental total cost of Pola-R-CHP versus R-CHOP was $25,301, and the ICER was $54,509/QALY gained. Further increase in the use of CAR-T would result in a favorable ICER for Pola-R-CHP versus R-CHOP. In the scenario analysis, applying the health utilities of the general population to patients who were progression free at Year 2 as well as at Year 5 had minimal impact on the ICER results ($70,685/QALY and $70,890/QALY, respectively).
Discussion
The current analysis used a 3-state partitioned survival CEA model to estimate life years, QALYs, and cost impacts of Pola-R-CHP versus R-CHOP based on data from the POLARIX study. In previously untreated patients with DLBCL, Pola-R-CHP was projected to be cost-effective (i.e. <$150,000/QALY) compared with R-CHOP from both a societal ($70,719/QALY) and payer ($88,855/QALY) perspective. An improvement of 0.46 QALYs with Pola-R-CHP versus R-CHOP was demonstrated, as well as cost savings related to disease progression. These findings suggest that the PFS advantage with frontline Pola-R-CHP versus R-CHOP translates to future cost savings that, in part, offset the original cost of Pola-R-CHP.
These results are in line with other published CEAs of Pola-R-CHP in frontline DLBCL. One such analysis, conducted by Kambhampati and colleagues, arrived at the same conclusion as our study—namely, that Pola-R-CHP was cost-effective compared with R-CHOP despite using a different methodological approachCitation47. Instead of a mixture-cure model, the authors developed a state-transition Markov model, including salvage chemotherapy and autologous SCT (ASCT) as second-line treatment and CAR-T as third-line treatment. In the base case, the authors modeled a scenario in which the PFS advantage observed with Pola-R-CHP did not change between Years 2 and 5; in this scenario, Pola-R-CHP therapy was cost-effective, with an ICER of $84,308/QALY, and improved further when the risk of relapse was decreased during Years 2 and 5 ($30,321/QALY)Citation47. This CEA included several sensitivity analyses evaluating the effects of varying the cost of Pola-R-CHP, the 5-year PFS rate, and the cost of CAR-TCitation47. Results of these sensitivity analyses underlined that a major contributor to the cost-effectiveness of frontline Pola-R-CHP was the reduction in the number of patients requiring subsequent CAR-T. A strength of this methodology was that it demonstrated how the cost-effectiveness of Pola-R-CHP depended on its long-term efficacy (measured in PFS). Conversely, the methodology used in our analysis is likely to be highly accurate at estimating the long-term PFS of Pola-R-CHP and R-CHOP, as evidenced by the overlapping PFS curves when GOYA trial data were used as a benchmark.
Our findings are also supported by the results of another CEA conducted by Fu et al. which used a decision-analytic Markov model and included salvage chemotherapy and ASCT or bendamustine/gemcitabine-based chemotherapy for transplant-ineligible patients in the second-line setting and polatuzumab vedotin in combination with bendamustine and rituximab in further lines to estimate QALYs, life years, and direct healthcare costs of a hypothetical cohort of patients aged 65 years with previously untreated DLBCLCitation48. The analysis reported an incremental total cost of $90,439 for Pola-R-CHP versus R-CHOP, improvements of 0.89 QALYs, and an ICER of $101,510/QALY assuming a drug treatment-effect of 5 yearsCitation48. Similar to the CEA conducted by Kambhampati et al.Citation47, and as would be expected, the ICER was sensitive to variations in long-term PFS, with the ICER ranging from $66,100 to $301,670/QALY when different survival functions were usedCitation48. However, the authors did not disclose whether they considered the impact of the cost offsets of therapies in the relapsed setting on the ICER.
In contrast with the two CEAs discussed above, a third study by Vijenthira and colleaguesCitation49, which also used a Markov model, concluded that Pola-R-CHP is not cost-effective at a willingness-to-pay threshold of $150,000/QALY. The higher ICER in this study may be explained by higher estimated incremental costs and lower incremental QALYs compared with other CEAs evaluating Pola-R-CHP. Compared with ours and the other two CEAsCitation47,Citation48, several factors appeared to contribute to the higher ICER in this studyCitation49. The study by Vijenthira et al.Citation49 assumed a much higher rate of granulocyte colony-stimulating factor (G-CSF) utilization with Pola-R-CHP (100%) than with R-CHOP (50%), whereas in the POLARIX study, G-CSF use was mandated for both arms, with comparable rates of febrile neutropenia occurring in both arms. The Vijenthira study also included adverse reaction management costs for febrile neutropenia only, mark-ups for hospital-related care (76%; including infusions, office visits, imaging, and laboratory tests), additional CAR-T hospital mark-up costs ($100,000), and model assumptions regarding quality of life. Assuming a strategy of second-line CAR-T for early relapse, the resulting total cost for frontline Pola-R-CHP was the highest in the Vijenthira study among all published CEAs, including oursCitation49. In terms of clinical outcomes, patients receiving Pola-R-CHP in the Vijenthira study had lower health utilities (0.63) than those receiving CAR-T (0.806) or ASCT (0.701). In contrast, a higher health utility (0.82) was assigned to progression-free patients (including those receiving Pola-R-CHP) in our analysis, and a lower health utility (0.74) was assigned to patients in the PD state (such as those receiving CAR-T or ASCT). We believe that the assignments of health utilities in patients in the progression-free and PD states are more in line with those of previous publicationsCitation50,Citation51, reflecting the impact of treatments for relapsed or refractory DLBCL on quality of life.
Similar to the base case of the CEA conducted by Kambhampati et al.Citation47, the Vijenthira studyCitation49 assumed that the treatment effect of Pola-R-CHP stopped at 2 years, whereas our mixture-cure model estimated sustained improvement in PFS with longer-term follow-up. The Vijenthira study required patients who were treated with Pola-R-CHP to be progression-free for 7 years in order to be considered cured; however, data suggest that patients who are free from progression 2 years after rituximab- and anthracycline-based therapy are highly likely to have similar life expectancy to that of a sex- and age-matched general populationCitation52–54.
A strength of our analysis is the fact that the model was based on primary data from a Phase III randomized trial. Our economic model demonstrated how the PFS advantage associated with Pola-R-CHP resulted in cost savings by avoiding the use of expensive subsequent therapies, including CAR-T and ASCT; these costs partially offset the increased drug costs of polatuzumab vedotin-containing frontline therapy. The mixture-cure model allows better-informed extrapolation of long-term results and appropriately captures the nature of frontline DLBCL in a curative setting. The approach has been validated by external sources (benchmarked against the GOYA study) and accepted by NICE for Pola R-CHP versus R-CHOP in previously untreated DLBCLCitation42. We also examined a societal perspective in keeping with guidance from the Second Panel on Cost-Effectiveness in Health and MedicineCitation20.
Limitations of our study include uncertainty around the OS extrapolation used in the model; however, the population-matched PFS curve of the R-CHOP arm in the GOYA study demonstrated good alignment with that of the R-CHOP curve from POLARIX. In addition, our model did not include recommended prophylactic medications, as it assumed prophylactic G-CSF use for all patients was equal for both treatment arms in POLARIX, more closely aligning our model with both study data and anticipated practice patterns. Previously discussed studies did not mandate G-CSF use for patients treated with R-CHOP despite comparable risks of febrile neutropenia for Pola-R-CHP and R-CHOP; therefore, this may be a contributor to a cost differential between the two regimens in our analysis. Another limitation of our analysis was that costs were derived from different sources and the model applied health utilities from a patient-matched population from the Phase III GOYA study given the longer follow-up duration of this study compared with POLARIXCitation14,Citation38. Further, we did not differentiate between ASCT and allogeneic SCT costsCitation36, and the costs associated with SCT in our model may be slightly overestimated based on recent cost estimates of ASCT and allogeneic SCT used in CEAs of CAR-T versus SCTCitation55,Citation56. We note that the overall average subsequent treatment costs were tested in a one-way sensitivity analysis (±20%), with the ICER results remaining robust. Another potential limitation was the estimation of productivity loss with inputs from the general population (which was not specific to patients with DLBCL) and the assumption of work productivity loss during the PD state (i.e. that patients with disease progression were unable to work at all). We acknowledge that further studies are needed to quantify the proportion of patients able to work due to effective treatments, as well as work productivity specifically in patients with DLBCL.
In conclusion, Pola-R-CHP was projected to be cost-effective in patients with previously untreated DLBCL compared with R-CHOP from both a societal and payer perspective. The economic model demonstrated an improvement in QALYs with Pola-R-CHP versus R-CHOP, and savings in costs related to progression of DLBCL. Based on this CEA, Pola-R-CHP is a cost-effective treatment, representing good value relative to R-CHOP in previously untreated DLBCL.
Transparency
Author contributions
All authors wrote the manuscript and provided the final approvals, executed the study, and performed data analysis.
Reviewer disclosures
All the peer reviewers on this manuscript have received an honorarium from JME for their review work. One of the reviewers on this manuscript has disclosed that they received consultancy fees from GILEAD srl.
The other peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Previous presentations
Presented as a poster at the 2022 Pan Pacific Lymphoma Conference Annual Meeting (July 18–22, 2022), the 10th Annual Meeting of the Society of Hematologic Oncology (September 28–October 1, 2022), and the 11th Annual Meeting of the Society of Hematologic Oncology (September 6–9, 2023).
Supplemental Material
Download MS Word (597.1 KB)Supplemental Material
Download TIFF Image (480 KB)Supplemental Material
Download TIFF Image (787 KB)Acknowledgements
This study was sponsored by F. Hoffmann-La Roche Ltd and Genentech, Inc. Third-party medical writing assistance, under the direction of all authors, was provided by Andrea Bothwell, BSc, and Anna Nagy, BSc, of Ashfield MedComms, an Inizio Company, and was funded by F. Hoffmann-La Roche Ltd.
Declaration of financial/other relationships
M.M. reports stock or other ownership with Merck Sharp & Dohme; honoraria from Genentech, Inc., F. Hoffmann-La Roche Ltd, GlaxoSmithKline, Bayer, Pharmacyclics, Janssen, Seattle Genetics, and ImmunoVaccine Technologies; research funding from Genentech, Inc., F. Hoffmann-La Roche Ltd, GlaxoSmithKline, Bayer, Pharmacyclics, Janssen, Seattle Genetics, Rocket Medical, and ImmunoVaccine Technologies. A.M., C.N., R.W., D.F., F.H., and J.L. are employees of Genentech, Inc., and receive F. Hoffmann-La Roche Ltd. stocks/stock options. R.H. and A.L. are employees of F. Hoffmann-La Roche Ltd. J.M.B. reports consulting or advisory roles with AbbVie, Adaptive Biotechnologies, AstraZeneca, BeiGene, Bristol-Myers Squibb, Epizyme, Kura, Kymera, MorphoSys, Nurix, F. Hoffmann-La Roche Ltd/Genentech, Inc., Seagen, TG Therapeutics, Verastem, and X4 Pharmaceuticals; speakers’ bureau from BeiGene and Seagen; research funding from MorphoSys.
Additional information
Funding
Notes
i Microsoft: Redmond, WA, USA.
ii POLIVY: South San Francisco, CA, USA.
iii TRUXIMA: Incheon, Republic of Korea.
iv RUXIENCE: Cork, Ireland.
v RIABNI: Thousand Oaks, CA, USA.
vi IBM Marketscan: New York, NY, USA
References
- Teras LR, DeSantis CE, Cerhan JR, et al. 2016 US lymphoid malignancy statistics by world health organization subtypes. CA Cancer J Clin. 2016;66(6):443–459. doi: 10.3322/caac.21357.
- Kanas G, Ge W, Quek RGW, et al. Epidemiology of diffuse large B-cell lymphoma (DLBCL) and follicular lymphoma (FL) in the United States and Western Europe: population-level projections for 2020–2025.Leuk Lymphoma. 2022;63(1):54–63. doi: 10.1080/10428194.2021.1975188.
- Sehn LH, Salles G. Diffuse large B-cell lymphoma. N Engl J Med. 2021;384(9):842–858. doi: 10.1056/NEJMra2027612.
- Crump M, Neelapu SS, Farooq U, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood. 2017;130(16):1800–1808. doi: 10.1182/blood-2017-03-769620.
- Neelapu SS, Jacobson CA, Ghobadi A, et al. 5-year follow-up supports curative potential of axicabtagene ciloleucel in refractory large B-Cell lymphoma (ZUMA-1). Blood. 2023;141(19):2307–2315. doi: 10.1182/blood.2022018893.
- Schuster SJ, Tam CS, Borchmann P, et al. Long-term clinical outcomes of tisagenlecleucel in patients with relapsed or refractory aggressive B-cell lymphomas (JULIET): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol. 2021;22(10):1403–1415. doi: 10.1016/S1470-2045(21)00375-2.
- Abramson JS, Palomba ML, Gordon LI, et al. Two-year follow-up of transcend NHL 001, a multicenter phase 1 study of lisocabtagene maraleucel (liso-cel) in relapsed or refractory (R/R) large B-Cell lymphomas (LBCL). Blood. 2021;138(Suppl 1):2840–2840. doi: 10.1182/blood-2021-148948.
- Salles G, Duell J, González Barca E, et al. Tafasitamab plus lenalidomide in relapsed or refractory diffuse large B-cell lymphoma (L-MIND): a multicentre, prospective, single-arm, phase 2 study. Lancet Oncol. 2020;21(7):978–988. doi: 10.1016/S1470-2045(20)30225-4.
- Dickinson MJ, Carlo-Stella C, Morschhauser F, et al. Glofitamab for relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2022;387(24):2220–2231. doi: 10.1056/NEJMoa2206913.
- Budde LE, Sehn LH, Matasar M, et al. Safety and efficacy of mosunetuzumab, a bispecific antibody, in patients with relapsed or refractory follicular lymphoma: a single-arm, multicentre, phase 2 study. Lancet Oncol. 2022;23(8):1055–1065. doi: 10.1016/S1470-2045(22)00335-7.
- Thieblemont C, Phillips T, Ghesquieres H, et al. Epcoritamab, a novel, subcutaneous CD3xCD20 bispecific T-Cell–engaging antibody, in relapsed or refractory large B-Cell lymphoma: dose expansion in a phase I/II trial. J Clin Oncol. 2023;41(12):2238–2247. doi: 10.1200/jco.22.01725.
- Kim W-S, Kim TM, Cho S-G, et al. Odronextamab in patients with relapsed/refractory (R/R) diffuse large B-Cell lymphoma (DLBCL): results from a prespecified analysis of the pivotal phase II study ELM-2. Blood. 2022;140(Suppl 1):1070–1071. doi: 10.1182/blood-2022-158406.
- Sehn LH, Hertzberg M, Opat S, et al. Polatuzumab vedotin plus bendamustine and rituximab in relapsed/refractory DLBCL: survival update and new extension cohort data. Blood Adv. 2022;6(2):533–543. doi: 10.1182/bloodadvances.2021005794.
- Tilly H, Morschhauser F, Sehn LH, et al. Polatuzumab vedotin in previously untreated diffuse large B-cell lymphoma. N Engl J Med. 2022;386(4):351–363. doi: 10.1056/NEJMoa2115304.
- Betts KA, Thuresson PO, Felizzi F, et al. US cost-effectiveness of polatuzumab vedotin, bendamustine and rituximab in diffuse large B-cell lymphoma. J Comp Eff Res. 2020;9(14):1003–1015. doi: 10.2217/cer-2020-0057.
- Bastos-Oreiro M, de Las Heras A, Presa M, et al. Cost-Effectiveness analysis of axicabtagene ciloleucel vs. Tisagenlecleucel for the management of relapsed/refractory diffuse large B-cell lymphoma in Spain. Cancers. 2022;14(3):538. doi: 10.3390/cancers14030538.
- Liu R, Oluwole OO, Diakite I, et al. Cost effectiveness of axicabtagene ciloleucel versus tisagenlecleucel for adult patients with relapsed or refractory large B-cell lymphoma after two or more lines of systemic therapy in the United States. J Med Econ. 2021;24(1):458–468. doi: 10.1080/13696998.2021.1901721.
- Felizzi F, Launonen A, Thuresson PO. Approximation of Long-Term survival with polatuzumab vedotin plus bendamustine and rituximab for patients with relapsed/refractory diffuse large B-Cell lymphoma: results based on the GO29365 trial. Pharmacoecon Open. 2023;7(1):37–46. doi: 10.1007/s41669-022-00339-1.
- Ho R, Launonen A. MSR38 comparison of mixture-cure model versus standard parametric survival model in 1L diffuse large B-cell lymphoma. Value in Health. 2022;25(7):S525. doi: 10.1016/j.jval.2022.04.1245.
- Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. 2016;316(10):1093–1103. doi: 10.1001/jama.2016.12195.
- Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness–the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371(9):796–797. doi: 10.1056/NEJMp1405158.
- Microsoft Corporation. Microsoft Excel. 2016 [cited 2023 June 28]. https://office.microsoft.com/excel
- Centers for Medicare & Medicaid Services. ASP Drug Pricing Files. 2023. [cited 2023 June 28]. https://www.cms.gov/medicare/medicare-part-b-drug-average-sales-price/2023-asp-drug-pricing-files
- Centers for Medicare & Medicaid Services. Physician fee schedule search. 2022 [cited 2023 Aug 08]. https://www.cms.gov/apps/physician-fee-schedule/overview.aspx
- Tkacz J, Garcia J, Gitlin M, et al. The economic burden to payers of patients with diffuse large B-cell lymphoma during the treatment period by line of therapy. Leuk Lymphoma. 2020;61(7):1601–1609. doi: 10.1080/10428194.2020.1734592.
- Chastek B, Harley C, Kallich J, et al. Health care costs for patients with cancer at the end of life. J Oncol Pract. 2012;8(6):75s–80s. doi: 10.1200/JOP.2011.000469.
- US Bureau of Labor Statistics. How do retirement plans for private industry and state and local government workers compare? 2023 [cited 2023 July 04]. https://www.bls.gov/opub/btn/volume-12/how-do-retirement-plans-for-private-industry-and-state-and-local-government-workers-compare.htm
- US Bureau of Labor Statistics. Average weekly hours and overtime of all employees on private nonfarm payrolls by industry sector, seasonally adjusted. 2023 [cited 2023 Mar 05]. https://www.bls.gov/news.release/empsit.t18.htm
- Guy GP, Jr, Yabroff KR, Ekwueme DU, et al. Economic burden of chronic conditions among survivors of cancer in the United States. J Clin Oncol. 2017;35(18):2053–2061. doi: 10.1200/jco.2016.71.9716.
- US Bureau of Labor Statistics. News release. 2023 [cited 2023 Mar 7]. https://www.bls.gov/news.release/pdf/empsit.pdf
- HCUP National Inpatient Sample (NIS). Healthcare cost and utilization project (HCUP). Rockville (MD): Agency for Healthcare Research and Quality; 2017 [cited 2023 June 28]. www.hcup-us.ahrq.gov/nisoverview.jsp
- US Food and Drug Administration. POLIVY® US Prescribing Information. 2023 [cited 2023 July 14]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/761121s008lbl.pdf
- US Bureau of Labor Statistics. CPI for all urban customers, medical care in U.S. city average. 2023 [cited 2023 May 05]. https://data.bls.gov/timeseries/CUUR0000SAM
- Genentech, Inc. Data on file.
- Keating SJ, Gu T, Jun MP, et al. Health care resource utilization and total costs of care among patients with diffuse large B cell lymphoma treated with chimeric antigen receptor T cell therapy in the United States. Transplant Cell Ther. 2022;28(7):404.e1–404.e6. doi: 10.1016/j.jtct.2022.03.021.
- Purdum A, Tieu R, Reddy SR, et al. Direct costs associated with relapsed diffuse large B-cell lymphoma therapies. Oncologist. 2019;24(9):1229–1236. doi: 10.1634/theoncologist.2018-0490.
- Felizzi F, Paracha N, Pöhlmann J, et al. Mixture cure models in oncology: a tutorial and practical guidance. Pharmacoecon Open. 2021;5(2):143–155. doi: 10.1007/s41669-021-00260-z.
- Sehn LH, Martelli M, Trněný M, et al. A randomized, open-label, phase III study of obinutuzumab or rituximab plus CHOP in patients with previously untreated diffuse large B-Cell lymphoma: final analysis of GOYA. J Hematol Oncol. 2020;13(1):71. doi: 10.1186/s13045-020-00900-7.
- Arias E, Xu J, Tejada-Vera B, et al. National vital statistics reports: from the centers for disease control and prevention, national center for health statistics, national vital statistics system. Natl Vital Stat Rep. 2022;71(2):1–18.
- Jakobsen LH, Bøgsted M, Brown P, et al. Minimal loss of lifetime for patients with diffuse large B-cell lymphoma in remission and event free 24 months after treatment: a Danish Population-based study. J Clin Oncol. 2017;35(7):778–784. doi: 10.1200/jco.2016.70.0765.
- Drummond MF, Sculpher MJ, Claxton K, et al. Methods for the economic evaluation of health care programmes. 4th ed. Oxford: Oxford University Press; 2015.
- National Institute of Health and Care Excellence (NICE) Guidance. Polatuzumab vedotin in combination for untreated diffuse large B-cell lymphoma. 2023 [cited 2023 Apr 06]. https://www.nice.org.uk/guidance/ta874/resources/polatuzumab-vedotin-in-combination-for-untreated-diffuse-large-bcell-lymphoma-pdf-82613673152197
- Morschhauser F, Feugier P, Flinn IW, et al. A phase 2 study of venetoclax plus R-CHOP as first-line treatment for patients with diffuse large B-cell lymphoma. Blood. 2021;137(5):600–609. doi: 10.1182/blood.2020006578.
- Pickard AS, Law EH, Jiang R, et al. United States valuation of EQ-5D-5L health states using an international protocol. Value Health. 2019;22(8):931–941. doi: 10.1016/j.jval.2019.02.009.
- Fryback DG, Dunham NC, Palta M, et al. US norms for six generic health-related quality-of-life indexes from the national health measurement study. Med Care. 2007;45(12):1162–1170. doi: 10.1097/MLR.0b013e31814848f1.
- Kite Pharma Inc. YESCARTA® U.S. prescribing Information. 2022 [2023 May 05]. https://www.fda.gov/media/108377/download
- Kambhampati S, Saumoy M, Schneider Y, et al. Cost-effectiveness of polatuzumab vedotin combined with chemoimmunotherapy in untreated diffuse large B-cell lymphoma. Blood. 2022;140(25):2697–2708. doi: 10.1182/blood.2022016624.
- Fu YH, Wu SJ, Tan ECH, et al. Cost-effectiveness analysis of frontline treatment with polatuzumab vedotin in diffuse large B-cell lymphoma. Blood. 2022;140(Suppl 1):10808–10809. doi: 10.1182/blood-2022-162980.
- Vijenthira A, Kuruvilla J, Crump M, et al. Cost-effectiveness analysis of frontline polatuzumab-rituximab, cyclophosphamide, doxorubicin, and prednisone and/or second-line chimeric antigen receptor T-cell therapy versus standard of care for treatment of patients with intermediate- to high-risk diffuse large B-cell lymphoma. J Clin Oncol. 2023;41(8):1577–1589. doi: 10.1200/jco.22.00478.
- Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380(1):45–56. doi: 10.1056/NEJMoa1804980.
- National Institute of Health and Care Excellence (NICE) Guidance. Tisagenlecleucel for treating relapsed or refractory diffuse large B-cell lymphoma after 2 or more systemic therapies. 2019 [cited 2023 May 02]. https://www.nice.org.uk/guidance/ta567/resources/tisagenlecleucel-for-treating-relapsed-or-refractory-diffuse-large-bcell-lymphoma-after-2-or-more-systemic-therapies-pdf-82607087377861
- Maurer MJ, Ghesquières H, Jais JP, et al. Event-free survival at 24 months is a robust end point for disease-related outcome in diffuse large B-cell lymphoma treated with immunochemotherapy. J Clin Oncol. 2014;32(10):1066–1073. doi: 10.1200/jco.2013.51.5866.
- Maurer MJ, Habermann TM, Shi Q, et al. Progression-free survival at 24 months (PFS24) and subsequent outcome for patients with diffuse large B-cell lymphoma (DLBCL) enrolled on randomized clinical trials. Ann Oncol. 2018;29(8):1822–1827. doi: 10.1093/annonc/mdy203.
- Shi Q, Schmitz N, Ou FS, et al. Progression-free survival as a surrogate end point for overall survival in first-line diffuse large B-cell lymphoma: an individual patient-level analysis of multiple randomized trials (SEAL). J Clin Oncol. 2018;36(25):2593–2602. doi: 10.1200/jco.2018.77.9124.
- Choe JH, Abdel-Azim H, Padula WV, et al. Cost-effectiveness of axicabtagene ciloleucel and tisagenlecleucel as second-line or later therapy in relapsed or refractory diffuse large B-cell lymphoma. JAMA Netw Open. 2022;5(12):e2245956-e2245956. doi: 10.1001/jamanetworkopen.2022.45956.
- Kelkar AM, Cliff ERS, Jacobson CA, et al. Cost-effectiveness of CD19 chimeric antigen receptor T-cell (CAR-T) therapy versus autologous stem cell transplantation (ASCT) for high-risk diffuse large B-cell lymphoma (DLBCL) in first relapse. J Clin Oncol. 2022;40(16 Suppl):7537–7537. doi: 10.1200/JCO.2022.40.16_suppl.7537.
