Abstract
Aim
The United States Preventive Services Taskforce (USPSTF) recently recommended lowering the age for average-risk colorectal cancer (CRC) screening from 50 to 45 years. While initiating screening at age 45 versus 50 provides a greater opportunity for CRC early detection and prevention, the full profile of benefits, risks, and cost-effectiveness of expanding the screen-eligible population requires further evaluation.
Materials and methods
The costs and clinical outcomes for screening at age 45 for triennial multi-target stool DNA [mt-sDNA], and other non-invasive stool-based modalities (annual fecal immunochemical test [FIT] and annual fecal-occult blood test [FOBT]), were estimated using the validated CRC-AIM microsimulation model over a lifetime horizon. Test sensitivity and specificity inputs were based on 2021 USPSTF modeling analyses; adherence rates were based on published real-world data and the costs of the screening test, follow-up colonoscopies, complications, and CRC care were included. Outcomes are reported from the perspective of a United States payer as clinical, life-years gained (LYG), and incremental cost-effectiveness ratio (ICER); stool-based and follow-up colonoscopy adherence ranges were explored in one-way, probabilistic and threshold analyses.
Results
When compared to initiation of CRC screening at age 45 versus 50, all modalities reduced both the incidence of and mortality from CRC and increased LYG. Initiating CRC screening at age 45 was cost-effective with an ICER of $59,816 and $35,857 per quality-adjusted life year (QALY) for mt-sDNA versus FIT and FOBT, respectively. In the threshold analyses, at equivalent rates to stool-based screening, mt-sDNA was always cost-effective at a willingness-to-pay threshold of $100,000 per QALY versus FIT and FOBT.
Conclusions
Initiating average-risk CRC screening at age 45 instead of age 50 increases the estimated clinical benefit by reducing disease burden while remaining cost-effective. Among stool-based screening modalities, mt-sDNA provides the most clinical benefit in a Commercial and Medicare population.
PLAIN LANGUAGE SUMMARY
Screening for colorectal cancer at an earlier age can provide additional benefits in terms of reducing disease complications and death. This study looked at the occurrence of disease complications and costs related to different types of colorectal cancer screening in 45 vs. 50 year old people. A model that has previously been used to project lifetime costs and disease complications in people receiving colorectal cancer screening was used in this study. We found that beginning screening at age 45 as compared to at age 50 reduced disease complications and death. In people who started screening at age 45, one particular screening type (multitarget stool DNA) was found to provide better economic value to a greater degree relative to other strategies. These findings were consistent even when many inputs into the model were changed over reasonable ranges. Therefore, our study helps show that starting screening in people at age 45 with average risk for developing colorectal cancer is beneficial by reducing disease complications and deaths, and that multitarget stool DNA is the strategy that provides the most benefits while being economically justifiable.
Introduction
Despite being one of the most preventable malignancies, colorectal cancer (CRC) remains the second most common cause of cancer death in the United States (US).Citation1 CRC screening has long been established at being effective in reducing both the incidence of and death from CRCCitation2 by identifying and removing precancerous polyps or altering the course of CRC by detecting invasive neoplasia at earlier, more effectively treatable stage of the disease. While the incidence of CRC in individuals aged 50 years and older has declined steadily since the 1980s, early-onset CRC, defined as CRC before the age of 50 years, has increased in recent years.Citation3 Further, the incidence of CRC observed now among 45-year-olds is similar to that observed among 50-year-olds when CRC screening was first recommended.Citation4 To that end, the US Preventive Services Taskforce (USPSTF) recently recommend lowering the screening age to 45 years of age in an effort to halt the rise in CRC in younger individuals.Citation5
While previous work suggests that the risks associated with screening for CRC at an earlier age are acceptable given the reduced incidence of CRCCitation6, the diversion of scarce resources, and the cost-effectiveness of initiating screening 5 years earlier, need to be further evaluated.Citation7 This is particularly true for non-invasive CRC screening strategies, given that patients have demonstrated a preference for non-invasive screening methods when afforded an opportunity to choose among different screening modalities.Citation8 Further, the effectiveness of stool based screening can be challenged by variable rates of adherence to follow-up colonoscopy.Citation9,Citation10 Some historic modeling studies have assumed perfect adherence rates, which limits the accuracy of the reported outcomes, as a benchmark of perfect adherence is not achieved in clinical practice. Among eligible individuals aged ≥45 years, only 59% are up to date with CRC screeningCitation11 and African Americans and those with lower socio-economic status are less able to access CRC screening.Citation12 While most CRC screening in the US is done by colonoscopy, the use of other less costly and more convenient alternatives has been increasing, notably in adults without risk factors. In light of the lowered age group and differential costs for guideline-recommended non-invasive CRC screening, the benefit and risk, and the cost-effectiveness of stool-based CRC screening modalities beginning at age 45 was assessed from the perspective of a United States (US) payer, using test-specific data, and real-world adherence rates.
Methods
Overview of model
The Colorectal Cancer and Adenoma Incidence and Mortality (CRC-AIM) microsimulation model was used to simulate an average-risk US cohort of 1,000,000 patients, free of a CRC diagnosis at age 40. Briefly, the model includes a natural history component, which follows the course of CRC using an adenoma-carcinoma sequence (), along with a CRC screening test component (Figure S1), which incorporates the sensitivity and specificity of each modality, the frequency of each test being used, adherence, and screening interval. The screening can benefit the population with CRC lesions with either removing adenoma and preventing the cancer or detecting the cancer in an earlier stage while more treatable.Citation14 Validation of the CRC-AIM model has been described previously in detailCitation14,Citation15 and has recently been calibrated (see the Supplementary for details).Citation13
Figure 1. Overview of CRC-AIM microsimulation model (Adapted from Vahdat et al.Citation13) Abbreviations. AJCC, American Joint Committee on Cancer; CRC, colorectal cancer; CRC-AIM, Colorectal Cancer-Adenoma Incidence and Mortality model.
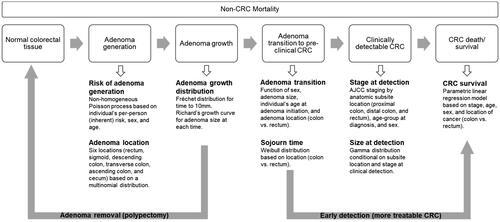
Individuals were screened from age 45 or age 50 until 75. Screening modalities explored in this analysis included guideline-recommended stool-based tests: either the annual fecal immunochemical test (FIT), the annual fecal-occult blood test (FOBT) or the triennial multi-target stool DNA test (mt-sDNA). For all positive stool-based tests, a follow-up colonoscopy is required; if patients are not adherent to follow-up colonoscopy, they were assumed to be non-adherent until they become symptomatic.
Inputs
Screening strategy performance parameters were based on the 2021 USPSTF modeling approach (Table S1).Citation6 The reported performance and the CISNET-CRC model outputs for all modalities are based on the results of one-time test performance, irrespective of the modality and screening interval. Complications associated with colonoscopy included gastrointestinal, serious gastrointestinal events and cardiovascular.Citation16 To derive cost inputs for CRC and complications, costs were first sourced for Medicare and were then inflated to 2022 dollars using the Medical Care Services component of the Consumer Price index.Citation17 To obtain commercial costs for treating CRC and managing complications, the Medicare-to-Commercial ratio of 1.38 was used.Citation18 Commercial costs were used for ages 45–65 and Medicare costs for ages 65 and over (Table S2).Citation16,Citation19–21 For lab-based screening tests, Protecting Access to Medicare Act (PAMA) rates were usedCitation20; colonoscopy costs were sourced from the literature.Citation22 Baseline utility inputs were included and were based on general US population utility values, adjusted for age (Table S3).Citation23 Utility loss for all colonoscopies and complications from colonoscopy were included as utility decrements per event, and were applied to baseline utility values.Citation24 Utility loss due to CRC was stratified by the level of CRC care, and stage of CRC; these utility decrements were applied on a per patient per year basis.Citation24
Analysis
The primary cost-effectiveness analysis compared the benefit of screening at age 45 versus screening at age 50 for guideline recommended stool-based tests.Citation5,Citation25–27 This was done by calculating the costs and outcomes for each stool-based screening test for ages 45–75 and for ages 50–75 and comparing the two. The secondary analysis compared the cost-effectiveness of guideline recommended stool-based screening tests when initiating screening at age 45.
Reported real-world adherence rates were used for both initial screening and follow-up colonoscopy after a positive stool-based screening test (where relevant) (). A sensitivity analysis for both the primary (Table S4) and secondary (Table S5) analysis was done using perfect adherence rates. Fixed adherence rates were assumed over time and similar follow-up colonoscopy rates were used for commercially insured and Medicare beneficiaries; given evolving data on follow-up colonoscopy rates across screening modalities, equal adherence to follow-up colonoscopy was assumed between FIT and FOBT. Adherence to surveillance and symptomatic colonoscopies was assumed to be 100%.
Table 1. Adherence parameters used in the model.
The primary outcome was the clinical benefit of CRC screening including incidence reduction, mortality reduction, and life years gained per 1,000 patients. Secondary outcomes included costs and quality-adjusted life years (QALYs), as well as the incremental cost-effectiveness ratio (ICER). The ICER was calculated for mt-sDNA versus other guideline recommended stool-based screening modalities (FIT or FOBT).
Threshold analysis
Threshold analyses were undertaken to explore the robustness of the adherence inputs on the ICER. In the first threshold analysis, stool-based screening test adherence rates were varied from 5 – 100% while adherence to follow-up colonoscopy was held constant at baseline real-world rates. In the second threshold analysis, follow-up colonoscopy adherence rates were varied from 5–100% while adherence to stool-based screening test adherence were held constant at baseline real-world rates.
Sensitivity analyses
One-way deterministic sensitivity analyses where adherence, costs and utilities were varied +/− 10% of their base case values were conducted. Probabilistic sensitivity analyses, where all parameters were varied simultaneously over 500 iterations, were also conducted.
Results
Screening from age 45 versus age 50
Lowering the screening age from age 50 to age 45 reduced CRC incidence and mortality across all modalities (). Screening at age 45 versus age 50 resulted in an additional 8 (FOBT) to 19 (mt-sDNA) life years gained (LYG) ().
Figure 2. Disease burden of lowering the screening to age 45 from 50, by stool-based screening test. Abbreviation. LYG, life years gained.
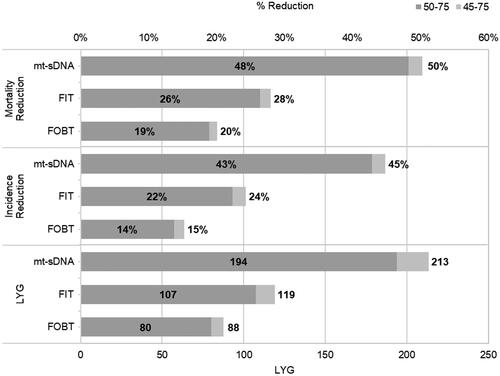
Clinical, cost, and utility outcomes of screening from age 45 versus age 50 are presented in . While screening costs were higher when screening from age 45 across all modalities due to an increased number of stool tests and subsequent follow-up colonoscopies, lowering the screening age reduced the cost associated with CRC (). Total QALYs were higher when initiating screening at age 45 versus age 50.
Table 2. Discounted clinical, cost, and utility outcomes of stool-based screening tests with real-world adherence rates.
Cost-effectiveness of stool-based screening modalities from age 45
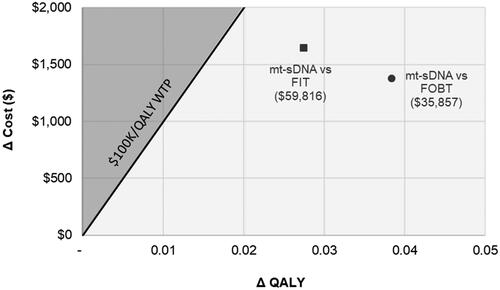
When assessing the cost-effectiveness of stool-based screening modalities when initiating screening at age 45, LYG per 1,000 patients were greatest for mt-sDNA (213) and lowest for FOBT (88); the greatest reduction in incidence and mortality from CRC were also observed with mt-sDNA (). At a WTP threshold of $100,000/QALY, mt-sDNA was cost effective versus either FIT ($59,816/QALY) or FOBT ($35,857) () due to lower CRC related costs in light of diagnosing earlier stage CRC.
Figure 3. Incremental cost-effectiveness plane of screening at age 45 under real-world reported adherence. Grey shading indicates that mt-sDNA is not cost-effective. Abbreviations. FIT, fecal immunochemical test; FOBT, fecal occult blood test; mt-sDNA, multi-target stool DNA; QALY, quality-adjusted life-years; WTP, willingness-to-pay
Sensitivity analysis
One-way sensitivity analyses when comparing stool-based screening modalities found that adherence to follow-up colonoscopy for mt-sDNA had the greatest impact on the ICER relative to both FIT and FOBT (Figure S2). The probabilistic analysis found that mt-sDNA was cost-effective at a WTP of $100K/QALY in 98% of the runs versus FIT and 100% of the runs versus FOBT (Figure S3).
Threshold analysis of variable adherence initiating screening at age 45
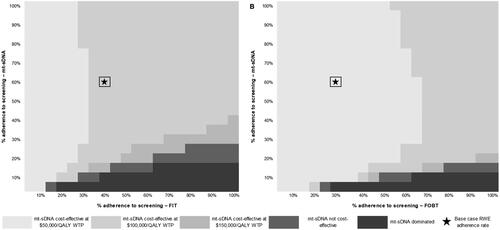
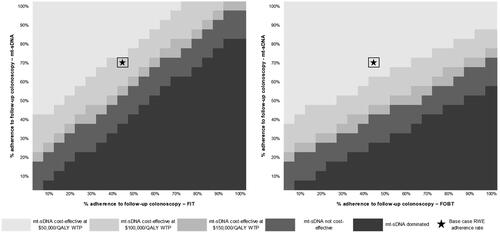
When adherence to stool-based screening was varied and adherence to follow-up colonoscopy was fixed at reported real-world values, mt-sDNA had the highest total costs at real-world performance (). However, mt-sDNA was cost-effective at a WTP threshold of $100,000/QALY versus FIT with real-world follow-up colonoscopy parameters (). Furthermore, mt-sDNA was cost-effective if adherence for mt-sDNA is greater than 40%, irrespective of the adherence for FIT (). mt-sDNA was always cost-effective at a WTP threshold of $100,000/QALY versus FOBT if adherence for mt-sDNA was greater than 20%, irrespective of the adherence for FOBT ().
Figure 4. Cost-effectiveness heatmap of mt-sDNA versus FIT (left) and FOBT (right) when varying screening test rates (follow-up colonoscopy adherence fixed at reported real-world values). Abbreviations. FIT, fecal immunochemical test; FOBT, fecal occult blood test; mt-sDNA, multi-target stool DNA; QALY, quality-adjusted life year; RWE, real-world evidence; WTP, willingness to pay.
When adherence to stool-based screening is fixed at real-world values and adherence to follow-up colonoscopy is varied, mt-sDNA was cost-effective at a WTP threshold of $100,000/QALY versus FIT if adherence to follow-up colonoscopy following mt-sDNA is 20% more than adherence to follow-up colonoscopy following FIT (). At a WTP threshold of $100,000/QALY, mt-sDNA was always cost-effective versus FOBT for equivalent or greater follow-up colonoscopy adherence rates up to WTP thresholds of $100,000/QALY ().
Figure 5. Cost-effectiveness heatmap of mt-sDNA versus FIT (left) and FOBT (right) when varying follow-up colonoscopy adherence rates (screening test rates fixed at reported real-world values). Abbreviations. FIT, fecal immunochemical test; FOBT, fecal occult blood test; mt-sDNA, multi-target stool DNA; QALY, quality-adjusted life year; RWE, real-world evidence; WTP, willingness to pay.
Discussion
The results observed in this modeling analysis support the cost-effectiveness of lowering the age for initiating average-risk CRC screening to 45 years, irrespective of the modality used to screen. Initiating screening at age 45 versus 50 results in increased clinical benefit (increased LYG and greater reduction in the incidence and mortality from CRC) while remaining cost-effective. Further, when exploring various stool-based screening modalities under real-world adherence rates among commercially insured and Medicare beneficiaries, mt-sDNA results in the highest number of LYG and is cost-effective versus FIT and FOBT.
Our results are similar to other models that have considered lowering the age of initiation of CRC screening, despite the use of different clinical and economic assumptions and inputs. Ladabaum et al.Citation30 found that initiating screening at age 45 versus age 50 with FIT averted CRC cases and deaths from CRC. Fisher et al.Citation31 found similar trends of improvement in clinical outcomes when lowering the screening age, as well as an increased magnitude of benefit when screening with mt-sDNA versus FIT. Using a microsimulation model, Peterse et al.Citation32 explored the optimal timing of initiating CRC screening with colonoscopy and/or stool-based screening modalities. When perfect adherence is assumed, the model-recommended strategy was screening every 10 years with colonoscopy from ages 45–75 years and FIT screening annually from ages 45–75 years, resulting in an efficiency ratio of 32 and 14 incremental colonoscopies per LYG, respectively.Citation32
Maximizing the allocation of healthcare resources is a key consideration in the provision of population-based prevention strategies. While lowering the screening age can increase the clinical benefit to patients, concerns regarding the use of scarce resources for screening patients at 45 have been raised. Ladabaum et al. stated that the resources used to screen for CRC at age 45 may be better placed at increasing adherence in those 50 and over.Citation30 However, the incidence of CRC has increased by nearly 50% at age 50 when compared with age 49, providing evidence that many previously undiagnosed cancers are detected when screening previously started at age 50.Citation33 There is no evidence that CRC in 45-year-olds is different to that of 50-year-olds, thus, knowledge gained to date on CRC can be safely extended from age 50 to 45, bypassing several years of clinical trials in the lower age group at a substantial cost.Citation4
Data from these analyses should be interpreted in the context of the assumptions. This analysis only considered non-invasive stool-based CRC screening modalities; screening with invasive tests such as colonoscopy every 10 years and sigmoidoscopy every 5 years has been determined to be effective from age 45.Citation30 While colonoscopy is sometimes regarded as the gold-standard for screening, the low capacity for colonoscopies coupled with low adherence has resulted in leveraging the use of non-invasive tests, as these latter only refer a fraction of the patient population for a follow-up colonoscopy. Another limitation is that the estimates in this analysis are based on the perspective of a Commercial followed by Medicare FFS payer and may not be reflective of the current landscape, where a majority of older patients have Medicare Advantage. However, based on a recent analysis of the cost-effectiveness of stool-based screening tests in a Medicare Advantage populationCitation34, we would expect findings to be similar. Based on available published data, the modeled reported real-world adherence rates used in this study are from those aged 50 and over and may not be reflective of adherence rates in those younger than 50. Given the recent recommendations by the USPSTF to lower the screening age to 45, data on adherence is not yet available for this age group. Interestingly, a recent survey among unscreened individuals 40 years of age and older demonstrates a preference for stool-based modalities.Citation35 As the use of reported real-world adherence rates impacts the results, long-term data is needed to substantiate the findings of this analysis. However, the threshold analyses conducted allow the determination of the cost-effectiveness endpoint across a potential range of adherence rates, for both the screening tests and follow-up colonoscopies, across all modalities. Further, the adherence rates used in this model are cross-sectional. The impact of intermittent and longitudinal adherence, and factors that impact adherence over time, on the cost-effectiveness of initiating screening at the age of 45 should be analyzed in detail in future studies. This model only estimated the cost-effectiveness of non-invasive stool tests; the cost-effectiveness of initiating screening at the age of 45 with invasive CRC screening modalities was not explored. Finally, the reported real-world adherence rates used in the model were not derived from the same source. While the adherence rates observed with annual FIT and FOBT are lower than mt-sDNA, it should be highlighted that mt-sDNA includes patient navigation services with every order (at no additional cost), which likely contribute to the higher adherence rates demonstrated in comparison to other stool-based screening tests.Citation9 While limitations may exist with the use of reported real-world adherence rates, it remains important that cost-effectiveness analyses base their models on these rates, given that adherence to CRC screening is much less than 100% in clinical practice, falling below the target goal of 80% in every community.Citation36
This study demonstrates that lowering the age to initiate CRC from 50 to 45 years increases the clinical benefit while remaining cost-effective, under real-world adherence rates.
Transparency
Author contributions
AMF, PL, BO and DL participated in the conception of the work. All authors participated in the analysis and interpretation of the data. LB drafted the article, with critical revisions provided by all authors. All authors approved the version of the manuscript to be published.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Previous presentations
Not applicable.
Supplemental Material
Download MS Word (373 KB)Supplemental Material
Download MS Word (623.4 KB)Acknowledgements
We would like to acknowledge Jing Voon Chen and Sushanth Jeyakumar for their contributions to the project.
Declaration of funding
Funding was received from Exact Sciences.
Declaration of financial/other interests
LB, RS, and NJS are employees of the Maple Health Group, which received consulting fees from Exact Sciences. CE, VV, ABO, and PL are employees and stock holders of Exact Sciences. DE has a professional service agreement with Exact Sciences serving as an independent contractor to provide guidance on study design and analysis. JK is an inventor of Mayo Clinic intellectual property under licence to Exact Sciences and may receive royalties paid to Mayo Clinic; he participates in a sponsored research agreement with Exact Sciences
References
- American Cancer Society. Key statistics for colorectal cancer. 2021. https://www.cancer.org/cancer/colon-rectal-cancer/about/key-statistics.html
- Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116(3):544–573. doi: 10.1002/cncr.24760.
- Siegel RL, Fedewa SA, Anderson WF, et al. Colorectal cancer incidence patterns in the United States, 1974–2013. J Natl Cancer Inst. 2017;109(8):djw322.
- Wender RC. Should routine screening for colorectal cancer start at 45 years of age? Yes: lowering the starting age is a settled issue. Am Fam Physician. 2022;105(2):120–121.
- Davidson KW, Barry MJ, Mangione CM, et al. Screening for colorectal cancer: US preventive services task force recommendation statement. JAMA. 2021;325(19):1965–1977. doi: 10.1001/jama.2021.6238.
- Knudsen AB, Rutter CM, Peterse EFP, et al. Colorectal cancer screening: an updated modeling study for the US preventive services task force. JAMA. 2021;325(19):1998–2011. doi: 10.1001/jama.2021.5746.
- Liang PS, Shaukat A. Assessing the impact of lowering the colorectal cancer screening age to 45 years. Lancet Gastroenterol Hepatol. 2020;5(6):523–524. doi: 10.1016/S2468-1253(20)30054-6.
- Inadomi JM, Vijan S, Janz NK, et al. Adherence to colorectal cancer screening: a randomized clinical trial of competing strategies. Arch Intern Med. 2012;172(7):575–582. doi: 10.1001/archinternmed.2012.332.
- Weiser E, Parks PD, Swartz RK, et al. Cross-sectional adherence with the multi-target stool DNA test for colorectal cancer screening: real-world data from a large cohort of older adults. J Med Screen. 2021;28(1):18–24. doi: 10.1177/0969141320903756.
- Miller-Wilson LA, Rutten LJF, Van Thomme J, et al. Cross-sectional adherence with the multi-target stool DNA test for colorectal cancer screening in a large, nationally insured cohort. Int J Colorectal Dis. 2021;36(11):2471–2480. doi: 10.1007/s00384-021-03956-0.
- Centers for Disease Control and Prevention. National Health Interview Survey (NHIS) 2021. 2023. Available from: https://www.cdc.gov/nchs/nhis/index.htm
- Warren Andersen S, Blot WJ, Lipworth L, et al. Association of race and socioeconomic status with colorectal cancer screening, colorectal cancer risk, and mortality in Southern US adults. JAMA Netw Open. 2019;2(12):e1917995-e. doi: 10.1001/jamanetworkopen.2019.17995.
- Vahdat V, Alagoz O, Chen J, et al. Calibration and validation of colorectal cancer and adenomia incidence and mortality (CRC-AIM) microsimulation model using deep neural networks. Med Decis Making. 2023;43(6):719–736. doi: 10.1177/0272989X231184175.
- Piscitello A, Saoud L, Fendrick AM, et al. Estimating the impact of differential adherence on the comparative effectiveness of stool-based colorectal cancer screening using the CRC-AIM microsimulation model. PLOS One. 2020;15(12):e0244431. doi: 10.1371/journal.pone.0244431.
- Piscitello A, Saoud L, Matney M, et al. Description and validation of the colorectal cancer and adenoma incidence & mortality (CRC-AIM) microsimulation model. bioRxiv. 2020.
- Hathway JM, Miller-Wilson LA, Jensen IS, et al. Projecting total costs and health consequences of increasing mt-sDNA utilization for colorectal cancer screening from the payer and integrated delivery network perspectives. J Med Econ. 2020;23(6):581–592. doi: 10.1080/13696998.2020.1730123.
- Consumer Price Index. Medical care services. 2021. https://beta.bls.gov/dataViewer/view/timeseries/CUSR0000SAM2
- MedPAC. Report to the congress: medicare payment policy. 2022. https://www.medpac.gov/document/march-2022-report-to-the-congress-medicare-payment-policy/
- Fisher DA, Princic N, Miller-Wilson L-A, et al. Healthcare costs of colorectal cancer screening and events following colonoscopy among commercially insured average-risk adults in the United States. Curr Med Res Opin. 2022;38(3):427–434. doi: 10.1080/03007995.2021.2015157.
- Centers for Medicare & Medicaid Services. Clinical laboratory fee schedule. 2022. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/PAMA-Regulations#where_to_find
- Mariotto AB, Yabroff KR, Shao Y, et al. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst. 2011;103(2):117–128. doi: 10.1093/jnci/djq495.
- Pyenson B, Scammell C, Broulette J. Costs and repeat rates associated with colonoscopy observed in medical claims for commercial and medicare populations. BMC Health Serv Res. 2014;14(1):92. doi: 10.1186/1472-6963-14-92.
- Szende A, Janssen B, Cabases J, editors. Self-reported population health: an international perspective based on EQ-5D. Dordrecht (NL): Springer; 2014.
- Goede SL, Rabeneck L, van Ballegooijen M, et al. Harms, benefits and costs of fecal immunochemical testing versus guaiac fecal occult blood testing for colorectal cancer screening. PLOS One. 2017;12(3):e0172864. doi: 10.1371/journal.pone.0172864.
- Wolf AMD, Fontham ETH, Church TR, et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin. 2018;68(4):250–281. doi: 10.3322/caac.21457.
- Shaukat A, Kahi CJ, Burke CA, et al. ACG clinical guidelines: colorectal cancer screening 2021. Am J Gastroenterol. 2021;116(3):458–479. doi: 10.14309/ajg.0000000000001122.
- Patel SG, May FP, Anderson JC, et al. Updates on age to start and stop colorectal cancer screening: recommendations from the U.S. multi-society task force on colorectal cancer. Gastroenterology. 2022;162(1):285–299. doi: 10.1053/j.gastro.2021.10.007.
- Akram A, Juang D, Bustamante R, et al. Replacing the guaiac fecal occult blood test with the fecal immunochemical test increases proportion of individuals screened in a large healthcare setting. Clin Gastroenterol Hepatol. 2017;15(8):1265.e1–1270.e1. doi: 10.1016/j.cgh.2017.01.025.
- Cooper GS, Grimes A, Werner J, et al. Barriers to follow-up colonoscopy after positive FIT or multitarget stool DNA testing. J Am Board Fam Med. 2021;34(1):61–69. doi: 10.3122/jabfm.2021.01.200345.
- Ladabaum U, Mannalithara A, Meester RGS, et al. Cost-effectiveness and national effects of initiating colorectal cancer screening for average-risk persons at age 45 years instead of 50 years. Gastroenterology. 2019;157(1):137–148. doi: 10.1053/j.gastro.2019.03.023.
- Fisher DA, Saoud L, Finney Rutten LJ, et al. Lowering the colorectal cancer screening age improves predicted outcomes in a microsimulation model. Curr Med Res Opin. 2021;37(6):1005–1010. doi: 10.1080/03007995.2021.1908244.
- Peterse EFP, Meester RGS, Siegel RL, et al. The impact of the rising colorectal cancer incidence in young adults on the optimal age to start screening: microsimulation analysis I to inform the American cancer society colorectal cancer screening guideline. Cancer. 2018;124(14):2964–2973. doi: 10.1002/cncr.31543.
- Abualkhair WH, Zhou M, Ahnen D, et al. Trends in incidence of Early-Onset colorectal cancer in the United States among those approaching screening age. JAMA Netw Open. 2020;3(1):e1920407. doi: 10.1001/jamanetworkopen.2019.20407.
- Bhatt J, Chen J, Vahdat V, et al. Cost-Effectiveness of mt-sDNA versus mailed FIT outreach for medicare advantage enrollees using the CRC-AIM microsimulation model. J Community Med Public Health. 2022;6:266.
- Makaroff KE, Shergill J, Lauzon M, et al. Patient preferences for colorectal cancer screening tests in light of lowering the screening age to 45 years. Clin Gastroenterol Hepatol. 2023;21(2):520–531.e10. doi: 10.1016/j.cgh.2022.07.012.
- National Colorectal Cancer Roundtable. 80% in every community strategic plan. [cited 2023]. Available from: https://nccrt.org/about/how-we-work/80-in-every-community-strategic-plan/.
