Abstract
Aim
Our study aimed to evaluate the cost-effectiveness of the chimeric antigen receptor (CAR) T-cell therapy, axicabtagene ciloleucel (axi-cel), compared to standard of care (SOC) in Sweden for second-line (2L) treatment of adult transplant-intended diffuse large B-cell lymphoma (DLBCL) patients who relapse within 12 months from completion of, or are refractory to (early r/r), first-line (1L) chemoimmunotherapy.
Methods
Cost-effectiveness was assessed using a three-state partitioned survival model. Mixture cure models were used to extrapolate time-to-event data from the ZUMA-7 trial (NCT03391466) beyond the observational period. Sensitivity and scenario analyses were performed to test the robustness of the base case results, including an analysis that assumed no switching to off-protocol CAR T-cell therapy in subsequent lines in the SOC arm.
Results
The model estimated an incremental cost-effectiveness ratio (ICER) of SEK 534,704 (EUR 50,303) per quality-adjusted life year (QALY) gained over a lifetime horizon of 50 years, with an incremental cost of SEK 812,944 (EUR 76,479) and incremental QALY of 1.52 for axi-cel compared with SOC. The probabilistic sensitivity analysis showed that axi-cel was cost-effective in 73% of the simulations when assuming a willingness-to-pay threshold of SEK 1,000,000 (EUR 94,077) per QALY. The ICER was SEK 694,351 (EUR 65,313) in the scenario analysis where the costs and effects of treatment switching were not included.
Conclusion
2L treatment with axi-cel in transplant-intended DLBCL patients with early r/r after completing 1L chemoimmunotherapy was cost-effective compared to SOC in a Swedish setting. Administering axi-cel in 2L is cost-effective as it enhances the possibility of curing more patients, resulting in not just a survival advantage, but also a reduction in the burden on quality of life and cost of subsequent therapy. This will be advantageous to both patients and society.
1. Introduction
Diffuse large B-cell lymphoma (DLBCL) is an aggressive and common subtype of lymphoma with an annual incidence of approximately 700 people in SwedenCitation1. The primary treatment in the first-line (1L) setting comprises chemoimmunotherapy consisting of rituximab and anthracycline-based chemotherapy (normally R-CHOP), which cures three in four patientsCitation2. For the quarter of patients who are not cured with 1L therapy, prognosis is determined by a range of factors, including response to 1L chemoimmunotherapy and timing of relapseCitation2.
Patients who relapse within 12 months from completion of, or are refractory to (early r/r), 1L chemoimmunotherapy have particularly poor survival outcomes. A Swedish study of 736 patients with relapsed or refractory (r/r) DLBCL treated during the period 2007–2018 found that patients with primary refractory disease, which was defined as stable or progressive disease as best response to primary therapy (28% of 736), had a median overall survival (OS) of 4.4 months and a two-year OS of 14%Citation2. Early relapse, which was defined as relapse within 12 months of primary diagnosis (62% of 736), was strongly associated with the selection of less intensive treatments, and the two-year OS was less than 20% in this patient group, compared to 55% for patients relapsing after more than two yearsCitation2.
For patients with r/r disease after 1L chemoimmunotherapy, the standard of care (SOC) is platinum-based chemoimmunotherapy followed by consolidation with high-dose therapy (HDT) and autologous stem cell transplantation (ASCT) for patients who are treated with curative intentCitation2,Citation3. However, among patients with early relapse after 1L chemoimmunotherapy, fewer than two-thirds of Swedish patients up to 70 years of age meet these criteria and initiate SOC therapy (i.e. are transplant-intended), and among those who start less than one in three complete ASCTCitation2. Overall, 22% of Swedish patients up to 70 years of age with early relapse completed ASCT compared to 57% for those with late relapseCitation2.
Axicabtagene ciloleucel (axi-cel) is a CAR T-cell therapy that received market authorization from the European Medicines Agency (EMA) in 2022 indicated for the treatment of adult patients with DLBCL and high-grade B-cell lymphoma with early r/r after completing 1L chemoimmunotherapy. Approval was granted based on results from the pivotal phase 3 trial ZUMA-7 (NCT03391466). The ZUMA-7 trial evaluated the efficacy and safety of axi-cel versus SOC (salvage chemoimmunotherapy with HDT followed by ASCT for responders) in transplant-intended DLBCL patients with early r/r after completing 1L chemoimmunotherapyCitation4. At a median follow-up of 24.9 months, the primary analysis found that axi-cel demonstrated a 60% improvement in centrally assessed event-free survival (EFS) compared to SOC (hazard ratio (HR): 0.40; 95% CI: 0.31, 0.51; p< .0001) Citation4. In an interim analysis (including survival data on eight patients that were lost to follow-up at the data cut-off for the primary analysis), the median OS was not reached in the axi-cel group and was 25.7 months in the SOC group (HR: 0.71; 95% CI: 0.52, 0.97)Citation5. Off-protocol treatment switching occurred in the ZUMA-7 trial and 56% of patients, i.e. 100 out of the 179 patients in the SOC arm received subsequent cellular therapyCitation4. A prespecified sensitivity analysis was performed in ZUMA-7, which adjusted OS for the confounding effect of treatment switching using the rank-preserving structural failure time model (RPSFTM) or the inverse probability of censoring weights (IPCW) methodCitation6. These analyses showed a greater OS benefit in favour of axi-celCitation4.
Axi-cel is currently recommended and funded in Denmark, Finland and Norway for the treatment of 2L DLBCL, while a decision is pending in Sweden. In third-line or later (3L+) DLBCL, axi-cel is recommended and funded in Norway, Finland and Sweden based on data from the ZUMA-1 trial. 5-year follow-up data from the ZUMA-1 trial showed that 43% of patients treated with axi-cel were alive after five years and a 5-year EFS rate of 30.3% (95% CI: 21.5%, 39.6%) was estimatedCitation7. 58% of the patients achieved a complete response and, in these patients, the 5-year OS rate was 64.4% (95% CI: 50.8%, 75.1%)Citation7. These findings support axi-cel as a curative treatment option for many DLBCL patientsCitation7.
Our study is the first study in the Nordic countries to evaluate the cost-effectiveness of CAR T-cell therapy using randomized clinical trial evidence, a gap that has been highlighted by all Nordic HTA bodiesCitation8–11. In addition, little is known about the cost-effectiveness of treating patients with CAR T-cell therapy when moving to an earlier line of treatment (from 3L+ to 2L). Therefore, the objective of this study was to evaluate the cost-effectiveness of axi-cel compared to the SOC and the long-term economic implications of introducing axi-cel in Sweden for the treatment of transplant-intended DLBCL patients with early r/r after completing 1L chemoimmunotherapy.
2. Methods
2.1. Model overview
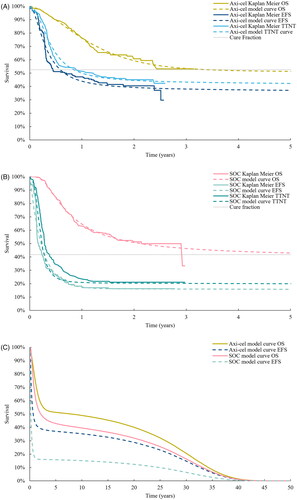
A three-state partitioned survival model was developed in Microsoft Excel and populated with clinical data from ZUMA-7 (NCT03391466) to reflect the target patient population of transplant-intended DLBCL patients with early r/r after completing 1L chemoimmunotherapy. The model simulated the proportions of a theoretical cohort transitioning between each of the three mutually exclusive health states (event-free, post-event and death) over time and assigned corresponding costs and utilities. The patient cohort entered the model in an event-free state. If patients experienced an event, defined as disease progression or initiation of the next line of therapy, they transitioned to the post-event state. To estimate treatment-related and adverse event (AE) costs, sub-states were included in the event-free and post-event health states to reflect whether patients were on or off treatment. illustrates the model structure. The proportion of patients in each health state at a given point in time was determined based on independently fitted parametric extrapolations of centrally-assessed EFS, OS and time-to-next treatment (TTNT), as illustrated in .
Figure 1. Model structure. The circles represent mutually exclusive health states, and the arrows represent transitions between or within states.
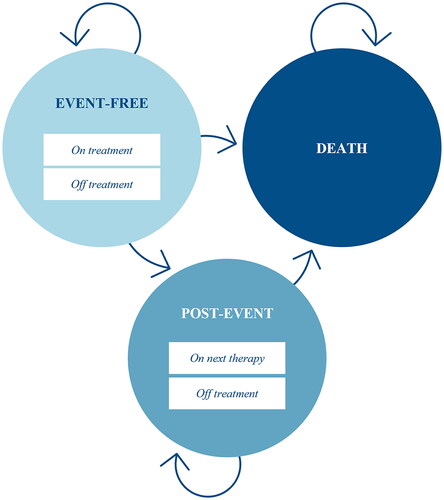
Figure 2. Health state occupancy. Implementation method of the partitioned survival model estimating health state occupancy through the disaggregation of sequential event curves (illustrative example). The health state occupancy in the event-free and death health states were derived from the centrally-assessed event-free survival (EFS) curve and overall survival (OS) curve, respectively. The health state occupancy in the post-event health state was calculated as the difference between the OS curve and the EFS curve (the proportion of patients who are still alive but are no longer event-free). The post-event subsequent therapy was determined by the time-to-next therapy (TTNT) curve.
Based on previous studies investigating mortality related to DLBCLCitation12,Citation13, it was assumed that patients who remain event-free after five years are considered long-term responders with resource utilization and utility equivalent to the general population. Furthermore, studies have shown that these patients have a similar or slightly higher mortality rate than the general populationCitation14,Citation15. Thus, a standardized mortality ratio (SMR) of 1.09 was applied to long-term survivors in the model based on the findings by Maurer et al.Citation15.
The analysis was conducted from a Swedish healthcare perspective with costs and health effects estimated over a lifetime horizon of 50 years and discounted by 3% per annum. The model had a monthly cycle length and half-cycle corrections were applied to account for within-cycle transitions. Finally, the model estimated total costs, life years (LYs), and quality-adjusted life years (QALYs) as well as the incremental cost-effectiveness ratio (ICER).
2.2. Time-to-event outcomes
Time-to-event data from the ZUMA-7 intention to treat (ITT) population (data cut-off date: 18 March 2021) was used to populate efficacy inputs in the model. A prior validation study using long-term data from ZUMA-1 determined that Mixture Cure Models (MCMs) were the most accurate at predicting long-term survival for DLBCL patients treated with axi-cel after at least two prior lines of therapyCitation16. Hence, MCMs were used to extrapolate time-to-event data (centrally-assessed EFS, OS, and TTNT) from the ZUMA-7 trial beyond the observational period. Statistical fit and clinical plausibilityCitation17 were used to determine the most appropriate extrapolations for the base case analysis. Independent survival models were fitted to individual patient level data from ZUMA-7 (diagnostic plots can be found in Supplementary Figure S4 and Figure S5). The Kaplan-Meier and the extrapolated survival curves applied in the model base case are illustrated in . More details on the extrapolation of time-to-event data can be found in the Supplementary Materials.
2.3. Health-related quality of life
Effects on health-related quality of life (HRQoL) was modelled using state-specific utility values derived from EuroQoL five-dimensions five-levels (EQ-5D-5L) instrument responses collected in ZUMA-7 (event-free state) and ZUMA-1 (post-event state)Citation18,Citation19. Health state utility values were age-adjusted based on Swedish general population utilities by Burström et al.Citation20 who used the UK EQ-5D index tariff by DolanCitation21.
2.3.1. Event-free utilities
In ZUMA-7, patient-reported outcomes (PROs) were routinely collected during the first 24 months after randomization. 82% (296 of 359) of patients enrolled in ZUMA-7 had reported at least two EQ-5D-5L instrument responses. EQ-5D-5L responses were cross-walked to EQ-5D-3L index values using the van Hout algorithmCitation22. The algorithm uses the UK value set developed by DolanCitation21, in line with TLV standard practiceCitation23. A mixed model with repeated measures (MMRM) was used to estimate state-specific utilities, accounting for multiple observations per patient.
The utility value for the event-free state was disaggregated into the periods on-treatment and off-treatment. This approach has been used in previous models for other CAR T-cell therapies in the 3L+ settingCitation24,Citation25. The on-treatment utility was applied for the first month for axi-cel and the first three months for the SOC. Patients who were still event-free after five years were reverted to the age- and gender-matched Swedish general population utilitiesCitation20. Utility decrements due to AEs were not applied in the model, as the potential influence of AEs was assumed to be captured by the event-free on-treatment utility values.
2.3.2. Post-event utilities
The collection of PROs post-event was not mandated in the ZUMA-7 trial, and data collection after switching to subsequent therapy did not usually include PRO reportsCitation26. Although some sites continued to collect PROs after EFS events, these comprised a minority of observations (less than 11% of total PRO observations). Because of these limitations, the post-event health state utility value was informed by the ZUMA-1 event-free utility valueCitation27. Utility values for patients treated with axi-cel in 3L+ represent a similar patient group, e.g. due to the high amount of subsequent CAR T-cell therapy use post-event in ZUMA-7. Finally, we made the conservative assumption of not including utility decrements due to subsequent therapy use.
2.4. Costs
Treatment specific costs (i.e. costs related to axi-cel treatment and treatment with SOC including subsequent treatments and management of AEs) and state-specific costs (e.g. costs related to monitoring) were included in the model. provides an overview of the resource use and unit costs applied in the model. All costs were reported in 2022 Swedish krona (SEK).
Table 1. Overview of model input values and sources.
2.4.1. Treatment costs
The cost of 2L treatment with axi-cel included the costs of leukapheresis, conditioning chemotherapy, CAR T-cell infusion and hospitalization following infusion in addition to the drug acquisition cost for axi-cel. The share of patients in the ZUMA-7 trial who underwent leukapheresis, received conditioning chemotherapy and completed the axi-cel infusion are presented in . The cost of 2L treatment with SOC included salvage chemoimmunotherapy followed by HDT and ASCT for patients with complete or partial response. In ZUMA-7, salvage chemoimmunotherapy consisted of R-ICE, R-GDP, R-DHAP and R-ESHAP regimens. According to the Swedish clinical guidelines for treatment of DLBCLCitation3, R-ESHAP is not used as salvage chemoimmunotherapy in Sweden, thus, patients receiving R-ESHAP in the trial were uniformly allocated to the other regimens in the model. The share of patients who received salvage chemoimmunotherapy (R-ICE, R-GDP and R-DHAP), stem cell harvest, HDT and who completed ASCT in the model are presented in . A weighted average cost of subsequent therapy (3L+) was applied in the model when a patient experienced a TTNT event. The proportion of patients receiving each subsequent therapy was collected in ZUMA-7 and applied in the model. Tender prices from Region StockholmCitation35 were applied in the model to calculate the drug acquisition costs. Pharmacy selling prices were used in cases where tender prices were not available (see Supplementary Materials). For axi-cel, the pharmacy purchasing price was used (derived from pharmacy selling priceCitation29). Unit costs were sourced from price lists from Region ÖstergötlandCitation31 and Södra RegionvårdsnämndenCitation32. The Swedish Regions’ Price Index (LPIK) 1980–2021Citation36 and forecast for LPIK in 2022Citation37 (excluding pharmaceutical costs) were used to adjust the cost of leukapheresis to a 2022 price level. Additional information on 2L and subsequent therapies is provided in the Supplementary Materials.
2.4.2. Health state costs
Health state resource utilization was assumed to be independent of treatment arm and included outpatient visits, nurse visits, inpatient stays, CT scans, PET-CT scans, and blood tests. Resource use was stratified into event-free and post-event and adjusted to reflect Swedish clinical practice. The event-free resource use included the resource use for follow-up after ASCT and axi-cel treatment. The resource use for patients who were still event-free after five years was reverted to zero based on the assumption that patients who are still event-free after five years are effectively considered long-term responders with minimal healthcare resource utilization. Patients transitioning to the death health state incurred a one-off end-of-life cost. The number of visits and tests included per month are presented in . Unit costs were sourced from price lists from Södra RegionvårdsnämndenCitation32 and Region SkåneCitation34. The Swedish Regions’ Price index (LPIK) 1980–2021Citation36 and forecast for LPIK in 2022Citation37 (excluding pharmaceutical costs) were used to adjust end-of-life cost to a 2022 price level.
2.4.3. Cost of adverse events
The model included the following Grade 3 and 4 AEs observed in ZUMA-7 that required treatment: cytokine release syndrome (CRS), neurologic events (i.e. immune effector cell-associated neurotoxicity syndrome (ICANS)), thrombocytopenia, anaemia and febrile neutropenia. Cost of managing AEs was not included for subsequent therapies. Unit costs were sourced from price lists from Södra RegionvårdsnämndenCitation32. More details can be found in the Supplementary Materials.
2.5. Sensitivity and scenario analyses
Deterministic sensitivity analyses (DSA) and a probabilistic sensitivity analysis (PSA) with 1,000 iterations were conducted to assess robustness of the results. In the DSA, baseline characteristics (proportion of females, mean age, and mean body weight and body surface area), resource use, utilities, AE rates, proportion of patients receiving each type of subsequent therapy and unit costs were varied. The PSA probability distributions are presented in the Supplementary Table S7.
Several scenario analyses were conducted to assess the impact of key inputs and assumptions, including the time horizon (5, 10, 20, 30 and 40 years), the hazard of death compared to the general Swedish population for those who were assumed to achieve long-term remission (SMRs of 1.18 and 1.00), the utility values for patients who were still event-free after five years (reverting to general population vs not reverting) and the perspective of the analysis (healthcare vs societal).
The societal perspective included costs of productivity loss, patient and caregiver time spent on treatment-specific activities, and transportation costs to and from the hospital. The patient time ascribed to each treatment-related activity is presented in . The applied hourly wages by age in Sweden are presented in . For the cost of transportation, it was assumed that patients have a one-hour drive each way to and from the hospital for any inpatient or outpatient visits. The transportation cost per visit was assumed to be SEK 300. For multi-day inpatient stays, the cost was only triggered once. AEs did not trigger additional transportation costs, as these were assumed to be treated during the inpatient stay at follow-up. As per TLV guidelines, the human capital approach was used to calculate the productivity lossCitation38. The productivity loss for patients in the pre- and post-event health states was included together with the productivity loss caused by premature death. The share of patients who are able to work per month is presented in .
Table 2. Patient time included in the scenario analysis, applying a societal perspective.
Table 3. Average age-adjusted hourly wage and workforce.
Table 4. Proportion (percentage) of patients being absent from work per month in the pre-event and post-event states in the model.
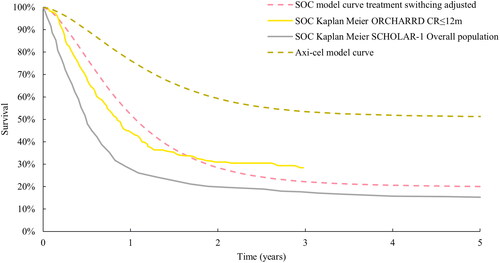
It was expected that the off-protocol treatment switching in ZUMA-7 heavily confounded the OS and costs. Therefore, a scenario analysis was conducted that accounted for the treatment switching from SOC to CAR T-cell therapy by not including the cost and effects of treatment switching in the SOC arm. Established statistical methods such as the RPSFTM and IPCW are generally regarded as appropriate for handling treatment switchingCitation41 and were applied to model the OS benefit in the absence of switchingCitation6. The two methods are described further in the Supplementary Materials. Both IPCW and RPSFTM were pre-specified in the ZUMA-7 protocol, and for the RPSFTM model full, partial or no re-censoring were performed following the guidance in the National Institute for Health and Care Excellence technical support document 16Citation6. Clinical validation and external controls were used to assess the plausibility of resultant curves. The resultant curve was expected to fall in between the OS curves from the ORCHARRDCitation42 and SCHOLAR-1Citation43 as these two trials represent pre-CAR T-cell therapy use in a similar population. The RPSFTM with full re-censoring (HR: 0.416) gave the most clinically plausible estimates and has been accepted by the National Institute for Health and Care Excellence in their assessment of axi-cel in 2L DLBCLCitation44. The resultant HR (0.416) was applied to the modelled axi-cel arm to estimate the OS curve for SOC in the absence of subsequent cellular therapy (). The proportion of patients who received subsequent cellular therapy (including axi-cel) were redistributed to the chemotherapy regimens to account for costs. As in the base case, the impact on HRQoL of subsequent therapy was not accounted for in the scenario analysis.
Figure 4. Validation of the modelled treatment switching adjusted SOC OS curve. ZUMA-7 modelled SOC OS curve adjusted for treatment switching using the RPSFTM model with full re-censoring and HR approach, ZUMA-7 modelled axi-cel OS curve and Kaplan Meier curves from ORCHARRDCitation49 and SCHOLAR-1Citation45.
3. Results
3.1. Cost-effectiveness analysis
Over a lifetime horizon of 50 years, the model estimated an ICER of SEK 534,704 per QALY gained with an incremental discounted cost of SEK 812,944 and incremental discounted QALY gain of 1.52 for axi-cel compared to SOC. The total discounted cost per patient was SEK 4,137,997 and SEK 3,325,053 for axi-cel and SOC, respectively, and the total discounted QALY gain was 7.51 and 5.99 for axi-cel and SOC, respectively. The model estimated an incremental undiscounted LY gain of 2.56 years for axi-cel compared to SOC, with an average of 14.24 and 11.68 LYs for axi-cel and SOC, respectively. A detailed overview of the results is provided in . Furthermore, the model estimated 5-year survival rates of 51.3% and 42.9% and 10-year survival rates of 48.9% and 39.6% for patients treated with axi-cel and SOC, respectively. The median OS gain for axi-cel versus SOC was 70 months.
Table 5. Base case cost-effectiveness results.
The costs included both 2L and subsequent therapy-related costs as well as costs related to disease management, AEs and end-of-life care. The cost of 2L therapy was highest for patients receiving axi-cel, whereas the subsequent therapy costs were highest for patients receiving SOC due to the high use of CAR T-cell therapy in subsequent treatment lines.
3.2. Sensitivity and scenario analyses
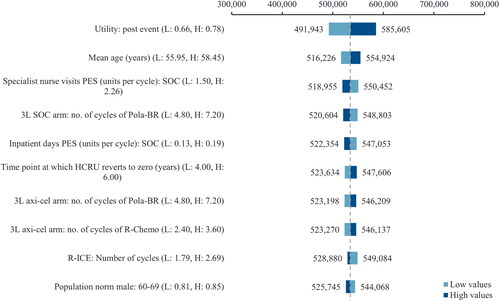
presents a tornado diagram with the 10 most influential model parameters from the DSA. The top three influential parameters were identified to be the post-event utility value, mean age and the number of specialist nurse visits in the post-event state for SOC.
Figure 5. Tornado diagram. The figure illustrates the 10 most influential parameters of the DSA. The base case parameters were varied based on their standard errors. If the standard errors were not reported, the parameters were varied by an arbitrary ±20% around the mean.
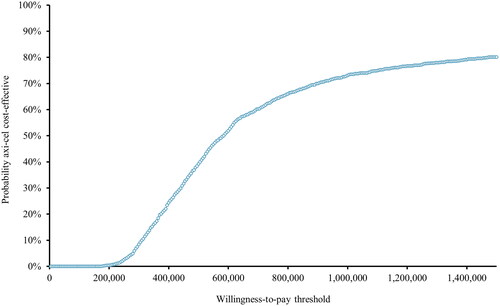
The PSA resulted in an incremental discounted cost of SEK 801,930 and an incremental QALY gain of 1.33. The estimated ICER from the PSA was SEK 491,868 per QALY gained. presents the PSA cost-effectiveness plane, and illustrates the probability of axi-cel being cost-effective at different willingness-to-pay thresholds. If assuming a willingness-to-pay threshold of SEK 1,000,000, axi-cel was cost-effective versus SOC in 73% of the simulations. The PSA results are presented in more detail in the Supplementary Table S8.
Figure 6. PSA cost-effectiveness plane (SEK). The figure illustrates that the majority of the alternative ICERs in the cost-effectiveness plane are located in the Northeast quadrant of the graph, where axi-cel is more effective and more costly compared to SOC. A small proportion is placed in the Northwest quadrant, where axi-cel is more costly and less effective compared to SOC.
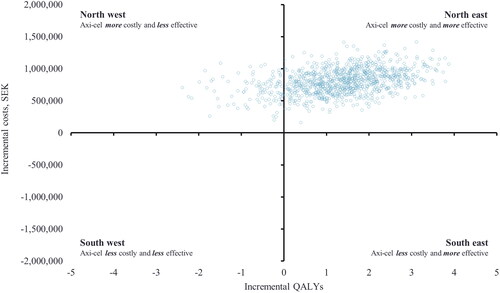
Figure 7. Cost-effectiveness acceptability curve (SEK). The figure illustrates the cost-effectiveness probability at different willingness-to-pay thresholds. At a willingness to pay threshold of SEK 1,000,000, axi-cel is cost-effective in 73% of the simulations.
The results of the scenario analyses are presented in . Most scenario analyses had a minor impact on the results, and the robustness of the base case result was confirmed. However, shortening the time horizon greatly increased the ICER, especially for time horizons of five or 10 years. The scenario analysis, where costs and effects for SOC were adjusted for off-protocol treatment switching, increased the ICER to SEK 694,351 per QALY gained.
Table 6. Results of scenario analysis.
4. Discussion
This study is the first study in the Nordic countries to evaluate the cost-effectiveness of axi-cel. The results of our analysis indicate that axi-cel is a cost-effective treatment option in 2L for transplant-intended DLBCL patients with early r/r after completing 1L chemoimmunotherapy.
In Sweden, the New Therapies Council, representing regional payers, has outlined criteria to determine the willingness to pay for new medicines. These criteria consider disease severity, clinical benefit, rarity of the disease and uncertainty in the clinical evidence. Based on these criteria and assuming a willingness to pay of SEK 1,000,000, axi-cel can be considered a cost-effective 2L treatment alternative for this patient population in Sweden. Axi-cel is currently recommended and funded by regional healthcare providers for the 3L+ treatment of r/r DLBCL in Sweden based on the ZUMA-1 trial. In the cost-effectiveness analysis of axi-cel compared to SOC for 3L+ r/r DLBCL, the estimated ICER was on par with the ICER estimated in the scenario analysis accounting for treatment switching in our studyCitation29. This is a conservative scenario, as 3L+ use of axi-cel is approved in Sweden but indicates that axi-cel is a cost-effective 2L treatment alternative and that the use should not be restricted to 3L+.
Efforts were made in our cost-effectiveness analysis to reflect Swedish clinical practice. Therefore, the patient characteristics from the ZUMA-7 trial and other parameters that informed the model were aligned with a Swedish clinical expert’s inputs as part of the model validation process. Several scenario analyses were undertaken to investigate the implication of using alternative model inputs and assumptions. The robustness of the base case result was confirmed as most scenario analyses had a minor impact on the result. The scenario analyses using different time horizons stood out, as the ICER was greatly impacted by the shorter (five- and 10-year) time horizons. As axi-cel is a one-off treatment, treatment costs are incurred upfront, whereas the benefits of receiving treatment are accumulated over the patients’ lifetime. Axi-cel has the potential to be a curative therapy for approximately 50% of DLBCL patients and shortening the time horizon will therefore significantly reduce the number of QALYs and LYs gained while most of the costs remain, leading to a higher incremental cost per QALY. The scenario analysis accounting for treatment switching was conducted because 56% of patients randomized to the SOC arm in ZUMA-7 received off-protocol CAR T-cell therapy as subsequent therapy. This is possibly higher than the use in Swedish clinical practice, meaning that the ICER could potentially be higher in a Swedish setting than the base case ICER reported in our study, due to the lower use of subsequent therapy in the SOC arm in Sweden. It is likely that the ICER for axi-cel compared to SOC in Sweden lies between the result of our base case analysis and the scenario analysis, where the OS for SOC was adjusted for treatment switching. However, the ICER is still relatively contained even in the extreme scenario of 0% CAR T-cell therapy use in subsequent treatment lines in the SOC arm. According to the Swedish HTA agency, TLV, cost-effectiveness analyses should include scenarios with and without indirect costsCitation47. We therefore performed a scenario analysis, changing the perspective of the analysis from a Swedish healthcare perspective to a social perspective. The analysis showed that changing the perspective reduces the ICER by SEK −81,732 compared to the base case.
Tisagenlecleucel, lisocabtagene maraleucel, tafasitamab, and glofitamab are all recently EU-approved treatments for use in r/r DLBCLCitation38,Citation48–50. However, none of these drugs are currently recommended or reimbursed in Sweden for r/r DLBCL. The New Therapies Council has issued negative reimbursement recommendations for tisagenlecleucel and tafasitamab, and recommendations are pending for lisocabtagene maraleucel and glofitamabCitation51. At the time of the analysis, these treatments were not available to patients in Sweden and were therefore not seen as relevant to the decision problem. In addition, these drugs are approved for different indications, and only lisocabtagene maraleucel and tafasitamab have EU approval in 2L DLBCL (tafasitamab only for transplant-ineligible patients)Citation33.
Using the ZUMA-1 event-free utility value as input for the post-event utility value in our analysis may overestimate the utility in the post-event health state. To address this, the post-event utility was included in the DSA, where the base case value was increased/decreased by 20%. The DSA showed that varying the post-event utility value impacted the ICER by less than SEK 51,000 (). Applying ZUMA-1 event-free utility values in the post-event state is further supported by the fact that CAR T-cell therapy is reimbursed and used in 3L in Sweden as well as by the fact that around 80% of ZUMA-7 SOC patients receiving 3L treatment ended up receiving CAR T-cell therapy.
The robustness of the results of our study was also supported by Perales et al. 2022Citation52, which evaluated the cost-effectiveness of axi-cel compared to SOC for 2L DLBCL in the US. Despite the application of different resource use and other costs for treating DLBCL due to the US setting as well as different utility values, Perales et al. 2022 reported comparable results: an ICER of USD 66,381 per QALY (approx. SEK 700,000 [converted to SEK in November 2022]) compared to the SEK 534,704 per QALY reported in our study. Furthermore, the US study demonstrated a discounted QALY gain for axi-cel of 7.08, which is close to the QALY gain of 7.51 in our study.
This cost-effectiveness analysis utilized data from the randomized controlled trial ZUMA-7. The ZUMA-7 trial is the first and largest (N = 359) head-to-head randomized trial comparing CAR T-cell therapy to SOC with a long follow-up duration. Previous CAR T-cell therapies have been investigated in clinical studies using a single-arm study design. The comparative and randomized trial design of the ZUMA-7 trial also supports the validity of the findings of this study. We explored various approaches to extrapolating survival outcomes beyond the ZUMA-7 trial data. These suggest that the existing OS trend observed in the ZUMA-7 trial will develop into substantial long-term benefits for patients. Furthermore, prolonged EFS as observed for treatment with axi-cel, is a key factor for the improvement in quality of life (QoL). These results are supported by the recently published primary PRO analysis of ZUMA-7, which shows a quicker return to baseline QoL in the axi-cel arm compared to SOCCitation53.
In this cost-effectiveness analysis, a comparison was made between the use of 2L treatment with axi-cel and SOC in transplant-intended DLBCL patients with early r/r after completing 1L chemoimmunotherapy in Sweden. The results show that introduction of axi-cel comes at a higher total cost, but that this cost is offset by the clear clinical benefit of axi-cel compared to SOC. Treating patients in 2L instead of 3L+ is cost-effective as it increases the possibility of curing more patients leading to not only a gain in survival, but also a reduction in the burden on QoL and cost of subsequent therapy. This will benefit both patients and society.
Transparency
Declaration of financial/other interests
Axicabtagene ciloleucel is a product of Gilead Sciences (funder). Gilead Sciences provided support in the form of payments to Incentive Denmark. Authors O.E, S.V and V.K are employees at Gilead Sciences and Kite Pharma, a Gilead company. Incentive Denmark was a paid vendor to Gilead Sciences, and authors A.L, E.M and A.D were paid employees of Incentive Denmark.
Author contributions
A.L, E.M and A.D: Methodology, formal analysis, validation, and writing - original draft. O.E: Methodology, validation, writing - review & editing, project administration and funding acquisition. S.V and V.K: Methodology, validation, and writing - review & editing. All authors have approved the final version of the manuscript to be published and agree to be accountable for all aspects of the work.
Reviewer disclosures
A reviewer on this manuscript has disclosed that they have done consulting for Gilead SRL. The other peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Supplemental Material
Download MS Word (75.2 KB)Supplemental Material
Download TIFF Image (1.9 MB)Supplemental Material
Download TIFF Image (27.3 KB)Supplemental Material
Download TIFF Image (923.4 KB)Supplemental Material
Download TIFF Image (64.8 KB)Supplemental Material
Download TIFF Image (59.1 KB)Acknowledgement
No assistance in the preparation of this article is to be declared.
Data availability statement
Due to ethical, legal and commercial reasons supporting data is not available beyond what is already disclosed in the Supplementary Materials.
Additional information
Funding
References
- Svenska nationella kvalitetsregistret för lymfom [Internet]. [cited 2022 Sep 5]. Available from: https://statistik.incanet.se/Lymfom/.
- Harrysson S, Eloranta S, Ekberg S, et al. Outcomes of relapsed/refractory diffuse large B‐cell lymphoma and influence of chimaeric antigen receptor T trial eligibility criteria in second line—a population‐based study of 736 patients. Br J Haematol. 2022;198(2):267–277. doi: 10.1111/bjh.18197.
- Regionala. Cancercentrum. Nationellt Vardprogram - aggressiva B-cellslymfom [Internet]. 2023 Jan [cited 2023 Mar 15]. Available from: https://kunskapsbanken.cancercentrum.se/globalassets/cancerdiagnoser/blod-lymfom-myelom/lymfom/vardprogram/nationellt-vardprogram-aggressiva-b-cellslymfom.pdf.
- Locke FL, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel as second-line therapy for large b-cell lymphoma. N Engl J Med. 2022;386(7):640–654. doi: 10.1056/NEJMoa2116133.
- Kite Pharma, A Gilead Company. ZUMA-7 trial data, CSR OS addendum [Data on File]. 2022.
- Latimer NR, Abrams KR. NICE DSU technical support document 16: adjusting survival time estimates in the presence of treatment switching [internet]. London: National Institute for Health and Care Excellence (NICE); 2014 [cited 2022 Apr 5]. (NICE Decision Support Unit Technical Support Documents). Available from: http://www.nicedsu.org.uk
- Neelapu SS, Jacobson CA, Ghobadi A, et al. 5-year follow-up supports curative potential of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1). Blood. 2023;141(19):2307–2315. 2022018893. doi: 10.1182/blood.2022018893.
- Medicinrådet. [Danish Medicines Council.]. Baggrund for Medicinrådets anbefaling vedrørende axicabtagene ciloleucel som mulig standardbehandling til diffust storcellet B-cellelymfom [Internet]. 2019 May [cited 2023 Aug 21]. Available from: https://medicinraadet.dk/media/oxclkejt/baggrund-for-medicinraadets-anbefaling-vedr-axicabtagene-ciloleucel-til-diffust-storcellet-b-celle-lymfom-vers-1-0-mbilag_adlegacy.pdf.
- Norwegian Medicines Agency. Axicabtagene ciloleucel (Yescarta) for the treatment of second or later relapsed/refractory diffuse large B cell lymphoma (DLBCL) and primary mediastinal large B-cell lymphoma (PMBCL) [Internet]. 2018 Jun. Available from: https://legemiddelverket.no/Documents/Offentlig%20finansiering%20og%20pris/Metodevurderinger/A/Axicabtagene%20ciloleucel%20(Yescarta)_ID2017_105%20-hurtig%20metodevurdering%20oppdatert.pdf.
- Tandvårds- och läkemedelsförmånsverket (TLV). [The Dental and Pharmaceutical Benefits Agency]. Underlag för beslut i landstingen, Yescarta (axicabtagene ciloleucel), Infusion av autolog chimär antigenreceptor T-cellsterapi Internet]. 2018 Nov. Available from: https://www.tlv.se/download/18.192533fa166f516fb27bb5ab/1542290744988/bes181107_underlag_yescarta.pdf.
- Fimea. Aksikabtageenisiloleuseeli (Yescarta) Aikuisten Suurisoluisten B-Solulymfoomien Hoidossa, Uusien sairaalalääkkeiden nopea arviointi [Internet]. 2018. Available from: https://www.fimea.fi/documents/160140/1454401/Fimea+KAI+16+2018+Aksikabtageenisiloleuseeli+(Yescarta)+aikuisten+suurisoluisten+B-solulymfoomien+hoidossa.pdf/9437de37-4d09-3b93-9c98-5981473a6b6b.
- Jacobson CA, Locke FL, Ghobadi A, et al. Long-Term (4- and 5-Year) Overall Survival in ZUMA-1, the Pivotal Study of Axicabtagene Ciloleucel in Patients with Refractory Large B-Cell Lymphoma. Available from: https://kitemedinfo.com/wp-content/uploads/2021/12/ASH2021_Jacobson_ZUMA-1-5-Yr-ASH_Poster-Layout_17Nov2021_FINAL_v2.pdf.
- Assouline S, Li S, Gisselbrecht C, et al. The conditional survival analysis of relapsed DLBCL after autologous transplant: a subgroup analysis of LY.12 and CORAL. Blood Adv. 2020;4(9):2011–2017. doi: 10.1182/bloodadvances.2020001646.
- Howlader N, Mariotto AB, Besson C, et al. Cancer-specific mortality, cure fraction, and noncancer causes of death among diffuse large B-cell lymphoma patients in the immunochemotherapy era: population-based outcomes in DLBCL. Cancer. 2017;123(17):3326–3334. Sep 1doi: 10.1002/cncr.30739.
- Maurer MJ, Ghesquières H, Jais JP, et al. Event-free survival at 24 months is a robust end point for disease-related outcome in diffuse large B-cell lymphoma treated with immunochemotherapy. J Clin Oncol. 2014;32(10):1066–1073. doi: 10.1200/JCO.2013.51.5866.
- Vadgama S, Mann J, Bashir Z, et al. Predicting survival for chimeric antigen receptor T-cell therapy: a validation of survival models using follow-up data from ZUMA-1. Value Health. 2022;25(6):1010–1017. doi: 10.1016/j.jval.2021.10.015.
- KOL validation interview Sweden 30 May. [transcript data on file]. 2022.
- Kite Pharma, A Gilead Company. A Phase 3, Randomized, Open-Label Study Evaluating Efficacy of Axicabtagene Ciloleucel Versus Standard of Care Therapy in Subjects with Relapsed/Refractory Diffuse Large B Cell Lymphoma [Internet]. clinicaltrials.gov; 2023 Feb [cited 2023 Mar 16]. Report No.: NCT03391466. Available from: https://clinicaltrials.gov/ct2/show/NCT03391466.
- Kite Pharma, A Gilead Company. A Phase 1/2 Multicenter Study Evaluating the Safety and Efficacy of KTE-C19 in Adults with Refractory Aggressive Non-Hodgkin Lymphoma [Internet]. clinicaltrials.gov; 2022 Oct [cited 2023 Mar 16]. Report No.: NCT02348216. Available from: https://clinicaltrials.gov/ct2/show/NCT02348216.
- Burström K, Johannesson M, Diderichsen F. Swedish population health-related quality of life results using the EQ-5D. Qual Life Res. 2001;10(7):621–635. doi: 10.1023/a:1013171831202.
- Dolan P. Modeling valuations for EuroQol health states. Med Care. 1997; 35(11):1095–1108. doi: 10.1097/00005650-199711000-00002.
- van Hout B, Janssen MF, Feng YS, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health. 2012;15(5):708–715. doi: 10.1016/j.jval.2012.02.008.
- Tandvårds-och läkemedelsförmånsverket (TLV). [The Dental and Pharmaceutical Benefits Agency]. Underlag för beslut i landstingen: libtayo (cemiplimab) [Internet]. Tandvårds- och läkemedelsförmånsverket; 2020. Available from: https://www.tlv.se/download/18.2baebd8016fa89b59b5176e2/1579177514292/bes_200113_underlag_libtayo.pdf.
- National Institute for Health and Care Excellence (NICE). Tisagenlecleucel for treating relapsed or refractory diffuse large B-cell lymphoma after 2 or more systemic therapies [TA567] [Internet]. 2019 [cited 2022 Jun 10]. Available from: https://www.nice.org.uk/guidance/ta567/history.
- National Institute for Health and Care Excellence (NICE). Axicabtagene ciloleucel for treating diffuse large B-cell lymphoma and primary mediastinal large B-cell lymphoma after 2 or more systemic therapies [TA559] [Internet]. 2019 [cited 2022 May 12]. Available from: https://www.nice.org.uk/guidance/ta559/evidence/appraisal-consultation-committee-papers-pdf-6661404973.
- Kite pharma, A Gilead Company. Supplemental analysis of patient reported outcomes (PRO) data collected from kite pharma’s ZUMA-7 phase III trial (KTE-C19-107). [Data on File]. 2020.
- National Institute for Health and Care Excellence (NICE). Axicabtagene ciloleucel for treating diffuse large B-cell lymphoma and primary mediastinal large B-cell lymphoma after 2 or more systemic therapies Technology appraisal guidance [TA872] [Internet]. 2023 [cited 2023 May 30]. Available from: https://www.nice.org.uk/guidance/ta872.
- Kite Pharma, A Gilead Company. ZUMA-7 trial data [Data on File]. 2022.
- Tandvårds- och läkemedelsförmånsverket. (TLV) [The Dental and Pharmaceutical Benefits Agency]. Räkna AUP och AIP [Internet]. [cited 2023 Mar 16]. Available from: https://www.tlv.se/apotek/rakna-aup.html.
- European Medicines Agency. Breyanzi (lisocabtagene maraleucel) [Internet]. 2021 [cited 2023 Sep 6]. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/breyanzi.
- Region Östergötland. Prislista 2022 - Klinisk immunologi och transfusionsmedicin [Internet]. 2022. Available from: https://vardgivarwebb.regionostergotland.se/pages/88036/Prislista%20klinisk%20immunologi%20och%20transfusionsmedicin%202021-2022.xlsx.
- Södra Regionvårdsnämnden. REGIONALA PRISER OCH ERSÄTTNINGAR FÖR SÖDRA SJUKVÅRDSREGIONEN [Internet]. 2022. Available from: https://sodrasjukvardsregionen.se/download/regionala-priser-och-ersattningar-for-sodra-sjukvardsregionen-2022/?wpdmdl=24791&masterkey=61decc148fc78.
- European Medicines Agency. Minjuvi (tafasitamab) [Internet]. 2021 [cited 2023 Sep 6]. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/minjuvi.
- Region Stockholm. Prislista Avtalsläkemedel Region Stockholm och Region Gotland juni 2022 [file] [Internet]. Region. Stockholm. [cited 2022 Jun 1]. Available from: https://contracts.tendsign.com/ContractArea/Details/1579312?eId=2XwXmS0zykSquwLRsSricwA%3d.
- Tandvårds- och läkemedelsförmånsverket (TLV) [The Dental and Pharmaceutical Benefits Agency]. Underlag för beslut i landstingen Yescarta (axicabtagene ciloleucel) [Internet]. 2018 [cited 2023 Mar 16]. Available from: https://www.tlv.se/download/18.192533fa166f516fb27bb5ab/1542290744988/bes181107_underlag_yescarta.pdf.
- NT-rådet. Nationellt ordnat införande av nya läkemedel [Internet]. 2023 [cited 2023 Sep 6]. Available from: https://www.janusinfo.se/nationelltinforandeavlakemedel.4.7c82b0fc1638b8db71b12b21.html.
- Swedish Association of Local Authorities and Regions. Prognosis for LPI and LPIK. [Internet]. [cited 2023 Mar 21]. Available from: https://skr.se/download/18.7bf04e091864ab301c4775b/1676374358017/LPIKtabell-20230216.xlsx.
- Statistikmyndigheten. [Statistics Sweden. ]. Genomsnittlig timlön för arbetare inom privat sektor [Internet]. [cited 2022 Jun 27]. Available from: https://www.scb.se/hitta-statistik/statistik-efter-amne/arbetsmarknad/loner-och-arbetskostnader/konjunkturstatistik-loner-for-privat-sektor-klp/pong/tabell-och-diagram/arbetare/genomsnittlig-timlon-for-arbetare-inom-privat-sektor.
- Perales MA, Kuruvilla J, Snider JT, et al. The cost-effectiveness of axicabtagene ciloleucel as second-line therapy in patients with large B-cell lymphoma in the United States: an economic evaluation of the ZUMA-7 trial. Transplant Cell Ther. 2022;28(11):750.e1–750.e6.;S2666636722015482. doi: 10.1016/j.jtct.2022.08.010.
- Elsawy M, Chavez JC, Avivi I, et al. Patient-reported outcomes in a phase 3, randomized, open-label study evaluating the efficacy of axicabtagene ciloleucel (Axi-Cel) versus standard of care therapy in patients with relapsed/refractory large B-Cell lymphoma (ZUMA-7). Blood. 2021;138(Supplement 1):430–430. doi: 10.1182/blood-2021-147598.
- Swedish Association of Local Authorities and Regions. LPI and LPIK 1980 - 2021 [Internet]. [cited 2023 Mar 21]. Available from: https://skr.se/download/18.fd18b1117fc1c31f5636714/1648730323474/2022-03-Tabellbilaga-LPI-LPIK-1980%E2%80%932021.xlsx.
- Skåne V. Region Skåne - Prislistor bild- och funktionsmedicin [Internet]. 2022 [cited 2022 Jun 27]. Available from: https://vardgivare.skane.se/patientadministration/avgifter-och-prislistor/prislistor-bild-funktionsmedicin/.
- European Medicines Agency. Columvi (glofitamab) [Internet]. 2023 [cited 2023 Sep 6]. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/columvi.
- 1177 Region Skåne. Stamcellstransplantation [Internet]. 2021 cited 2022 Jun 27]. Available from: https://www.1177.se/Skane/behandling–hjalpmedel/operationer/transplantationer/stamcellstransplantation/#section-95356.
- Neelapu SS, Locke FL, Bartlett NL, et al. Comparison of 2-year outcomes with CAR T cells (ZUMA-1) vs salvage chemotherapy in refractory large B-cell lymphoma. Blood Adv. 2021;5(20):4149–4155. Oct 26doi: 10.1182/bloodadvances.2020003848.
- Tandvårds-och läkemedelsförmånsverket (TLV). [The Dental and Pharmaceutical Benefits Agency]. Yervoy (ipilimumab) Hälsoekonomiskt kunskapsunderlag [Internet]. TLV; Available from: https://www.tlv.se/download/18.467926b615d084471ac33ac1/1510316361032/halsoekonomiskt-kunskapsunderlag-yervoy_forsta_linjen.pdf.
- Kunskapsbanken. Knowledge support for those who work in cancer care - RCC Kunskapsbanken [Internet]. [cited 2022 Jun 27]. Available from: https://kunskapsbanken.cancercentrum.se/.
- Ishak KJ, Proskorovsky I, Korytowsky B, et al. Methods for adjusting for bias due to crossover in oncology trials. Pharmacoeconomics. 2014;32(6):533–546. doi: 10.1007/s40273-014-0145-y.
- van Imhoff GW, McMillan A, Matasar MJ, et al. Ofatumumab versus rituximab salvage chemoimmunotherapy in relapsed or refractory diffuse large B-cell lymphoma: the ORCHARRD study. J Clin Oncol. 2017;35(5):544–551. doi: 10.1200/JCO.2016.69.0198.
- National Institute for Health and Care Excellence (NICE). Single Technology Appraisal Axicabtagene ciloleucel for treating relapsed or refractory diffuse large B-cell lymphoma after first-line chemoimmunotherapy [ID1684] Committee Papers [Internet]. 2023 [cited 2023 May 15]. Available from: https://www.nice.org.uk/guidance/gid-ta10580/documents/committee-papers-2.
- Statistikmyndigheten. [Statistics Sweden]. Population aged 15-74 (Labor Force Survey), percent by sex, age, labour status and year [Internet]. 2022. [cited 2023 Aug 20]. Available from: https://www.statistikdatabasen.scb.se/pxweb/en/ssd/START__AM__AM0401__AM0401A/NAKUBefolkning2Ar/table/tableViewLayout1/.
- Tandvårds-och läkemedelsförmånsverket (TLV). [The Dental and Pharmaceutical Benefits Agency]. Tandvårds- och läkemedelsförmånsverkets allmänna råd [Internet]. 2017 [cited 2022 Jun 10]. Available from: https://www.tlv.se/download/18.467926b615d084471ac3230c/1510316374332/TLVAR_2017_1.pdf.
- European Medicines Agency. Kymriah (tisagenlecleucel) [Internet]. 2021 cited 2023 Sep 6]. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/kymriah.