Abstract
Background
Hepatorenal syndrome (HRS) is characterized by severely reduced renal perfusion that precipitates rapid morbidity and mortality. Terlipressin is the only US Food and Drug Administration-approved treatment to improve kidney function for adults with HRS with a rapid reduction in kidney function. Prior to the approval of terlipressin, unapproved vasoconstrictive agents used in HRS treatment were octreotide/midodrine and norepinephrine with albumin.
Methods
A cohort decision-tree model representing a US hospital perspective assessed the clinical outcomes and direct medical costs (based primarily on hospital charges) of treating HRS with terlipressin + albumin (ALB) versus midodrine/octreotide (MID/OCT)+ALB, or norepinephrine (NorEp)+ALB. Treatment efficacy was defined by clinical response (complete/HRS reversal, partial, or no response) based on change of serum creatinine derived from published clinical trial reports. The proportions of patients with complete response were: terlipressin + ALB (36.2%), NorEp + ALB (19.1%), and MID/OCT + ALB (3.1%). Model outcomes included utilization of HRS-related healthcare resources (hospital and intensive care, outpatient and emergency department, dialysis, and transplantations), adverse events, and HRS-related mortality. Outcomes were assessed for the initial hospitalization in the base case and at 30, 60, and 90 days post-discharge.
Results
Total costs incurred over the initial hospitalization with terlipressin + ALB were lower vs NorEp + ALB, primarily due to higher ICU costs with NorEp + ALB ($7,433 vs $61,897). TER + ALB was associated with higher total costs vs MID/OCT + ALB due to higher pharmacy costs with terlipressin + ALB. The cost per complete response achieved of terlipressin + ALB ($451,605) was half that of NorEp + ALB ($930,571) and one-tenth that of MID/OCT + ALB ($4,942,123).
Conclusions
HRS patients treated with terlipressin experienced better clinical outcomes and a lower cost per treatment response vs other unapproved treatments. ICU days and pharmacy costs were key cost drivers distinguishing the treatment groups. These outcomes suggest that terlipressin is cost-effective on the basis of total cost per response achieved.
PLAIN LANGUAGE SUMMARY
Hepatorenal syndrome (HRS) is a rare and sudden life-threatening complication of the liver. Patients with HRS should receive immediate treatment with a drug that narrows blood vessels known as a vasoconstrictor. Terlipressin is the most common vasoconstrictor used for patients with HRS. Other common vasoconstrictors are midodrine with octreotide and norepinephrine. This study aimed to compare the cost of terlipressin with those of midodrine with octreotide and norepinephrine while also considering how well each of them worked to reverse HRS.
This was done using an economic model. This economic model assessed the costs of the vasoconstrictor drugs and the costs of treating HRS, including costs attributable to drug acquisition, adverse events, organ transplantation, dialysis, and institutional encounters (i.e. hospitalization, ICU, emergency department, and outpatient visits). The magnitude of these costs depends on how well each drug reversed HRS. Based on inputs derived from their respective clinical trials, 36% of patients who were given terlipressin had a complete response (HRS was reversed), 19% of patients who were given norepinephrine had a complete response, and 3% of patients who were given midodrine with octreotide had a complete response. The total cost per patient was approximately $163,481 for terlipressin, $177,298 for norepinephrine, and $155,030 for midodrine with octreotide. When the costs were evaluated against how well the drugs worked to reverse HRS, the lowest cost per HRS reversal was $451,605 when treated with terlipressin. The cost per reversal for norepinephrine was $930,571 and for midodrine with octreotide was $4,942,123. These results show that terlipressin works well and is more cost-effective for US hospitals compared with the other unapproved treatment options for HRS with rapid reduction in kidney function.
Introduction
Hepatorenal syndrome (HRS) is a rare, acute, life-threatening complication of cirrhosis and is associated with poor prognosis, with >80% mortality within 3 monthsCitation1–4. The annual probability of HRS occurrence among persons with chronic liver disease is 18%Citation5, with a prevalence of 40,000 cases in the United States (US)Citation6,Citation7.
Clinically, HRS is characterized by a precipitous decline in acute kidney function and an inability to sustain adequate kidney perfusion. This is often evidenced by an increase in serum creatinine (SCr) ≥0.3 mg/dL within 2 days or ≥50% within 7 daysCitation8. Without effective bridging or definitive treatment, HRS will rapidly progress. Progressing patients require substantial healthcare resources and the associated direct medical costs for services including inpatient hospital stays and intensive care unit (ICU) stays; potentially with mechanical ventilation, dialysis, and liver transplantation.
Treatment guidelines recommend prompt receipt of vasoconstrictive drugs in combination with albumin (ALB)Citation8. Terlipressin is currently the only pharmacologic treatment approved in the United States for HRS and is listed as the preferred treatment for patients with rapid reduction in kidney function by the ACG guidelines 2022, AASLD guidance 2021, and international guidelines 2018Citation8–14. Alpha-adrenergic agonists such as midodrine with octreotide (MID/OCT) or norepinephrine (NorEp) are commonly used as standard of care in combination with albumin (ALB)Citation15, although these treatments are not approved by the US Food and Drug Association. The objective of this analysis was to compare the clinical and economic benefits of terlipressin in combination with ALB, MID/OCT + ALB, and NorEp + ALB for the treatment of HRS in US hospitals. Additionally, the ALB alone scenario reflecting no vasopressor administration was evaluated.
Methods
Economic model overview
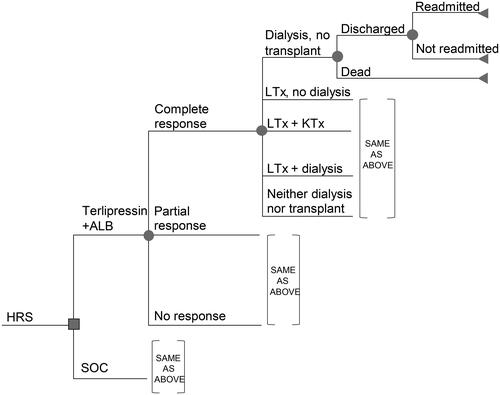
A cohort decision-tree model was constructed in Microsoft Excel 365 to estimate the expected clinical events and costs up to 90 days from the hospital discharge of an HRS patient with rapid reduction in kidney function treated with terlipressin + ALB, MID/OCT + ALB, or NorEp + ALB. Upon treatment, patients will experience either complete response, partial response, or no response based on change in serum creatinine ().
Figure 1. HRS economic model decision tree framework.
Abbreviations. ALB, albumin; HRS, hepatorenal syndrome; KTx, kidney transplant; LTx, liver transplant; SOC, standard of care.
HRS-related mortality and the utilization of healthcare resources to manage HRS from the US hospital perspective were estimated for each level of treatment response. Costs for each treatment included the initial costs of HRS drug acquisition as well as the costs for HRS management and adverse events of special interest associated with HRS treatment. Model outcomes are reported as total costs per patient and the proportion of patients alive at the end of the initial hospitalization and 30, 60, and 90-days after discharge from the initial hospitalization. All costs are expressed in 2021 USD.
Clinical outcomes
Complete treatment response was defined as HRS reversal with SCr ≤ 1.5 mg/dL while on treatment, by day 14, or discharge. Partial response was defined as reduction of SCr to at least 0.3 mg/dL above lowest value obtained within the 14 days prior to randomization. Probabilities of treatment response and adverse events for terlipressin + ALB were those observed within the CONFIRM clinical trialCitation16. Note that we used probabilities for treatment response sourced from data on file and not the Phase 3 clinical trial publication because the former provided the requisite values to model three levels of treatment response. The data on file is available upon request. Probabilities for treatment response for MID/OCT + ALB were estimated from the Cavallin et al.Citation17trial, which reported 55.5% and 4.8% of participants had complete response on terlipressin + ALB and MID/OCT + ALB, respectively (relative risk = 11.54). Similarly, 14.8% and 23.8% of participants in the two treatment groups experienced partial response (relative risk = 0.62). Efficacy for MID/OCT + ALB was then estimated by multiplying these relative risks by the respective terlipressin + ALB response proportions. A pooled meta-analysis of four trials was used to estimate the relative risk of TERLI + ALB versus NorEp + ALBCitation18–21. Overall, terlipressin + ALB had a probability of complete response of 52.8% versus 27.4% for NorEp + ALB, resulting in a relative risk = 1.9 that was used to adjust the complete and partial response proportions for terlipressin + ALB ().
Table 1. Efficacy and healthcare resource utilization model inputsCitation16.
The mortality risk by treatment response over time from the CONFIRM trialCitation16 () was used to estimate the mortality risk at each time point for all treatments.
Probabilities of experiencing selected adverse events were those observed in the CONFIRM trial for terlipressin + ALB and reported in the prescribing labelCitation22 (). Data to estimate similar adverse event probabilities for MID/OCT + ALB and NorEp + ALB were taken from a systematic literature review and meta-analysis reported by Pitre et al.Citation23
Table 2. Probability of adverse event of interest by treatment.
Drug costs
Drug costs were obtained from the RED BOOKCitation24 for all comparators using wholesale acquisition cost. No discounts or rebates were assumed in the analysis. The number of treatment days were those observed within the CONFIRM trialCitation25 for TERLI + ALB and a randomized trial of MID/OCT + ALB versus NorEp + ALBCitation26 (). Drug administration costs are implicitly included in daily hospital day costs and were not modeled separately to avoid double counting.
Table 3. Drug cost, dosing, and duration used in the model.
Data to estimate the costs of treatment-related adverse events above those of the typical HRS treatment patterns were scarce. The cost estimates for each adverse event is the summation of median cost for facility claim and professional claims from a database analysisCitation27 (Supplementary Table S1).
Healthcare utilization and costs
Days of hospitalization, probabilities and duration of dialysis, and probabilities of transplant procedures and HRS readmissions were calculated from the CONFIRM trial by treatment response level ().
The total number of general ward and intensive care unit (ICU) inpatient days are equal for all treatments for each response strata except for NorEp + ALB, which is required to be administered within the ICU. Therefore, all treatment days for NorEp + ALB are assigned to the ICU, with the remaining post-treatment days assigned to the general ward (). It is of note that the overall duration of hospital stay for non-responders is shortest. This is likely a result of the higher likelihood of death during the initial hospitalization for non-responders, resulting in a shorter length of stay.
The probabilities of HRS readmission observed within the CONFIRM trial were also stratified by treatment response (). All HRS readmissions were assumed to come from emergency department visits.
Per event costs were obtained from the published literature and adjusted to 2021 USD (Supplementary Table S1). Costs of continuous and intermittent dialysis were estimated using the mean number of continuous dialysis days (3.44 days), intermittent dialysis days during the initial hospitalization (5.48 days), and intermittent dialysis days after the initial hospitalization (9.85 days) observed in the CONFIRM trial multiplied by the per dialysis cost of each type. All patients, regardless of response or readmission status, were assumed to have one outpatient follow-up visit. Costs associated with mortality during the initial hospitalization were assumed to be higher than costs associated with mortality occurring after discharge, since those deaths are more likely to be at home versus in the hospital.
Model outputs
The primary model output is the expected per patient cost of the initial hospitalization for each treatment. To estimate these costs, the expenditures for drugs, hospitalization days, clinical events, and deaths were tabulated according to their expected frequency. Cumulative costs per patient for the 30-, 60-, and 90-day follow-up times are also reported as well as the incremental cost per responder of terlipressin + ALB relative to MID/OCT + ALB and NorEp + ALB. One-way univariate sensitivity analyses were conducted to evaluate the impact of input uncertainty on cost-effectiveness model outcomes on the cost per responder metrics.
Results
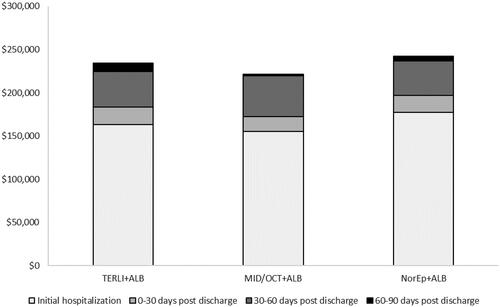
Costs incurred over the initial hospitalization were lower for terlipressin + ALB ($163,481) versus NorEp + ALB ($177,298) and higher than MID/OCT + ALB ($155,030) (). These results were consistent through the 30-, 60-, and 90-day follow-up periods (). Cost savings relative to NorEp + ALB were driven by the lower costs of ICU hospitalization. Comparing the total costs associated with each treatment to its clinical efficacy, the average cost per complete response was lowest for terlipressin + ALB ($451,605) versus NorEp + ALB ($930,571) versus MID/OCT + ALB ($4,942,123). With its higher probability of complete response (36.2% vs 19.1%) and lower associated per patient cost ($163,481 vs $177,298 for the initial hospitalization), terlipressin + ALB was the dominant treatment strategy compared to NorEp + ALB. Terlipressin + ALB was more effective (36.2% vs 3.1% complete response rate) and more costly ($163,481 vs $155,030 for the initial hospitalization) than MID/OCT + ALB, resulting in an incremental cost of $25,559 per each additional complete responder for the initial hospitalization. At 90 days post-discharge the incremental cost per responder of terlipressin + ALB was $37,918 relative to MID/OCT + ALB and continued to be the dominant (i.e. more effective and less expensive) treatment strategy compared to NorEp + ALB.
Table 4. Total costs per patient and outcomes for each treatment during the initial HRS hospitalization.
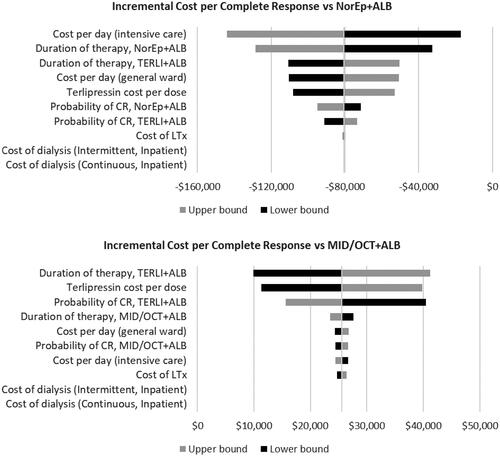
Sensitivity analysis varying key model inputs by 20% revealed costs associated with treatment (e.g. duration of therapy, intensive care hospitalization, drug acquisition) were most influential to the relative value of terlipressin ().
Discussion
In the CONFIRM trial of patients with HRS type 1 (HRS-1 or HRS-acute kidney injury (AKI)), terlipressin + ALB was shown to have significantly higher efficacy to reverse HRS over ALB alone, thereby reducing the need for and cost of dialysis. Further, over 65% of participants of the trial had previously received – and failed – MID and/or OCTCitation25, suggesting that there is a need for more effective HRS treatment. Clinical data suggest NorEp + ALB has higher efficacy than ALB and MID/OCT + ALB but is also associated with high hospitalization costs due to its ICU administration requirement.
Our economic model suggests that treating adult HRS patients with rapid reduction in kidney function with terlipressin + ALB is cost-effective with lower cost per complete responder than MID/OCT + ALB or NorEp + ALB from the initial HRS hospitalization through 90-days post-discharge. Further, terlipressin + ALB dominates NorEp + ALB with higher efficacy and lower overall costs. Given the severity and high mortality of HRS, and by extension the long-term value of avoiding negative outcomes, it is likely that the model underestimates the economic benefits of terlipressin, as possible long-term outcomes such as ongoing dialysis and some transplants cannot accrue within the model’s relatively short time horizon.
This model relied on efficacy inputs from the CONFIRM trial and other published international randomized clinical trials, implicitly assuming that these populations are representative of the target eligible patient population in the United States and trial outcomes are representative of those that would be observed in real-world clinical practice. We assert the similarity assumption is reasonable since (1) 90% of the population of the CONFIRM trial was from the United States, suggesting the demographics would be similar to that of clinical practice and (2) a recently published real-world data analysis found a 15.9% HRS reversal under current standard-of-care treatmentsCitation15, which is similar to that of the ALB arm in the CONFIRM trial (16.8%).
The advantage of utilizing clinical trial data is that the potential impact of patient comorbidities on the efficacy and resource utilization outcomes is minimized due to the randomization between the arms. However, clinical data does result in response-based healthcare resource utilization that did not always follow an intuitive pattern of higher utilization for lower responses. For example, the proportion of partial responders requiring renal replacement therapy is less than the proportion of complete responders. These are the observed data from the trial but are based on a relatively small number of patients (n = 22 partial responders).
The CONFIRM trial could not supply every input and we relied on other sources to estimate the probabilities of adverse events associated with NorEp + ALB and MID/OCT + ALB. Estimates of adverse event costs were taken from the published literature and pertained other conditions which may not accurately depict the event costs incurred by HRS patients. Other costs were sourced from the US Medicare reimbursement tables, which may be lower than the actual cost of care.
In interpreting these findings, it is also important to consider that terlipressin is not appropriate for all patients with HRS. As per the terlipressin prescribing informationCitation22 there is a black box warning indicating that terlipressin can cause serious or fatal respiratory failure, and patients with volume overload or with ACLF Grade 3 are at increased risk. It is recommended not to initiate terlipressin in patients experiencing hypoxia (e.g. SpO2 < 90%) until oxygenation levels improve. In addition to patients with hypoxia (oxygen saturation < 90%), at-risk groups include patients with fluid overload, and patients with acute-on chronic liver failure Grade 3. Also, patients treated with terlipressin who experience treatment-related respiratory failure or ischemia may become ineligible for liver transplantation, and the risk of this should be carefully considered. It is recommendedCitation22 that the benefits of terlipressin do not outweigh the risks for patients listed for liver transplantation with high priority (i.e. MELD ≥ 35). The present model does not consider these subpopulations or contingencies explicitly and so the benefits reported here should not be assumed to extend to the aforementioned subgroups and clinical scenarios.
Conclusions
HRS patients treated with terlipressin+ALB exhibited superior clinical outcomes compared to those treated with MID/OCT+ALB and NorEp+ALB, as evidenced by the results from the head-to-head randomized international trials. Furthermore, our in-depth analysis also shows that terlipressin has a lower cost per treatment response vs unapproved treatments, namely MID/OCT+ALB and NorEp+ALB. As such, terlipressin is cost-effective and a value-based treatment option for appropriate adults with HRS with rapid reduction in kidney function in US hospitals.
Transparency
Author contributions
JAC, XH, SC, VE, and JN were responsible for conception and design of the study. JAC and SC were responsible for the analysis and development of the model. All authors participated in critically reviewing and interpreting the data. All authors critically reviewed the manuscript for intellectual content and all authors approved the final version for publication submission. All authors agree to be accountable for all aspects of the work.
Reviewer disclosures
Peer reviewers on this manuscript have received an honorarium from JME for their review work but have no other relevant financial relationships to disclose.
Previous presentations
Study results were previously presented in part at the American Association for the Study of Liver Diseases: The Liver Meeting, November 4–8, 2022, Washington, DC, USA.
Supplemental Material
Download MS Word (36 KB)Acknowledgements
Medical writing support was provided by Sonya J. Snedecor, PhD (OPEN Health) and funded by the study sponsor.
Declaration of funding
This work was sponsored by Mallinckrodt Pharmaceuticals, Hampton, NJ, the manufacturer of terlipressin; the sponsors were involved in all stages of the work and in the manuscript preparation.
Declaration of financial/other relationships
XH, KJ, and JN are employees and/or shareholders of Mallinckrodt Pharmaceuticals. JAC, SC, and VE are or were employees of OPEN Health, which received funding from Mallinckrodt Pharmaceuticals to conduct this study.
References
- Boyer TD, Sanyal AJ, Wong F, et al. Terlipressin plus albumin is more effective than albumin alone in improving renal function in patients with cirrhosis and hepatorenal syndrome type 1. Gastroenterology. 2016;150(7):1579–1589.e2. doi: 10.1053/j.gastro.2016.02.026.
- Erly B, Carey WD, Kapoor B, et al. Hepatorenal syndrome: a review of pathophysiology and current treatment options. Semin Intervent Radiol. 2015;32(4):445–454. doi: 10.1055/s-0035-1564794.
- Salerno F, Gerbes A, Ginès P, et al. Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Gut. 2007;56(9):1310–1318.
- Jamil K, Huang X, Lovelace B, et al. The burden of illness of hepatorenal syndrome (HRS) in the United States: a retrospective analysis of electronic health records. J Med Econ. 2019;22(5):421–429. doi: 10.1080/13696998.2019.1580201.
- Gines A, Escorsell A, Gines P, et al. Incidence, predictive factors, and prognosis of the hepatorenal syndrome in cirrhosis with ascites. Gastroenterology. 1993;105:229–236.
- Pant C, Jani BS, Desai M, et al. Hepatorenal syndrome in hospitalized patients with chronic liver disease: results from the nationwide inpatient sample 2002-2012. J Investig Med. 2016;64(1):33–38. doi: 10.1136/jim-d-15-00181.
- US Census Bureau. Quick facts 2021 [cited 2022 Nov 29]. Available from: https://www.census.gov/quickfacts/fact/table/US/TST045218.
- Angeli P, Bernardi M, Villanueva C, et al. EASL clinical practice guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69(2):406–460. doi: 10.1016/j.jhep.2018.03.024.
- Bajaj JS, O'Leary JG, Lai JC, et al. Acute-on-chronic liver failure clinical guidelines. Am J Gastroenterol. 2022;117(2):225–252. doi: 10.14309/ajg.0000000000001595.
- Biggins SW, Angeli P, Garcia-Tsao G, et al. Diagnosis, evaluation, and management of ascites, spontaneous bacterial peritonitis and hepatorenal syndrome: 2021 practice guidance by the American Association for the study of liver diseases. Hepatology. 2021;74(2):1014–1048. doi: 10.1002/hep.31884.
- Acevedo JG, Cramp ME. Hepatorenal syndrome: Update on diagnosis and therapy. World J Hepatol. 2017;9(6):293–299.
- Glass L, Sharma P. Evidence-based therapeutic options for hepatorenal syndrome. Gastroenterology. 2016;150(4):1031–1033.
- O'Leary JG, Levitsky J, Wong F, et al. Protecting the kidney in liver transplant candidates: Practice-Based recommendations from the American Society of Transplantation Liver and Intestine Community of Practice. Am J Transplant. 2016;16(9):2516–2531.
- Papaluca T, Gow P. Terlipressin: Current and emerging indications in chronic liver1. Acevedo JG, Cramp ME. Hepatorenal syndrome: Update on diagnosis and therapy. World J Hepatol. 2017;9(6):293–299.
- Sanyal AJR, Brown KA, Landis CS, et al. Hepatorenal syndrome patient characteristics, treatment, and clinical response by disease severity: real-world practice patterns from 11 US hospitals. American Association for the Study of Liver Disease (AASLD) Annual Meeting, November 12–15, 2021; Virtual.
- Mallinckrodt Pharmaceuticals. Data on File. Clinical study report: a multi-center, randomized, placebo-controlled, double-blind study to confirm efficacy and safety of terlipressen in subjects with hepatorenal syndrome type 1 (The CONFIRM Study), 2020.
- Cavallin M, Kamath PS, Merli M, et al. Terlipressin plus albumin versus midodrine and octreotide plus albumin in the treatment of hepatorenal syndrome: a randomized trial. Hepatology. 2015;62(2):567–574. doi: 10.1002/hep.27709.
- Arora V, Maiwall R, Rajan V, et al. Terlipressin is superior to noradrenaline in the management of acute kidney injury in acute on chronic liver failure. Hepatology. 2020;71(2):600–610. doi: 10.1002/hep.30208.
- Nayyar S, Kaur R, Mohan G, et al. A prospective study to compare the efficacy of noradrenaline verses terlipressin in hepatorenal syndrome in patients with advanced cirrhosis. Int J Adv Med. 2021;8(9):7.
- Saif RU, Dar HA, Sofi SM, et al. Noradrenaline versus terlipressin in the management of type 1 hepatorenal syndrome: a randomized controlled study. Indian J Gastroenterol. 2018;37(5):424–429. doi: 10.1007/s12664-018-0876-3.
- Singh V, Ghosh S, Singh B, et al. Noradrenaline vs. terlipressin in the treatment of hepatorenal syndrome: a randomized study. J Hepatol. 2012;56(6):1293–1298. doi: 10.1016/j.jhep.2012.01.012.
- Mallinckrodt Pharmaceuticals. TERLIVAZ [package insert]; Bedminster, NJ. 2022.
- Pitre T, Kiflen M, Helmeczi W, et al. The comparative effectiveness of vasoactive treatments for hepatorenal syndrome: a systematic review and network meta-analysis. Crit Care Med. 2022;50(10):1419–1429. doi: 10.1097/CCM.0000000000005595.
- Red Book Online (2021) [Internet]. 2021.
- Wong F, Pappas SC, Curry MP, et al. Terlipressin plus albumin for the treatment of type 1 hepatorenal syndrome. N Engl J Med. 2021;384(9):818–828. doi: 10.1056/NEJMoa2008290.
- El-Desoki Mahmoud EI, Abdelaziz DH, Abd-Elsalam S, et al. Norepinephrine is more effective than midodrine/octreotide in patients with hepatorenal syndrome-acute kidney injury: a randomized controlled trial. Front Pharmacol. 2021;12:675948. doi: 10.3389/fphar.2021.675948.
- Mallinckrodt Pharmaceuticals. Data on File. Average AE (identified by DX codes) per-Day Cost per Outpatient Encounter from MarketScan Database. 2022.