Abstract
Aims
The study objectives were to 1) characterize the cost drivers of patients with Helicobacter pylori (HP) and 2) estimate HP-related cost savings following lab-confirmed HP eradication with US guideline-recommended treatment compared to failed eradication.
Methods
We identified adults newly diagnosed with HP between 1/1/2016–12/31/2019 in the Veradigm Electronic Health Record Database linked to claims data (earliest HP diagnosis = index date). For the overall costs analysis, we required patients to have data available for ≥12 months before and after the index date. Then, we used multivariable modeling to assess the marginal effects of comorbidities on all cause-healthcare costs in the 12 months following HP diagnosis. For the eradication savings analysis, we identified patients with ≥1 HP eradication regimen, a subsequent HP lab test result, and ≥1 year of data after the test result. Then we used multivariable modeling to estimate HP-related cost while adjusting for eradication status, demographics, post-testing HP-related clinical variables, and the interactions between eradication status and each HP-related clinical variable.
Results
The overall cost analysis included 60,593 patients with HP (mean age 54.2 years, 65.5% female). Mean (SD) 12-month unadjusted all-cause costs were $23,693 ($78,089). Rare comorbidities demonstrated the highest marginal effect. The marginal effects of gastric cancer and PUD were $15,705 and $7,323, respectively. In the eradication savings analysis, 1,835 (80.0%) of the 2295 patients had lab test-confirmed HP eradication. Compared to failed eradication, there were significant one-year cost savings among patients with successful HP eradication and select conditions: $1,770 for PUD, $518 for atrophic gastritis, $494 for functional dyspepsia, and $352 for gastritis.
Conclusions
The healthcare costs of patients with HP are partially confounded by their burden of high-cost comorbidities. In the subset of patients with available results, confirmed vs. failed eradication of HP was associated with short-term cost offsets among those with specific to HP-related sequelae.
PLAIN LANGUAGE SUMMARY
Helicobacter pylori (HP) is a common infection. We aimed to better understand healthcare costs for people infected with HP. Specifically, we were interested in 1) investigating whether complications from HP were causing high costs. 2) whether successful eradication of HP would lead to lower healthcare costs. We captured data on adults diagnosed with HP between 2016 and 2019. The data used in this study came from medical records and insurance bills. In the first part of the study, we found that patients with HP often have other health issues, and these other health issues were driving high healthcare costs. The majority of cost savings associated with HP eradication accrue from the prevention of potential complications of long-term infection, such as peptic ulcer disease and, rarely, gastric cancer.
Introduction
Helicobacter pylori (HP) is a common gastrointestinal (GI) infection that infects approximately 36% of the United States (US) populationCitation1. While asymptomatic in the majority of patients, chronic HP infection may result in benign complications, ranging from mild dyspepsia to uncomplicated and complicated peptic ulcer disease (PUD) to malignant complications, including gastric cancerCitation2,Citation3. The American College of Gastroenterology recommends offering HP eradication therapy to all patients who test positive for active HP infection since eradication is associated with a reduced risk of complications, specifically gastric cancerCitation4. Unfortunately, HP eradication treatment failure is increasingly common and exposes patients to the risk of ongoing complications of persistent HP infection and subjects them to additional antibiotic treatment and high-dose gastric acid suppression.
Despite its high prevalence and potentially serious consequences, the burden of HP on the US healthcare system is unstudied and focuses on the costs of complications associated with HP infection. One analysis found that costs for patients with dyspepsia were $1,144 in the week following HP diagnosis and $5,401 in the subsequent six monthsCitation5. Using data from the National Inpatient Sample, the mean cost in 2011 was $13,803 for a PUD-related hospitalization and $24,706 for a gastric cancer hospitalizationCitation6,Citation7. The total expenses for the management of gastroduodenal ulcers in the US in 2015 were estimated to be $777 million, with hospital stays accounting for 43.5% of those costsCitation8. The cost of gastric cancer in the US in 2020 was projected to be $2.3 billionCitation9.
While there is some evidence about the costs of HP-associated complications, there is currently no direct evidence of the healthcare costs for patients with confirmed HP and no direct data regarding the short-term cost savings associated with HP eradication failure versus success. The primary aim of this study was to estimate healthcare costs among patients with clinically confirmed HP. We secondarily aimed to evaluate factors driving costs among patients with clinically confirmed HP, to compare costs in the year following successful versus failed HP eradication, and to differentiate factors associated with higher costs that are potentially modifiable by the treatment of comorbid disease or impacted by HP management.
Methods
Data sources
We conducted a retrospective, observational study using electronic health records (EHR) from Veradigm Network EHR linked with privacy-preserving tokens to closed insurance claims data from Komodo Health between 1 January 2015, and 31 July 2021. The EHR dataset consists of de-identified patient records sourced from ambulatory/outpatient primary care and specialty settings. The insurance claims data contain de-identified inpatient, outpatient, and pharmacy claims. All data used in this study was sourced from healthcare encounters that took place in the US. This study did not include an analysis of patient notes.
The linked dataset only contains de-identified data as per the de-identification standard defined in Section §164.514(a) of the Health Insurance Portability and Accountability Act of 1996 (HIPAA) Privacy Rule. The process by which the data is de-identified is attested to through a formal determination by a qualified expert as defined in Section §164.514(b)(1) of the HIPAA Privacy Rule. Because this study used only de-identified patient records, it is no longer subject to the HIPAA Privacy Rule and is therefore exempt from Institutional Review Board approval and for obtaining informed consent according to US law. This study was conducted in compliance with the Declaration of Helsinki and used only de-identified data.
The diagnosis, laboratory, and procedure codes used in this study are reported in Supplementary Table 1. The generic medications used in the study are reported in Supplementary Table 2.
Patient selection
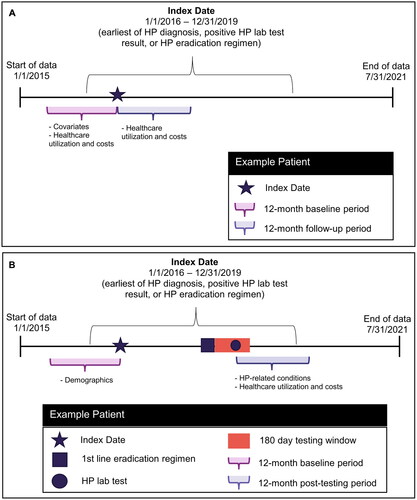
We used a two-stage patient selection strategy to create the two analytic cohorts used in this study: those with clinically confirmed HP and the subset with eradication testing results. This is illustrated in . First, we constructed a cohort of newly diagnosed adults with clinically confirmed HP infection to perform an overall cost analysis. To do this, we identified patients who met the criteria for one of the following three categories between 1 January 2016, and 31 December 2019: (1) lab-identified HP, (2) diagnosis-identified HP, or (3) treatment-identified HP. The earliest date on which patients met the criteria for at least one of these three categories was set as the index date.
Figure 1. Study cohort construction for the primary analysis and secondary analysis.
HP: Helicobacter pylori; EHR: electronic health record. aSerology tests were not included as they are not used to confirm active HP infection
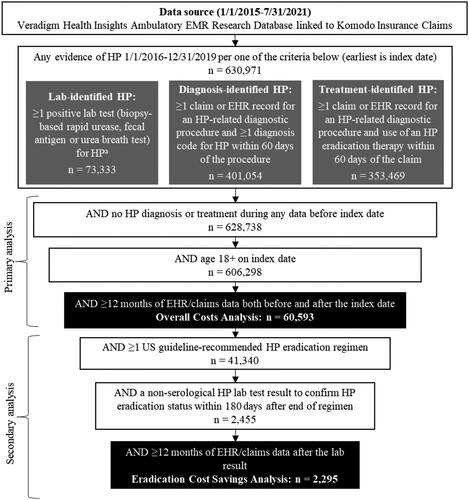
Lab identification of HP was determined in the subset of patients who had results available in the EHR for appropriate lab tests for diagnosing active HP infection (i.e. rapid urease, fecal antigen, or urea breath tests [UBT]). Patients who had at least one claim or record for an HP-related diagnostic test or procedure (esophagogastroduodenoscopy [EGD], rapid urease, fecal antigen, or UBT) but no corresponding test result could qualify as diagnosis-identified or treatment-identified according to the respective following algorithms. Diagnosis-identified HP required at least one EHR encounter or medical claim with a diagnosis code for HP (International Classification of Diseases, 9th Edition and 10th Edition, Clinical Modification Codes: 041.86 or B96.81) and an HP-related diagnostic test or procedure in the 60 days before the diagnosis. Whereas treatment-identified HP required that patients had pharmacy claims indicating that they received a US guideline-recommended HP eradication regimen (see Supplementary Table 2) and an HP-related diagnostic test or procedure in the 60 days before the diagnosisCitation4. We categorized patients as having received a specific HP regimen when there was record of prescription fills for all drugs in the regimen within a 14-day period. Proton pump inhibitors (PPIs) are available over the counter and often paid for out-of-pocket. Therefore, regimens that included PPIs were considered complete as long as all non-PPI medications in the eradication regimens were present.
We excluded patients younger than 18 years old on the index date and individuals with any prior history of lab-, diagnosis-, or treatment-identified HP. Additionally, we required continuous enrollment in the claims database and activity in the EHR for at least 12 months before and after the index date (hence, the earliest index date allowable in the cohort was 1 January 2016). This final cohort of patients was used for the overall HP cost analysis.
Next, we constructed a subcohort of patients for the eradication cost savings analysis (). The subcohort included patients from the main cohort with at least one US guideline-recommended HP eradication regimen between the index date and the earliest of any of the following: the end of continuous claims enrollment, the end of activity in the EHR or the end of the study period (31 July 2021). We required that patients have a non-serological HP lab test (rapid urease, fecal antigen, or UBT) result within 180 days after the end of their first-line eradication regimen. We also required that patients have at least 12 months of continuous claims enrollment and activity in the EHR following the lab result. Patients were stratified into two cohorts based on the result of their non-serological HP lab test: lab-confirmed eradication success (if negative test result) or failure (if positive test result).
Study design
The study designs for the overall cost analysis, and the HP eradication cost savings analysis are illustrated in . For the overall cost analysis, the baseline period was the 12 months preceding the index period, and the follow-up period was the 12 months following the index date. For the eradication cost savings analysis, the baseline period was 12 months preceding the index date, and the post-eradication testing period was the 12 months following the HP lab test used to assess eradication success or failure.
Study variables
Demographic characteristics were recorded at the index date and included age, sex, and geographic region. We recorded body mass index (BMI), tobacco use, general clinical conditions, and HP-associated diagnoses in the baseline period (see Supplementary Table 1). For patients in the HP eradication cost savings analysis, we also captured HP-related conditions and HP-related symptoms in the 12-month post-eradication testing period.
Healthcare utilization and costs
All-cause healthcare utilization and costs, including office visits, laboratory services, radiology services, and outpatient prescriptions, were recorded for the 12-month baseline period and the 12-month follow-up period for all patients. HP-related healthcare utilization and costs were measured in the 12-month baseline and 12-month post-eradication testing period among patients in the eradication cost savings analysis. HP-related medical claims included outpatient claims with an HP diagnosis in any position, inpatient claims with HP as the admitting diagnosis, and pharmacy claims for antacids, antibiotics, bismuth, H2-receptor antagonists, PPIs, and sucralfate.
Utilization and costs were measured using medical and drug claims data. All costs are reported as per person per year and were adjusted to 2021 US dollars using the medical care component of the Consumer Price IndexCitation10.
Statistical analysis
For the overall cost analysis, we employed an average marginal effects analysisCitation11. Marginal in this context means “additional” or “incremental” change in healthcare costs associated with having a particular demographic characteristic or clinical indication. The average marginal effects analysis makes use of a generalized linear model (GLM) to investigate cost patterns in the dataset by estimating the marginal effect on costs for a variety of independent variables that represent various clinical and demographic markers. The regression model, in this instance, was linear with no transformations or interactions. The estimated coefficients are interpretable as the change in the dependent variable (cost) for a unit change in the associated independent variable (clinical or demographic marker).
We first conducted a stepwise regression to identify statistically significant independent variables. Variables considered for inclusion in the overall costs model were age, sex, geographic region of residence, BMI, and smoking status, along with both the non-HP and HP-associated clinical conditions listed in Supplementary Table 1. We opted to include comorbidities as individual independent variables rather than as an aggregated comorbidity index or disease severity measure to identify the specific factors influencing cost in these complex patients. For this model, all independent variables were measured in the 12-month baseline period prior to the index date. The dependent variable was the total all-cause healthcare costs in the 12-month period following the index date. The study population was all patients with HP who qualified for the overall cost analysis ().
All independent variables identified as significant in the stepwise regression were included in the GLM to estimate the average marginal effects of identified variables on total all-cause healthcare costs among patients with HP. Predicted costs were estimated for each patient based on the sum of marginal effects. The data set was then split into groups of patients whose estimated costs were less than or equal to the median (low-cost group) or greater than the median (high-cost group). The marginal effects were averaged across all patients in each group and then compared between groupsCitation12. This approach enables the identification of the clinical markers that drive patients to have above-median costs.
For the eradication cost savings analysis, we constructed a separate GLM to investigate the relationship between HP-related costs and the HP eradication outcome (success vs. failure) following treatment with a guideline-recommended HP eradication regimen. We focused on HP-related costs incurred after eradication testing and did not include costs for first-line therapy and follow-up visits prior to eradication confirmation testing.
The study population was all patients with HP who qualified for the eradication cost savings analysis. For this model, the dependent variable was the total HP-related healthcare costs in the 12-month period following the first available eradication confirmation lab test result (i.e. the first non-serological HP test following first-line eradication treatment).
Covariates included age, sex, geographic region of residence, BMI, eradication status, and the following clinical conditions: atrophic gastritis, dyspepsia not otherwise specified (NOS), esophagitis, functional dyspepsia, gastric polyp, gastritis, gastroesophageal reflux disease (GERD), idiopathic thrombocytopenic purpura, and PUD. For this model, the clinical conditions of interest were measured in the 12-month post-eradication testing period. We also tested for interactions between each clinical condition of interest and eradication status.
We presented continuous measures as mean and standard deviation (SD). For cost and utilization measures when 50% or more of the individuals used a service category, we also reported the median with interquartile range (IQR) and categorical measures as counts and frequencies. For all cost data where we adjusted for relevant covariates, we presented point estimates with 95% confidence intervals (CI). All data processing and statistical analyses were conducted using SAS V9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Characteristics of patients with HP
A total of 60,593 patients with clinically confirmed HP met inclusion criteria (mean age 54.2 years, 65.5% female, 81.8% non-Hispanic; Supplementary Table 3). During the baseline period, the most common general comorbidities were cardiovascular disease (62.4%), obesity (32.7%), and type 2 diabetes (32.0%). The most common HP-associated diagnoses were abdominal pain or tenderness (59.3%), GERD (49.2%), gastritis (39.4%), and dyspepsia NOS (30.5%).
Unadjusted all-cause healthcare utilization and costs
Overall, 6.4% of patients with clinically confirmed HP had at least one admission in the baseline period, while 8.1% of patients had at least one admission in the follow-up period (). More than 90% of patients had at least one claim for outpatient services, outpatient office visits, and outpatient pharmacy fills in both the baseline period and the follow-up period. The mean (SD) number of pharmacy claims was 38.2 (45.0) in the baseline period and 44.5 (48.6) in the follow-up period. Mean (SD) and median (IQR) total costs were $17,513 ($69,298) and $5,696 ($11,868) in the baseline period and $23,693 ($78,089) and $6,808 ($14,361) in the follow-up period.
Table 1. Unadjusted all-cause healthcare costs and Resource utilization in patients with HP.
Marginal effects cost analysis
The high degree of variability in costs among patients with clinically confirmed HP suggested that factors other than HP were driving high post-diagnosis costs among a subset of patients. To better understand cost drivers for these patients, we used a GLM approach to evaluate factors that might contribute to higher healthcare costs among those diagnosed with HP. The top three conditions associated with high costs in the follow-up period were rare comorbidities unrelated to HP: end-stage renal disease ($92,988; 95%CI: $86,422 to $99,553), osteomyelitis ($41,026; 95%CI: $30,111 to $51,941), and cancer ($25,475: 95%CI: $19,710 to $31.241) (). Some other top contributors to higher costs were more common chronic conditions such as type 2 diabetes ($22,901; 95%CI: $9,996 to $35,806) and chronic obstructive pulmonary disease ($10,496; 95%CI: $8,179 to $12,814).
Table 2. Average marginal effects of comorbidities and demographic factorsa on healthcare costs of patients with Helicobacter pylori (HP) infection (descending order).
Several diagnoses potentially related to HP contributed to high healthcare costs. Notably, hematemesis and gastric cancer were in the top 10 contributors to higher costs, with the average marginal effect of a diagnosis of hematemesis being $19,100 (95%CI: $13,652 to $24,549) and the average marginal effect of a diagnosis of gastric cancer being $15,705 (95%CI: $1,509 to $29,902). The marginal effects of PUD and vomiting were $7,323 (95%CI: $4,679 to $9,967) and $7,118 (95%CI: $5,165 to $9,072), respectively.
Next, we compared the average marginal effects for patients above and below the median to examine which clinical markers were associated with having above-median costs (). Type 2 diabetes had the largest difference in marginal effect between the low and high-cost groups ($3,968 vs. $10,708; difference = $6,740). By comparison, the difference in marginal effect for the remaining top 10 ranged from $975 to $2,139. Among potential HP-related diagnoses, the differences in marginal effect between the low and high-cost groups were $1,381, $750, and $559 for vomiting, PUD, and hematemesis, respectively. Conditions that are costly but rare, such as gastric cancer, were not associated with having above-median costs.
Table 3. Average marginal effects of Covariates on healthcare costs of patients above and below the median Predicted costa.
Characteristics of patients with eradication confirmation test results following First-Line eradication therapy
Overall, 2,295 patients with clinically confirmed HP met the criteria for inclusion in the eradication cost savings analysis. Of these, 80.0% had lab-confirmed eradication success, and 20.0% had lab-confirmed eradication failure (Supplementary Table 3). Patients in the eradication cost savings analysis were of similar age to those in the overall analysis; however, patients with successful eradication appeared to have a lower frequency of baseline comorbidities compared to the primary analytic cohort, including a lower frequency of cardiovascular disease (54.8% vs. 62.4%), obesity (27.8% vs. 32.7%), and type 2 diabetes (24.7% vs. 32.0%).
The frequency of many HP-related clinical conditions decreased pre vs. post-HP eradication treatment in those patients with successful eradication; in contrast, among those with HP eradication failure following treatment, the observed frequencies of HP-related clinical conditions were either relatively unchanged, decreased only slightly, or, in some cases, increased (Supplementary Table 3). For example, the percentage of patients with a diagnosis of dyspepsia NOS decreased by 8.0% following successful eradication (26.7% vs. 18.7%) but only decreased by 2.2% following failed eradication (29.6% vs. 27.4%). Notably, the percentage of patients with a diagnosis of gastritis decreased by 6.4% following successful eradication (33.1% vs. 26.7%) but increased by 13.9% following failed eradication (34.3% vs. 48.3%).
Unadjusted HP healthcare Resource utilization and costs
For patients with successful eradication, HP-related mean (SD; median [IQR]) costs were $357 ($1,098; $63 [$211]) in the 12-month baseline period and $233 ($696; $30 [$136]) in the 12 months following eradication testing (Supplementary Table 4). For patients with failed eradication, HP-related mean (SD; median [IQR]) costs were $418 ($1,335; $68 [$259]) in the 12-month baseline period and $705 ($1,285; $257 [$693]) in the 12 months following eradication testing.
Among those with successful eradication, 67.7% had at least one outpatient service in the baseline period, whereas 36.1% had at least one outpatient service in the 12 months following eradication testing. Among those with failed eradication, 64.3% had at least one pharmacy claim in the baseline period, whereas 93.3% had at least one outpatient service in the 12 months following eradication testing.
Model of HP-related costs following eradication testing
In the model of costs following first-line HP eradication treatment and subsequent eradication testing, several conditions were associated with higher HP-related costs. The marginal effect on HP-related costs was highest for PUD ($2,181, 95%CI: $1,715 to $2,647), gastric polyp ($1,585, 95%CI: $809 to $2,362), and functional dyspepsia ($714, 95%CI: $477 to $952) (). From a geographic standpoint, the northeast region of residence was associated with the highest costs ($189, 95%CI: $46 to $333) compared to other US geographic regions
Table 4. Marginal effects of reduction in Helicobacter pylori (HP)-related costs associated with HP eradication.
After controlling for other variables, eradication alone was not associated with a difference in HP-related costs in the 12 months following eradication testing. However, eradication in patients with PUD was associated with $1,770 (95%CI: -$2,290 to -$1,249) in cost savings. Significant cost offsets with successful eradication were also observed for atrophic gastritis (-$518, 95%CI: -$978 to -$58), functional dyspepsia (-$494, 95%CI: -$780 to -$208), and gastritis (-$352, 95%CI: -$528 to -$176).
Discussion
In this analysis of patients newly diagnosed with clinically confirmed HP, the total unadjusted median all-cause costs were $5,696 in the baseline period and $6,808 in the follow-up period. There appeared to be increased spending on outpatient services and pharmacy claims in the follow-up period, and this may be attributed to the management of both HP and any concurrent diagnoses that precipitated the HP diagnosis. Multivariable analysis demonstrated that patients with clinically confirmed HP often have accompanying comorbid conditions that impact their overall healthcare utilization and costs. Notably, patients with confirmed successful HP eradication had lower costs attributed to specific short-term HP-related complications compared to patients with eradication failure following HP treatment.
Several approaches were taken to understand fixed and non-fixed cost drivers among patients with HP to identify and differentiate non-fixed costs that are modifiable through the treatment of comorbid disease versus those potentially modifiable through HP eradication. First, a GLM approach was used to evaluate factors that might contribute to higher healthcare costs. The highest marginal costs were associated with uncommon conditions that are unrelated to HP, such as end-stage renal disease and osteomyelitis. There were also more common chronic conditions associated with higher costs, including type 2 diabetes and chronic obstructive pulmonary disease, as well as conditions associated with HP, including hematemesis, gastric cancer, and PUD.
A marginal effects approach was used to compare patients with costs above versus below the median and the average costs associated with specific conditions. Type 2 diabetes had the greatest marginal effect difference, making it the single biggest driver of whether or not a patient’s costs were above versus below the median. Due to the rarity of events during the study timeframe, gastric cancer was not a driver of high costs. While the marginal effects approach is informative, it offers little in the way of specific actions to directly lower the potential burden of HP-related costs, and the potential benefits derived from HP treatment are obscured by the costs of comorbidities. For instance, there is some evidence that HP eradication can improve glycemic control in patients with type 2 diabetesCitation13, but our analysis is not structured to determine if there are cost benefits of HP eradication in patients with diabetes secondary to improved glycemic control.
While our first analysis highlighted the complexity of cost drivers among patients with HP, there are subgroups of patients for whom HP infection and related conditions or symptoms represent a substantial portion of their healthcare burden. Focusing on patients with higher post-index costs can help reduce the overall cost burden associated with HP. Therefore, we performed a separate marginal effects model to describe the costs associated with specific HP-related events during follow-up and focused specifically on conditions/symptoms that have a higher likelihood of improvement with successful eradication compared to failure.
On average, patients with a diagnosis of PUD had costs $2,181 higher than patients without a diagnosis of PUD. Among patients with PUD, successful eradication, on average, was associated with cost savings of $1,770 in the 12 months following eradication. Functional dyspepsia (-$494), gastritis (-$352), and atrophic gastritis (-$518) also had statistically significant marginal cost offsets associated with successful eradication.
Limitations
Our study has many strengths as well as limitations. Most notably, there are no prior studies directly measuring the cost of clinically–confirmed HP in the US using claims data. In addition, our study pulls from a large nationwide sample, and we were able to account for geographic differences in spending. Finally, our study combines the clinical details of EHR laboratory data with the comprehensive cost data from claims records.
One limitation of this study was the inability to capture the benefits of HP eradication over a longer post-eradication testing period. This would be most relevant for evaluating the cost savings associated with preventing gastric cancer; however, gastric cancer is a very rare outcome and the benefits of HP eradication with respect to reduced gastric cancer incidence and mortality would not be observed for several years post-eradicationCitation14–16. In addition, because there was a high level of missingness for race and ethnicity, we are unable to comment on differential costs based on race and ethnicity.
This study is also limited by the lack of an appropriate control group to provide additional context for the observed costs. The main challenge in identifying an appropriate control group is that the majority of patients with HP go undiagnosed. They may be undiagnosed because they are asymptomatic and don’t meet the criteria for testing, or they are symptomatic but experience barriers to diagnosis. Therefore, any population of patients without a diagnosis of HP would likely include a confounding proportion of patients with undiagnosed HP due to the endemic nature of this infection. Therefore, we focused on segmenting costs in a population with known HP status.
This study is also subject to the limitations common to studies of routinely collected healthcare data, such as data entry errors, missing data, and coding specificity limitations. As we could only leverage data available in the structured portion of the EHR, we could not capture patients based on positive histology testing. Therefore, we may be missing some clinically–confirmed HP cases. Future studies could leverage the clinical notes to add to data richness and inform the study’s interpretation. For instance, the notes might give insight into the reason for testing as well as the use of over-the-counter PPIs.
Limitations to generalizability include that less than six percent of patients had post-eradication test results available; as these patients were not randomly selected, they may not be representative of the full dataset or the population as a whole. As this study leveraged the cost data available in administrative claims data, only insured patients were eligible for study inclusion. In addition, this study is not representative of costs in the majority of patients with HP who are never tested for HP.
Finally, we are likely underestimating the portion of costs attributable to HP as our analysis only captured the costs of encounters with an HP diagnosis and did not include the costs of managing HP-related complications. For example, while the estimated marginal effect of PUD on HP-related costs was $7,323, this marginal effect would not include the cost of encounters coded for PUD unless the encounter had a concurrent diagnosis of HP. This analysis also does not capture the downstream indirect costs of repeated antibiotic use, including increased community antibiotic resistance, patient inconvenience, and decreased quality of lifeCitation17.
Conclusions
Taken as a whole, these findings not only detail the healthcare utilization and cost complexity of patients with clinically confirmed HP infection but also provide a potential profile of patients with higher health resource utilization. Patients with HP often have other substantial comorbid conditions that can be associated with meaningfully increased costs, some of which are modifiable in the course of managing HP. Among patients with specific HP-related sequelae, there may be short-term cost offsets associated with confirmed HP eradication vs. failure.
Transparency
Declaration of financial/other interests
SS is a consultant for Phathom Pharmaceuticals and RedHill Biopharma. RY is a consultant for Phathom Pharmaceuticals. KC, RS, and MB are employees of Veradigm, which received funding from Pathom Pharmaceuticals to conduct this study. CP and RJ are employees of Phathom Pharmaceuticals
Author contributions
SS contributed to conceptualization, investigation, methodology, visualization, and lead development of the manuscript. RY contributed to conceptualization, investigation, methodology, visualization, and writing of the manuscript. MB and KC contributed to conceptualization, formal analysis, funding acquisition, investigation, methodology, project administration, supervision, validation, data visualization and writing of the manuscript. RJ and CP contributed to conceptualization, funding acquisition, investigation, methodology, project administration, supervision, data visualization and writing of the manuscript. RS contributed to conceptualization, data curation, formal analysis, investigation, methodology, software, validation, visualization, and critical review and editing of the manuscript.
Reviewer disclosures
A reviewer on this manuscript has disclosed that they have done unpaid consultancy for Phathom Pharma who sponsored this study. The other peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Previous presentations
Portions of this work were presented at AMCP Nexus 2022 in National Harbor, Maryland.
Supplemental Material
Download PDF (433.4 KB)Acknowledgements
Medical writing support was provided by Jessamine Winer-Jones, an employee of Veradigm. This support was funded by Phathom Pharmaceuticals. Guidance on design and interpretation of statistical models was provided by Norman H Sedgley, Professor of Economics at Loyola University Maryland.
Data availability statement
The data that support the findings of this study are available from Veradigm and Komodo Health but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of Veradigm and Komodo Health.
Additional information
Funding
References
- Hooi JKY, Lai WY, Ng WK, et al. Global prevalence of Helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology. 2017;153(2):420–429. doi: 10.1053/j.gastro.2017.04.022.
- Crowe SE. Helicobacter pylori infection. N Engl J Med. 2019;380(12):1158–1165. doi: 10.1056/NEJMcp1710945.
- de Martel C, Georges D, Bray F, et al. Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis. Lancet Glob Health. 2020;8(2):e180-90–e190. doi: 10.1016/S2214-109X(19)30488-7.
- Chey WD, Leontiadis GI, Howden CW, et al. ACG clinical guideline: treatment of Helicobacter pylori infection. Am J Gastroenterol. 2017;112(2):212–239. doi: 10.1038/ajg.2016.563.
- Mapel D, Roberts M, Overhiser A, et al. The epidemiology, diagnosis, and cost of dyspepsia and Helicobater pylori gastritis: a case–control analysis in the Southwestern United States. Helicobacter. 2013;18(1):54–65. doi: 10.1111/j.1523-5378.2012.00988.x.
- Solanki S, Chakinala RC, Haq KF, et al. Inpatient burden of gastric cancer in the United States. Ann Transl Med. 2019;7(23):772–772. doi: 10.21037/atm.2019.11.54.
- Kanotra R, Ahmed M, Patel N, et al. Seasonal variations and trends in hospitalization for peptic ulcer disease in the United States: a 12-year analysis of the nationwide inpatient sample. Cureus. 2016;8(10):e854. doi: 10.7759/cureus.854.
- Peery AF, Crockett SD, Murphy CC, et al. Burden and cost of gastrointestinal, liver, and pancreatic diseases in the United States: update 2018. Gastroenterology. 2019;156(1):254–272.e11. doi: 10.1053/j.gastro.2018.08.063.
- Shah S, Hubscher E, Pelletier C, et al. Helicobacter pylori infection treatment in the United States: clinical consequences and costs of eradication treatment failure. Expert Rev Gastroenterol Hepatol. 2022;16(4):341–357. doi: 10.1080/17474124.2022.2056015.
- Measuring price change in the CPI: medical care. Consumer price index. Published March 16, 2022. Accessed September 20, 2022. https://www.bls.gov/cpi/factsheets/medical-care.htm.
- Onukwugha E, Bergtold J, Jain R. A primer on marginal effects-part II: health services research applications. Pharmacoeconomics. 2015;33(2):97–103. doi: 10.1007/s40273-014-0224-0.
- Otani K, Baden WW. Healthcare cost and predictive factors: high- and low-utilization model development. Health Mark Q. 2009;26(3):198–208. doi: 10.1080/07359680903263599.
- Song X, Cai C, Jin Q, et al. The efficacy of Helicobacter pylori eradication in diabetics and its effect on glycemic control: a systematic review and meta-analysis. Helicobacter. 2021;26(2):e12781. doi: 10.1111/hel.12781.
- Kumar S, Metz DC, Ellenberg S, et al. Risk factors and incidence of gastric cancer after detection of Helicobacter pylori infection: a large cohort study. Gastroenterology. 2020;158(3):527–536.e7. doi: 10.1053/j.gastro.2019.10.019.
- Graham DY, Shiotani A. The time to eradicate gastric cancer is now. Gut. 2005;54(6):735–738. doi: 10.1136/gut.2004.056549.
- Ford AC, Yuan Y, Moayyedi P. Helicobacter pylori eradication therapy to prevent gastric cancer: systematic review and meta-analysis. Gut. 2020;69(12):2113–2121. doi: 10.1136/gutjnl-2020-320839.
- Shah SC, Bonnet K, Schulte R, et al. Helicobacter pylori management is associated with predominantly negative patient experiences: results from a focused qualitative analysis. Dig Dis Sci. 2022;67(9):4387–4394. doi: 10.1007/s10620-021-07320-8.