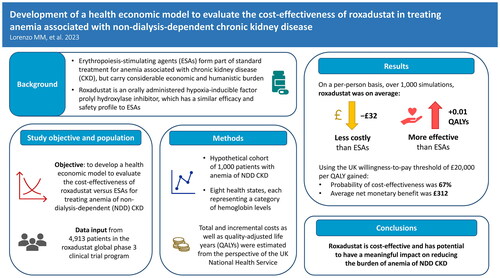
Abstract
Background
Treatment for anemia of chronic kidney disease (CKD) largely consists of erythropoiesis-stimulating agents (ESAs) with iron supplementation. Although ESAs are well-established and efficacious, their use has been associated with considerable economic and humanistic burdens. Roxadustat, an oral medication, is a hypoxia-inducible factor prolyl hydroxylase inhibitor that targets multiple causes of CKD and has a similar efficacy and safety profile to ESAs. The cost-effectiveness of this treatment, however, has yet to be investigated.
Objective
The study objective was to develop a health economic model to evaluate the cost-effectiveness of roxadustat compared with ESAs for treating anemia of non-dialysis-dependent (NDD) CKD.
Methods
A cohort-based model was developed for a hypothetical cohort of 1,000 patients with anemia of NDD CKD, incorporating eight health states, representing the hemoglobin level of each patient. The model was informed by individual patient-level data from the roxadustat global phase 3 clinical trial program. Total and incremental costs as well as quality-adjusted life-years (QALYs) associated with roxadustat versus ESAs were estimated from the perspective of the UK National Health Service. Sensitivity analyses were performed to assess the robustness of the model. Analyses exploring alternative scenarios were also conducted.
Results
On a per-person basis, over 1,000 simulations, roxadustat was found to be on average less costly (−£32) and more effective (+0.01 QALYs) than ESAs, with a dominant incremental cost-effectiveness ratio. The probability of cost-effectiveness at a £20,000 per QALY willingness-to-pay threshold from the UK perspective was 67%.
Conclusion
The model developed may be a useful instrument that, alongside expert clinical opinion, can inform clinical and policy decision-making regarding treatment of anemia of NDD CKD. The model highlights the cost-effectiveness of roxadustat, as well as its potential to have a meaningful impact in reducing the burden of anemia of NDD CKD.
Introduction
Chronic kidney disease (CKD) is a complex and multifaceted condition resulting in the gradual loss of kidney function and is defined by reduced estimated glomerular filtration rate (eGFR), proteinuria, or structural kidney diseaseCitation1. CKD can be categorized into five stages according to eGFR levels. People with early CKD have kidney damage with eGFR levels that are considered normal (stage 1: ≥ 90 mL/min/1.73 m2) or mildly decreased (stage 2: 60–89 mL/min/1.73 m2), while those with stage 3 have moderately decreased eGFR levels (30–59 mL/min/1.73 m2)Citation2. Severely decreased eGFR levels are indicative of advanced disease (stage 4: 15–29 mL/min/1.73 m2) and kidney failure (stage 5: <15 mL/min/1.73 m2)Citation2. When kidney function is significantly impaired, dialysis or a transplant is required. The global prevalence of CKD has been estimated at approximately 9%, with CKD stages 1 and 2 accounting for 5%, stage 3 for 4%, stage 4 at 0.16%, and stage 5 at 0.07%; the prevalence of dialysis is 0.04%, and kidney transplantation 0.01%Citation3.
Anemia, a reduction in red blood cell count and consequently in hemoglobin (Hb) levels (<13.0 g/dL in males and <12.0 g/dL in femalesCitation4) is a common complication of CKDCitation5. The prevalence and severity of anemia increase with CKD progression. For example, in an observational study of over 1,000,000 people registered at general practices in England, the proportion with anemia (defined as Hb ≤11.0 g/dL, as per National Institute for Health and Care Excellence [NICE] guidelines) was approximately 5.3%, 17.1%, 34.3% and 42.8% in patients with CKD stage 3a, 3b, 4 and 5, respectivelyCitation6. In the US, a survey of the general population calculated the percentage of anemia to be 17.4% in patients with CKD stage 3 to over 50% in those with stages 4 and 5Citation7.
Initial signs and symptoms of anemia in individuals with CKD include low energy, fatigue, pale skin, poor appetite, headaches, dizziness, rapid heartbeat, and shortness of breathCitation8. As anemia progresses, it is associated with significant fatigue, cognitive dysfunction, and reduced quality of life, with an increased risk of progression to adverse cardiovascular (CV) and kidney outcomesCitation9–14. Among patients with anemia of CKD in the same observational study from the UK, the prevalence of CV disease (CVD), such as heart failure (14.5%) and peripheral vascular disease (7.0%), was approximately twice that of patients with CKD and no anemia (6.1% and 3.6%, respectively), while ischemic heart disease (27.2% vs 17.1%) and stroke (18.2% vs 10.5%) were also more commonCitation6.
Current anemia management strategies include a combination of oral or intravenous (IV) iron, short or long-acting erythropoiesis-stimulating agents (ESAs), and red blood cell transfusion when necessaryCitation4,Citation15. Despite the clinical value of ESAs, their use is associated with a considerable economic burdenCitation16. In addition to the drug acquisition price and costs associated with refrigerated storage and transit, plus the disposal of sharps, the logistics of administration (subcutaneous or IV injection) is another cost consideration, particularly for the non-dialysis-dependent (NDD) population who do not have regularly scheduled dialysis appointments during which ESAs can be administeredCitation16. These direct healthcare costs may be further compounded by the treatment burden on both patients and caregivers, including the need for parenteral administration, as well as work productivity loss costs (due to patients’ time spent receiving therapy). Consequently, novel oral treatments have the potential to significantly alleviate such burdens.
Hypoxia-inducible factor prolyl hydroxylase (HIF-PH) inhibitors are a newer class of oral medication for CKD-related anemia that stimulate erythropoiesis by increasing both endogenous erythropoietin and iron availabilityCitation17. Roxadustat was the first HIF-PH inhibitor approved for the management of anemia associated with CKD in multiple countries including the UK and the European UnionCitation18,Citation19. The ALPINE global phase 3 clinical trial program comprised eight studies (both placebo- and active-controlled), enrolling >9,000 patients, designed to assess the efficacy and safety of roxadustat in different sub-populations with anemia of CKDCitation20. In trials evaluating roxadustat versus placebo in people with anemia associated with NDD CKD (ALPS, ANDES, OLYMPUS; ), roxadustat demonstrated superior efficacy versus placebo at increasing Hb while decreasing the need for rescue therapy, including red blood cell transfusion and IV iron, and with an adverse event (AE) profile comparable to placeboCitation21–27. In a trial comparing roxadustat with the ESA darbepoetin alfa in an NDD CKD population (DOLOMITES), roxadustat was non-inferior to darbepoetin alfa in achieving Hb response, while having a lower iron supplementation requirement (6.2% of patients randomized to roxadustat used IV iron during weeks 1–36, compared to 12.7% patients randomized to darbepoetin alfa)Citation28,Citation29. In addition, the safety profile of roxadustat was comparable to that of darbepoetin alfa, with no new safety signals observed over the study duration. Similar results were observed in trials with the dialysis-dependent (DD) CKD population (HIMALAYAS, ROCKIES, SIERRA, PYRENEES), in which roxadustat was efficacious for correcting and maintaining Hb levels with an AE profile comparable to ESAsCitation30–37.
Table 1. Roxadustat clinical trials in populations with NDD CKD stages 3–5.
While the efficacy and safety of roxadustat for managing anemia associated with CKD have been well-established, the cost-effectiveness of this treatment remains unexplored. In this study, a de novo health economic model was developed to evaluate the cost-effectiveness of roxadustat in treating anemia associated with NDD CKD.
Methods
Model overview
We developed a cohort-based proportion in-state model to estimate the cost-effectiveness of roxadustat compared to ESAs for treating anemia associated with CKD in NDD individuals, from the perspective of the UK National Health Service (NHS) and Personal Social Services. Ethics approval was not sought for this study as primary data were not collected; instead, we conducted a post hoc analysis of previously collected individual patient data. Data from the underlying studies for this cost-effectiveness model were collected in accordance with the appropriate ethical standards. The model employed a lifetime horizon (25 years based on a starting age of 63 years) to accommodate the chronic and progressive nature of CKD with a cycle length of 3 months and application of a half-cycle correction. Cycle length was chosen to capture the multiple changes in Hb levels a patient may experience during the year, and to align with guidelines that recommend monitoring of Hb levels in patients with NDD CKD every 3 monthsCitation38. The future value of both costs and benefits was discounted at a rate of 3.5% per annumCitation39. The model incorporated eight health states, representing different Hb levels (<7, 7 to 7.99, 8 to 8.99, 9 to 9.99, 10 to 10.99, 11 to 11.99, 12 to 12.99, and ≥13 g/dL); Hb categories were chosen based on those used for the relative risk of blood transfusion in a previous cost-effectiveness study of anemia treatment targets in patients with CKD stages 3–4Citation40.
To obtain the baseline utility value for the model population, utility decrements for CKD (using kidney complaints as a proxy) and dialysis were applied to age- and sex-adjusted general population utility norms calculated by Kind et al. Citation41. A CKD utility decrement of 0.033 was calculated by subtracting the absolute utility value of someone with kidney complaints (0.845) from the utility value of a similarly aged population without kidney complaints (0.878) Citation42. Utility decrements of 0.35 and 0.26 were applied for patients receiving hemodialysis and peritoneal dialysis, respectivelyCitation43.
Absolute utility values for each Hb category (health state) were derived from individual patient-level data using a generalized linear mixed model (GLMM) with a Gaussian distribution and an identity link, controlling for CVD history and diabetes mellitus status at baseline. Study IDs and subject IDs were used as random factors to control for nesting effects from using multiple studies and repeated measures of subjects. Utility decrements were then calculated for each health state by subtracting each calculated utility value from the utility value of reference Hb level >13 g/dL (Supplementary Table 1). These decrements were then applied to the model population utility values, independently of the treatment arm, throughout the lifetime horizon of the model to ensure that those with anemia never reported utility values higher than the general population.
The economic model included drugs under the class of epoetins within the British National Formulary (BNF): darbepoetin alfa, epoetin alfa, epoetin beta, epoetin zeta, and methoxy polyethylene glycol-epoetin beta. The roxadustat clinical trial program included two of these ESAs (epoetin alfa and darbepoetin alfa); however, all available ESAs were included to allow for future adaptation of the model to suit localized ESA use. An ESA class effect was assumed in the model design, with all ESAs expected to have equal effectiveness when prescribed at equivalent doses, based on supporting clinical dataCitation44. Following clinical input, it was assumed in the base case that 20% of NDD patients would require a home visit by a nurse, with an appointment time of 15 min, to aid ESA administration.
A series of interrelated statistical equations were used to derive health state occupancy, as well as generate estimates of the lifetime costs and benefits associated with treatment for anemia associated with CKD. The model structure () and settings () were informed by individual patient-level data from the roxadustat global phase 3 clinical trial program. Regression analysis was used to predict outcomes beyond the data collected in the clinical trials. Costs were informed by published sources, including list prices for roxadustat and ESAs (), although it is acknowledged that both would be offered to the NHS at a discount. Modelling assumptions and inputs, and the applicability of the model to the UK population, were validated by consultation with clinical and health economic experts at different phases of the model development process.
Figure 1. Model overview. Abbreviations. ESA, Erythropoiesis-stimulating agent; Hb, Hemoglobin; IV, Intravenous; QALY, Quality-adjusted life-year; TRAE, Treatment-related adverse event.
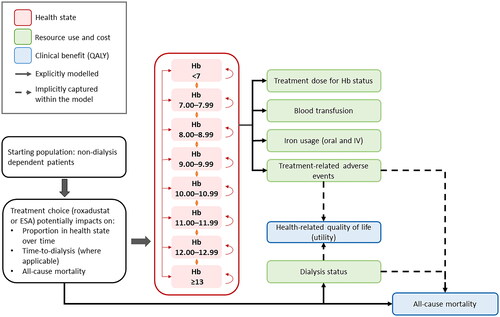
Table 2. Summary of model settings included in the base case and scenario analysis.
Table 3. Summary of unit costs in the economic model.
As the baseline patient characteristics were balanced across individual studies (Supplementary Table 2), data from the four NDD clinical trial datasets were pooled at the individual patient level. To account for any limitations with this approach, all statistical models accounted for any potential differences between clinical trials by using a hierarchical model structure and used study identification (ID) as a random effect to control for any impacts of “nesting” (i.e. patients from the same study are more likely to behave similarly compared to patients from another study).
The patient populations at the start of the model were distributed between the eight health states based on baseline Hb levels in the clinical trials (Supplementary Table 3). Mortality at each cycle, taking into account treatment type, diabetes, CVD history, eGFR at baseline, and interaction between treatment type and baseline eGFR, was calculated using a parametric survival function. Six functions were fitted to the data (exponential, Weibull [proportional hazard], Gompertz, log-normal, log-logistic, and generalized gamma). On the basis of the Akaike Information Criterion (AIC) and Bayesian Information Criterion (BIC) score, as well as long-term clinical plausibility (validated by clinical experts), the exponential distribution was used in the base case. The second-best choice (a Weibull function) was used to inform a sensitivity analysis. The coefficients from the all-cause mortality analysis that were used in the economic model are presented in Supplementary Table 4. The model has the option to include or exclude a treatment effect for roxadustat (hazard ratio [HR]) in the survival calculations. The HR was set to one in the base case calculations (i.e. no difference in mortality between arms) to align with clinical opinion, but a treatment effect was included in a scenario analysis.
Within each model cycle, all individuals who are alive are distributed across Hb categories using a multinomial regression equation (informed by individual patient-level data from the clinical trials), with the treatment type, time, diabetes at baseline, CVD history at baseline, and interaction between treatment type and time impacting the proportion of patients in each health state over time (Supplementary Figures 1–2). The health state 10 g/dL ≤ Hb < 10.99 g/dL was used as the reference case in all statistical analyses as it is within the clinical target Hb range (10 g/dL ≤ Hb < 12 g/dL). The coefficients and corresponding 95% confidence intervals from the proportion in state analysis that were used in the economic model are presented in Supplementary Tables 5a and b, respectively.
A further parametric survival function was used to estimate the proportion of living individuals who had progressed to dialysis at any time in the model, accounting for diabetes, CVD history, and eGFR at baseline. Treatment type was assumed to have no impact on time to dialysis and, as such, was not included as a covariable. From the six functions that were fitted to the data (exponential, Weibull [proportional hazard], Gompertz, log-normal, log-logistic, and generalized gamma), the log-logistic function was found to be the best in terms of visual fit to the Kaplan-Meier plot and long-term clinical plausibility (validated by clinical experts) and, as such, was used in the base case (an exponential distribution was explored in scenario analysis). The coefficients from the time to dialysis analysis used in the economic model are presented in Supplementary Table 6.
Additional statistical equations were used to quantify the dose of active therapy (roxadustat or ESA) received (GLMM with a Gamma distribution, accounting for Hb level, CVD history at baseline, and diabetic status at baseline), proportion needing IV iron (GLMM with a binomial distribution, accounting for Hb level, treatment type, CVD history at baseline, diabetic status at baseline, study ID, and an interaction between Hb level and treatment type), mean IV iron dose (GLMM with a Gaussian distribution, controlling for treatment type, CVD history at baseline, and diabetic status at baseline), proportion needing blood transfusions (GLMM with a binomial distribution, accounting for Hb level, treatment type, CVD history at baseline, and diabetic status at baseline), and red blood cell transfusion dose (GLMM with a Gamma distribution controlling for treatment type, CVD history at baseline, and diabetic status at baseline) (Supplementary Tables 7–11; Supplementary Figures 3–4).
Once the distribution between Hb levels in surviving patients was determined, the associated health-related quality of life (HRQoL), resource use, and cost were assigned according to the health state and calculated for each arm. All equations were re-run at the start of each new cycle with the total costs and quality-adjusted life-years (QALYs) calculated over the time horizon of the model.
Relationships between model inputs and outcomes that were implicitly (rather than explicitly) captured in the model were not analyzed directly but were based on cohort averages as observed in the roxadustat clinical trials. Therefore, as survival was an explicitly modeled outcome, the impacts of dialysis status and treatment-related adverse events (TRAEs) on mortality were implicitly captured. Similarly, as HRQoL was explicitly modelled, the impact of TRAEs on HRQoL was implicitly captured. This approach reduced the possibility of double counting outcomes with multiple inputs, resulting in a more conservative estimate of cost-effectiveness.
Three AEs were included in the economic model – stroke, myocardial infarction (MI), and vascular access thrombosis (VAT). Stroke and MI were chosen as AEs because the pre-existing literature notes their prevalence in CKD and end-stage kidney disease populationsCitation45. VAT was included following readout of the clinical trials, with data demonstrating that roxadustat was associated with an increased probability of VATCitation26,Citation29. Other TRAEs had either a low incidence rate or showed no significant difference between the roxadustat and ESA arms. The choice of AEs included in the model was validated by clinical experts. Owing to insufficient numbers of events for a robust regression analysis of TRAEs, the 3-monthly probability of a stroke (hemorrhagic, ischemic, and cerebellar), MI, or VAT was derived for each arm from the clinical trial data using the event rate and total patient exposure time (independent of Hb level) (Supplementary Table 12).
The likelihood of roxadustat being cost-effective was calculated using a probabilistic sensitivity analysis, in which inputs in the model were selected from an underlying probability distribution and the model was run 1,000 times, with each iteration using a different set of values for the inputs. The mean costs and QALY outcomes per patient were calculated and plotted on a cost-effectiveness plane. A willingness-to-pay (WTP) threshold of £20,000–£30,000 was applied to assess cost-effectiveness. Based on the NICE threshold range of £20,000–£30,000 per QALY for its standard technology appraisalsCitation46, we conducted probabilistic analyses (probability of cost-effectiveness and probabilistic sensitivity) at the lower (£20,000) and upper (£30,000) ranges of the WTP threshold.
Scenario analyses
Additional analyses were conducted exploring several alternative model settings/inputs, including an alternative mortality distribution (Weibull) and an alternative time-to-dialysis distribution (exponential).
In the clinical trials, a slight mortality advantage was observed with roxadustatCitation26,Citation29, but this was not considered clinically plausible and not included in the base case. However, a scenario was run whereby the treatment type all-cause mortality HR was applied to each population.
An additional scenario analysis was conducted to take into account the utility gains associated with different modes of treatment administration (injection vs. oral administration). Utility gains were based on the results of a discrete choice experiment (DCE) previously conducted to elicit the anemia treatment preferences of patients with NDD CKD, which found a statistically significant improvement in utility value with oral administration at home versus fortnightly subcutaneous injection at homeCitation47. The present study used incremental utilities of +0.041 for an oral pill three times weekly at home and +0.017 for a subcutaneous injection once every 4 weeks at home, with the reference level set as a subcutaneous injection once every 2 weeks at home. When weighted by the proportion of patients expected to be prescribed each regimen in the DCE (roxadustat: oral pill three times weekly at home, 100%; ESA: subcutaneous injection once every two or four weeks at home, 65% and 35%, respectively), the weighted utilities for patients receiving roxadustat and ESAs were 0.041 and 0.006, respectively.
Owing to the low level of TRAEs in the clinical trial data, another scenario analysis was conducted using published data to inform rates of stroke, MI, and VATCitation40,Citation48,Citation49, and generate Hb category-specific event probabilities rather than use a common value across all Hb categories (Supplementary Table 12).
In the UK, ESAs are made available as single-use pre-filled vialsCitation50–54. In the base case, a cost per microgram was used for the calculated ESA dose. In another scenario analysis, as each ESA contains different micrograms per injection, the number of injections required per cycle was calculated by rounding up the expected dose for each ESA to the smallest possible vial size of the ESA that would cover the full required dose (i.e. to administer a dose of 16.33 mcg of epoetin alfa, a 16.8 mcg epoetin alfa injection size would be used instead of a larger or smaller available vial size).
The functionality to maintain the proportion in state at any given time point was built into the model, which allowed us to test the sensitivity of the results to changes in proportion in state over time. To maintain proportion in the state after 5, 10, and 15 years, three scenarios were conducted.
Results
Base case cost-effectiveness analysis
In the base case analysis, on a per-person basis, over 1,000 simulations, roxadustat was found to be on average less costly (−£32) and more effective (+0.01 QALYs) than ESAs. Considering the NICE threshold of £20,000 per QALY gained, roxadustat was cost-effective, with a dominant incremental cost-effectiveness ratio (ICER), and an average net monetary benefit (NMB) of £312 (). From 1,000 simulations, 67.3% were cost-effective at a WTP threshold of £20,000/QALY gained (). It is noteworthy that 48% of simulations were cost-effective at a threshold of zero, representing the proportion of simulations that were cost-saving. At the threshold of £30,000, 75% of 1,000 simulations were found to be cost-effective (; Supplementary Figure 5).
Figure 2. Probabilistic results. Probabilistic sensitivity results are shown for incremental costs and benefits on the cost-effectiveness plane (a). individual points represent separate simulations, and the dashed line corresponds to the willingness-to-pay threshold of £20,000. Points below the dashed line would be considered cost-effective, while points to the right of the y-axis (positive change in QALY) and below the x-axis (decreased costs) are considered dominant. In the cost-effectiveness acceptability curve (B), the percentage of simulations is shown as a function of varying the willingness-to-pay threshold. Abbreviations. ICER, Incremental cost-effectiveness ratio; QALY, Quality-adjusted life-year.
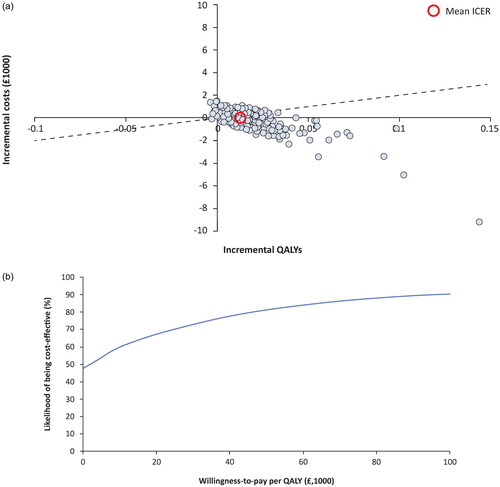
Table 4. Probabilistic results at WTP thresholds £20,000 and £30,000 per QALY gained.
Scenario analyses
Exploring alternative settings in the model demonstrated largely consistent results with the base case (). Overall, roxadustat remained either cost-effective or dominant compared to ESAs in all scenarios analyzed, except one: the scenario including a treatment effect on mortality. In this scenario, the ICER was £34,063, which is marginally over the upper limit commonly used by NICE in its decision-making process (£30,000 per QALY). This ICER was likely to be driven by higher life expectancy and treatment costs with roxadustat.
Table 5. Summary of scenario results at WTP thresholds £20,000 and £30,000 per QALY gained.
Discussion
The purpose of this study was to develop an economic model to evaluate the cost-effectiveness of roxadustat in treating anemia associated with NDD CKD. When considering evidence reported from the large roxadustat phase 3 clinical trial program at the established UK WTP threshold of £20,000–£30,000 per QALY gained, the model demonstrated that roxadustat is a more cost-effective treatment for anemia associated with NDD CKD compared to ESAs. The model was employed in NICE’s recent appraisal of roxadustat for treating anemia associated with CKD (Technology appraisal TA807), and consequently roxadustat was the first drug in class recommended for routine NHS use for treating symptomatic anemia associated with CKD in adults who have stage 3–5 CKD with no iron deficiency and are not on dialysis at the start of treatmentCitation55.
Exploratory scenario analyses suggested that the model was robust to changes in input parameters. With plausible variations to the input parameters, the ICER was likely to remain within the bounds that would typically be considered cost-effective in the UK. In the scenarios explored, roxadustat was, or was very nearly, cost-saving or cost-effective, compared to ESAs. The NMB associated with roxadustat was greatest when including a utility increment for the treatment administration method, highlighting the economic benefits that an oral treatment for anemia associated with CKD can provide over injectables. Beyond the economic benefits, the mode of treatment administration is considered by patients with NDD-CKD to be an important aspect of their anemia management, with a preference for oral medication over injectable treatmentsCitation47.
It is common for cost-effectiveness outcomes to vary across countries and geographic areas owing to numerous clinical and socioeconomic factors. It is therefore reassuring that our study results are supported by a study assessing the efficacy, tolerance, and cost-effectiveness of roxadustat treatment for anemia associated with NDD CKD from the perspective of the Chinese medical systemCitation56. Using a Markov model, the study found that roxadustat was more cost-effective than placebo. Although the time horizon was less than in the study reported here (5 years) and placebo, not ESAs, was used as the comparator, the data support the current findings in demonstrating the potential of roxadustat to be a cost-effective treatment for anemia associated with CKD.
Limitations of this study include those common to economic modelling analyses. Treatment discontinuation rates were not directly modelled owing to a lack of long-term follow-up data, which precluded accurately modelling its impact. Instead, treatment discontinuation was assumed to be captured, where possible, indirectly via the unique patient ID random effect included in all statistical analyses. Within the cost-effectiveness model, it is therefore assumed that the rate of treatment discontinuation that has been captured indirectly in the available trial data will continue at the same rate throughout the remainder of the time horizon. Furthermore, it is expected that treatment discontinuation and treatment switching will have negligible impacts on treatment outcomes. For example, if an individual receiving ESA switches treatment type, they would move to another ESA with equivalent efficacy and TRAE rates and, therefore, the impact would be negligible. At present, a person receiving roxadustat can only switch to an ESA, the only alternative treatment type currently available. As our model assumes that both treatments have the same survival profile and similar list prices and impacts on proportion in state, switching from roxadustat to an ESA would probably not have a substantial impact on the cost-effectiveness outcomes. However, there are costs associated with refrigerated storage and transit, disposal of sharps, and administration of a subcutaneous or IV injection that were not factored into our model and which would likely incur cost savings in favor of roxadustat. In addition, the model used in this study assumes that the effectiveness of roxadustat or any ESA remains constant throughout the time horizon in the base case. An additional limitation relates to the probability of the AEs stroke, MI, or VAT being derived for each arm from the clinical trial data using the event rate and total patient exposure time, independent of Hb level. As Hb levels have an impact on CV events, this limitation has the potential to influence the results.
A strength of this study is that we have used patient-level data from the roxadustat global phase 3 clinical trials in the NDD population to parameterize the model, including close to 5,000 patients. However, as in all health economic analyses based on clinical trials, the requirement to extrapolate outcomes beyond the observations of the clinical trials inherently introduces uncertainty. This uncertainty, though, has been addressed using robust statistical approaches and clinical and health economic expert validation. Furthermore, the scenario analysis exploring sensitivity of the results to changes in proportion in state over time (5, 10, 15 years) demonstrated that the model does not need long-term extrapolation to reach its conclusions. In addition, data from the UK Renal Registry show a similar proportion of patients in the target Hb state as that predicted by the modelCitation57. In terms of unit costs, national publicly available databases were utilized (NHS cost collection, Prescription Cost Analysis, and BNFCitation58–60) Remaining cost parameters were sourced from UK literature and adjusted to 2020 costs where necessary.
In conclusion, anemia associated with CKD places significant burden on the individual, their family and caregivers, as well as the healthcare system. Roxadustat, an oral medication, offers an alternative to ESAs, avoiding injections and hospital visits while also reducing IV iron requirements. We have developed a novel model to evaluate the cost-effectiveness of roxadustat in comparison to ESAs for treating anemia associated with CKD. The results of this de novo model highlight the cost-effectiveness of roxadustat, as well as its potential to have a meaningful impact in reducing the burden of anemia associated with CKD.
Transparency
Declaration of financial/other interests
MA and MML are employees of Astellas Pharma, Inc.
SM and JM are employees of York Health Economic Consortium, which received funds from Astellas Pharma Inc. for building the economic model.
Author contributions
Conceptualization: MA, MML, JM, SM.
Formal analysis and interpretation of data: JM, SM.
Writing – review and editing: JM, MA, MML, SM.
All authors have given final approval of the version to be published, and all authors agree to be accountable for all aspects of the work.
Reviewer disclosures
All peer reviewers on this manuscript have received an honorarium from JME for their review work.
One of the reviewers was on the NICE committee which evaluated Roxadustat in this indication.
Geolocation information
United Kingdom
Supplemental Material
Download MS Word (231.1 KB)Acknowledgements
The authors extend their thanks to Matthew Hall, of Nottingham University Hospitals NHS Trust, for clinical input, and John Cairns, of The London School of Hygiene & Tropical Medicine, for health economics expertise. Statistical support was provided by Martin Blogg, of Astellas Pharma, Inc. Model construction and design, and development of the study report was provided by Heather Davies, Angel Varghese, and Hannah Baker of York Health Economics Consortium, funded by Astellas Pharma, Inc. Medical writing support for this publication was provided by Angharad Morgan, and Iona Easthope, of Lumanity, funded by Astellas Pharma, Inc.
Data availability statement
The datasets analyzed during the current study were sourced from the original trial publications, the results of which have been fully referenced and are publicly available.
Researchers may request access to anonymized participant-level data, trial-level data and protocols from Astellas-sponsored clinical trials at www.clinicalstudydatarequest.com. For the Astellas criteria on data sharing see: https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Astellas.aspx.
Additional information
Funding
References
- Evans M, Lewis RD, Morgan AR, et al. A narrative review of chronic kidney disease in clinical practice: current challenges and future perspectives. Adv Ther. 2022;39(1):33–43. doi: 10.1007/s12325-021-01927-z.
- Levey AS, Eckardt KU, Tsukamoto Y, et al. Definition and classification of chronic kidney disease: a position statement from kidney disease: improving global outcomes (KDIGO). Kidney Int. 2005;67(6):2089–2100. doi: 10.1111/j.1523-1755.2005.00365.x.
- GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2020; 395(10225):709–733.
- Kidney Disease: improving Global Outcomes (KDIGO) Anemia Work Group. KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney International. 2012;Suppl. 2:279–335.
- Mikhail A, Brown C, Williams JA, et al. Renal association clinical practice guideline on anaemia of chronic kidney disease. BMC Nephrol. 2017;18(1):345. doi: 10.1186/s12882-017-0688-1.
- Dmitrieva O, de Lusignan S, Macdougall IC, et al. Association of anaemia in primary care patients with chronic kidney disease: cross sectional study of quality improvement in chronic kidney disease (QICKD) trial data. BMC Nephrol. 2013;14(1):24. doi: 10.1186/1471-2369-14-24.
- Stauffer ME, Fan T. Prevalence of anemia in chronic kidney disease in the United States. PLOS One. 2014;9(1):e84943. doi: 10.1371/journal.pone.0084943.
- National Kidney Foundation. Anemia and Chronic Kidney Disease 2015. Available from: https://www.kidney.org/atoz/content/what_anemia_ckd.
- van Haalen H, Jackson J, Spinowitz B, et al. Impact of chronic kidney disease and anemia on health-related quality of life and work productivity: analysis of multinational real-world data. BMC Nephrol. 2020;21(1):88. doi: 10.1186/s12882-020-01746-4.
- Hoshino J, Muenz D, Zee J, et al. Associations of hemoglobin levels with health-related quality of life, physical activity, and clinical outcomes in persons with stage 3-5 nondialysis CKD. J Ren Nutr. 2020;30(5):404–414. doi: 10.1053/j.jrn.2019.11.003.
- Toft G, Heide-Jørgensen U, van Haalen H, et al. Anemia and clinical outcomes in patients with non-dialysis dependent or dialysis dependent severe chronic kidney disease: a Danish population-based study. J Nephrol. 2020;33(1):147–156. doi: 10.1007/s40620-019-00652-9.
- Thorp ML, Johnson ES, Yang X, et al. Effect of anaemia on mortality, cardiovascular hospitalizations and end-stage renal disease among patients with chronic kidney disease. Nephrology (Carlton). 2009;14(2):240–246. doi: 10.1111/j.1440-1797.2008.01065.x.
- Locatelli F, Pisoni RL, Combe C, et al. Anaemia in haemodialysis patients of five European countries: association with morbidity and mortality in the dialysis outcomes and practice patterns study (DOPPS). Nephrol Dial Transplant. 2004;19(1):121–132. doi: 10.1093/ndt/gfg458.
- Eriksson D, Goldsmith D, Teitsson S, et al. Cross-sectional survey in CKD patients across Europe describing the association between quality of life and anaemia. BMC Nephrol. 2016;17(1):97. doi: 10.1186/s12882-016-0312-9.
- National Institute for Health and Care Excellence. NICE guideline [NG203]: chronic kidney disease: assessment and management. 2021. Available from: https://www.nice.org.uk/guidance/ng203.
- Gauthier-Loiselle M, Michalopoulos SN, Cloutier M, et al. Costs associated with the administration of erythropoiesis-stimulating agents for the treatment of anemia in patients with non-dialysis-dependent chronic kidney disease: a US societal perspective. J Manag Care Spec Pharm. 2021;27(12):1703–1713. doi: 10.18553/jmcp.2021.27.12.1703.
- Koury MJ, Haase VH. Anaemia in kidney disease: harnessing hypoxia responses for therapy. Nat Rev Nephrol. 2015;11(7):394–410. doi: 10.1038/nrneph.2015.82.
- Li QY, Xiong QW, Yao X, et al. Roxadustat: do we know all the answers? Biomol Biomed. 2023;23(3):354–363. doi: 10.17305/bb.2022.8437.
- National Institute for Health and Care Excellence. Roxadustat for treating symptomatic anaemia in chronic kidney disease. Technology appraisal guidance [TA807] 2022 [cited 2023 28/06/2023]. Available from: https://www.nice.org.uk/guidance/ta807/chapter/2-Information-about-roxadustat.
- European Medicines Agency. Evrenzo European Public Assessment Report 2021 [updated 08 June 2023; cited 2023 15 August 2023]. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/evrenzo.
- ClinicalTrials.gov. Roxadustat in the treatment of anemia in chronic kidney disease patients not requiring dialysis (ALPS) [10 July 2023]. Available from: https://clinicaltrials.gov/ct2/show/NCT01887600.
- Shutov E, Sułowicz W, Esposito C, et al. Roxadustat for the treatment of anemia in chronic kidney disease patients not on dialysis: a phase 3, randomized, double-blind, placebo-controlled study (ALPS). Nephrol Dial Transplant. 2021;36(9):1629–1639. doi: 10.1093/ndt/gfab057.
- ClinicalTrials.gov. A study of roxadustat for the treatment of anemia in participants with chronic kidney disease and Not Receiving Dialysis [10 July 2023]. Available from: https://clinicaltrials.gov/ct2/show/NCT01750190.
- Coyne DW, Roger SD, Shin SK, et al. Roxadustat for CKD-related anemia in non-dialysis patients. Kidney Int Rep. 2021;6(3):624–635. doi: 10.1016/j.ekir.2020.11.034.
- ClinicalTrials.gov. Safety and efficacy study of Roxadustat to treat anemia in patients with chronic kidney disease (CKD), not on dialysis [10 July 2023]. Available from: https://clinicaltrials.gov/ct2/show/NCT02174627.
- Fishbane S, El-Shahawy MA, Pecoits-Filho R, et al. Roxadustat for treating anemia in patients with CKD not on dialysis: results from a randomized phase 3 study. J Am Soc Nephrol. 2021;32(3):737–755. doi: 10.1681/ASN.2020081150.
- Provenzano R, Szczech L, Leong R, et al. Efficacy and cardiovascular safety of roxadustat for treatment of anemia in patients with non-dialysis-dependent CKD: pooled results of three randomized clinical trials. Clin J Am Soc Nephrol. 2021;16(8):1190–1200. doi: 10.2215/CJN.16191020.
- ClinicalTrials.gov. Roxadustat in the treatment of anemia in chronic kidney disease (CKD) patients, not on dialysis, in comparison to darbepoetin Alfa (dolomites). Available from: https://clinicaltrials.gov/ct2/show/NCT02021318.
- Barratt J, Andric B, Tataradze A, et al. Roxadustat for the treatment of anaemia in chronic kidney disease patients not on dialysis: a phase 3, randomized, open-label, active-controlled study (DOLOMITES). Nephrol Dial Transplant. 2021;36(9):1616–1628. doi: 10.1093/ndt/gfab191.
- ClinicalTrials.gov. Safety and efficacy study of roxadustat (FG-4592) for the treatment of anemia in end-stage Renal disease (ESRD) newly initiated dialysis participants (Himalayas) [10 July 2023]. Available from: https://clinicaltrials.gov/ct2/show/NCT02052310.
- Provenzano R, Shutov E, Eremeeva L, et al. Roxadustat for anemia in patients with end-stage renal disease incident to dialysis. Nephrol Dial Transplant. 2021;36(9):1717–1730. doi: 10.1093/ndt/gfab051.
- ClinicalTrials.gov. Safety and efficacy study of roxadustat to treat anemia in patients with chronic kidney disease, on dialysis [10 July 2023]. Available from: https://clinicaltrials.gov/ct2/show/NCT02174731.
- Fishbane S, Pollock CA, El-Shahawy M, et al. Roxadustat versus epoetin alfa for treating anemia in patients with chronic kidney disease on dialysis: results from the randomized phase 3 ROCKIES study. J Am Soc Nephrol. 2022;33(4):850–866. doi: 10.1681/ASN.2020111638.
- ClinicalTrials.gov. Study to evaluate the efficacy and safety of roxadustat in the treatment of anemia in participants with ESRD on stable dialysis [10 July 2023]. Available from: https://clinicaltrials.gov/ct2/show/NCT02273726.
- Charytan C, Manllo-Karim R, Martin ER, et al. A randomized trial of roxadustat in anemia of kidney failure: SIERRAS study. Kidney Int Rep. 2021;6(7):1829–1839. doi: 10.1016/j.ekir.2021.04.007.
- ClinicalTrials.gov. Roxadustat in the treatment of anemia in end stage renal disease (ESRD) patients on stable dialysis (Pyrenees) [10 July 2023]. Available from: https://clinicaltrials.gov/ct2/show/NCT02278341.
- Csiky B, Schömig M, Esposito C, et al. Roxadustat for the maintenance treatment of anemia in patients with end-stage kidney disease on stable dialysis: a European phase 3, randomized, open-label, active-controlled study (PYRENEES). Adv Ther. 2021;38(10):5361–5380. doi: 10.1007/s12325-021-01904-6.
- KDIGO. Chapter 1: diagnosis and evaluation of anemia in CKD. Kidney Int Suppl (2011). 2012;2(4):288–291.
- Attema AE, Brouwer WBF, Claxton K. Discounting in economic evaluations. Pharmacoeconomics. 2018;36(7):745–758. doi: 10.1007/s40273-018-0672-z.
- Yarnoff BO, Hoerger TJ, Simpson SA, et al. The cost-effectiveness of anemia treatment for persons with chronic kidney disease. PLOS One. 2016;11(7):e0157323. doi: 10.1371/journal.pone.0157323.
- Kind P, Hardman G, Macran S. UK population norms for EQ-5D. Discussion paper 172 cited 2023 15 August 2023]. Available from: https://www.york.ac.uk/che/pdf/DP172.pdf.
- Ara R, Brazier JE. Using health state utility values from the general population to approximate baselines in decision analytic models when condition-specific data are not available. Value Health. 2011;14(4):539–545. doi: 10.1016/j.jval.2010.10.029.
- National Institute for Health and Care Excellence. Single technology appraisal (STA). Specification for manufacturer/sponsor submission of evidence. 2012 cited 2023 17 August 2023]. Available from: https://www.nice.org.uk/guidance/ta358/documents/kidney-disease-autosomal-dominant-polycystic-tolvaptan-id652-committee-papers2.
- Chung EY, Palmer SC, Saglimbene VM, et al. Erythropoiesis-stimulating agents for anaemia in adults with chronic kidney disease: a network meta-analysis. Cochrane Database Syst Rev. 2023;2(2):Cd010590.
- Jankowski J, Floege J, Fliser D, et al. Cardiovascular disease in chronic kidney disease: pathophysiological insights and therapeutic options. Circulation. 2021;143(11):1157–1172. doi: 10.1161/CIRCULATIONAHA.120.050686.
- National Institute for Health and Care Excellence. Guide to the methods of technology appraisal 2013 [10 July 2023]. Available from: https://www.nice.org.uk/process/pmg9/chapter/the-appraisal-of-the-evidence-and-structured-decision-making.
- Alexandre AF, Morga A, Thomas C, et al. Preferences for anaemia treatment attributes among patients with non-dialysis-dependent chronic kidney disease. Adv Ther. 2023;40(2):641–657. doi: 10.1007/s12325-022-02367-z.
- Theidel U, Asseburg C, Giannitsis E, et al. Cost-effectiveness of ticagrelor versus clopidogrel for the prevention of atherothrombotic events in adult patients with acute coronary syndrome in Germany. Clin Res Cardiol. 2013;102(6):447–458. doi: 10.1007/s00392-013-0552-7.
- Lok CE, Bhola C, Croxford R, et al. Reducing vascular access morbidity: a comparative trial of two vascular access monitoring strategies. Nephrol Dial Transplant. 2003;18(6):1174–1180. doi: 10.1093/ndt/gfg122.
- National Institute for Health and Care Excellence. British National Formulary. Epoetin alfa [10 July 2023]. Available from: https://bnf.nice.org.uk/medicinal-forms/epoetin-alfa.html.
- National Institute for Health and Care Excellence. British National Formulary. Darbepoetin alfa [10 July 2023]. Available from: https://bnf.nice.org.uk/medicinal-forms/darbepoetin-alfa.html.
- National Institute for Health and Care Excellence. British National Formulary. Epoetin beta [10 July 2023]. Available from: https://bnf.nice.org.uk/medicinal-forms/epoetin-beta.html.
- National Institute for Health and Care Excellence. British National Formulary. Epoetin zeta [10 July 2023]. Available from: https://bnf.nice.org.uk/medicinal-forms/epoetin-zeta.html.
- National Institute for Health and Care Excellence. British National Formulary. Methoxy polyethylene glycol-epoetin beta [10 July 2023]. Available from: https://bnf.nice.org.uk/medicinal-forms/methoxy-polyethylene-glycol-epoetin-beta.html.
- National Institute for Health and Care Excellence. Technology appraisal guidance [TA807]: Roxadustat for treating symptomatic anaemia in chronic kidney disease 2022 [10 July 2023]. Available from: https://www.nice.org.uk/guidance/ta807.
- Hu Z, Tao H, Shi A, et al. The efficacy and economic evaluation of roxadustat treatment for anemia in patients with kidney disease not receiving dialysis. Expert Rev Pharmacoecon Outcomes Res. 2020;20(4):411–418. doi: 10.1080/14737167.2020.1747436.
- UK Renal Registry. 20th Annual Report of the Renal Association 2018 [10 July 2023]. Available from: https://ukkidney.org/sites/renal.org/files/publication/file-attachments/Full%20Annual-Report_0.pdf.
- NHS England. 2018/19 National Cost Collection Data Publication 2022 [10 July 2023]. Available from: https://www.england.nhs.uk/publication/2018-19-national-cost-collection-data-publication/.
- NHS Business Service Authority. Prescription Cost Analysis - England 2019 2021 [10 July 2023]. Available from: https://www.nhsbsa.nhs.uk/statistical-collections/prescription-cost-analysis/prescription-cost-analysis-england-2019.
- National Institute for Health and Care Excellence. British National Formulary 2023 [10 July 2023]. Available from: https://bnf.nice.org.uk/.
- National Institute for Health and Care Excellence. British National Formulary. Roxadustat [10 July 2023]. Available from: https://bnf.nice.org.uk/drugs/roxadustat/medicinal-forms/.
- Personal Social Services Research Unit. Unit Costs of Health and Social Care 2019 [10 July 2023]. Available from: https://www.pssru.ac.uk/project-pages/unit-costs/unit-costs-2019/.
- Personal Social Services Research Unit. Unit Costs of Health and Social Care 2020 [10 July 2023]. Available from: https://www.pssru.ac.uk/project-pages/unit-costs/unit-costs-2020/.
- National Institute for Health and Care Excellence. British National Formulary. Ferrous fumarate [10 July 2023]. Available from: https://bnf.nice.org.uk/medicinal-forms/ferrous-fumarate.html.
- National Institute for Health and Care Excellence. British National Formulary. Ferrous gluconate [10 July 2023]. Available from: https://bnf.nice.org.uk/medicinal-forms/ferrous-gluconate.html.
- National Institute for Health and Care Excellence. British National Formulary. Ferrous sulfate [10 July 2023]. Available from: https://bnf.nice.org.uk/medicinal-forms/ferrous-sulfate.html.
- National Institute for Health and Care Excellence. British National Formulary. Iron dextran [10 July 2023]. Available from: https://bnf.nice.org.uk/medicinal-forms/iron-dextran.html.
- National Institute for Health and Care Excellence. British National Formulary. Iron sucrose [10 July 2023]. Available from: https://bnf.nice.org.uk/medicinal-forms/iron-sucrose.html.
- National Institute for Health and Care Excellence. British National Formulary. Iron carboxymaltose [10 July 2023]. Available from: https://bnf.nice.org.uk/medicinal-forms/ferric-carboxymaltose.html.
- Xu XM, Vestesson E, Paley L, et al. The economic burden of stroke care in England, Wales and Northern Ireland: using a national stroke register to estimate and report patient-level health economic outcomes in stroke. Eur Stroke J. 2018;3(1):82–91. doi: 10.1177/2396987317746516.
- National Institute for Health and Care Excellence. Technology appraisal guidance [TA317]: Prasugrel with percutaneous coronary intervention for treating acute coronary syndromes 2014 [10 July 2023]. Available from: https://www.nice.org.uk/guidance/ta317.