Abstract
Aims
French guidelines for the management of non-muscle-invasive bladder cancer recommend that blue-light cystoscopy should be used in patients where the risk of missing residual tumor is highest. Despite evidence for its cost-effectiveness, budgetary concerns have limited uptake in France. The aim of this analysis was to model the cost-consequences of adopting the recommendations in a French urology unit.
Materials and methods
A budget impact model was developed in Excel, using a decision tree approach derived from guidelines issued by L’Academie franҫaise d’urologie. Risk profiles were derived from an analysis of studies using white-light cystoscopy; estimates for the impact of blue-light cystoscopy were derived from a published Cochrane Review. Costs were based on published tariff prices from L’Agence Technique de L’Information sur L’Hospitalisation. The model allowed results to be tailored to activity levels and projected blue-light usage in the chosen urology unit.
Results
Two scenarios were evaluated, based on a 3-year time horizon. Full implementation of all recommendations within a large public hospital was estimated to yield incremental costs of €269 per procedure (∼10% increase overall); a more targeted approach within a smaller private hospital yielded incremental costs of €133 per procedure (5% increase overall).
Limitations
The basis of the model is a change in the time to first recurrence. There are no data available for subsequent recurrences or progression, both of which could have an influence on expenditure. Secondly, recurrence rates for blue-light cystoscopy were not specifically available for each patient group identified in the guidelines: extrapolation of data may have resulted in bias. Finally, the data were derived from clinical trials, which may not be generalisable to real-world clinical practice.
Conclusions
The model has shown that the additional expenditure required to implement blue-light cystoscopy is modest and not disproportionate to the overall cost of care.
Introduction
Bladder cancer (BC) is the tenth most common cancer worldwide with more than 573,000 new cases in 2020, representing around 3% of all new global cancer diagnosesCitation1. Europe and North America have a relatively high incidence with ≥17.3 men and ≥4.5 women per 100,000 people diagnosed each yearCitation2. In France, BC is the fifth most common cancer, accounting for around 3.5% of new cancer diagnoses; in 2022, 16,492 people were diagnosed with BC in France, of whom 13,321 cases were menCitation3.
BC is predominantly a disease of the elderly – the average age of diagnosis being 73 yearsCitation4. In consequence, almost half of deaths recorded in the first 3 years after a bladder cancer diagnosis are not attributable to the cancer itselfCitation5. Having said that, bladder cancer cannot be considered an indolent tumor, especially for those patients with more advanced features at the time of diagnosisCitation6. In patients in whom the tumor is initially limited to the urothelium or lamina propria (non-muscle-invasive bladder cancer; NMIBC), 5-year cause-specific survival (excluding other causes of death) is around 91%Citation6. For those with a disease that has spread locally into the deeper bladder tissues (muscle-invasive bladder cancer; MIBC) this drops to around 48%, while for those patients with metastatic disease at the time of diagnosis, cause-specific survival falls to just 8%Citation6.
Reflecting this steep gradient of risk, the key objective of management for those diagnosed with NMIBC – approximately 75% of all new cases – is to maintain patients in this category for as long as possible, whilst maintaining the option to escalate treatment at an early stage of disease progression. The basis of this strategy is a combination of transurethral resection of bladder tumor (TURBT), coupled with ongoing routine surveillance using flexible cystoscopy. Where surveillance cystoscopy following initial treatment identifies potential areas of disease recurrence, further TURBT is indicated, doubling as both a diagnostic/staging procedure and a therapeutic intervention for those lesions that remain non-muscle-invasiveCitation7.
The strategy adopted for management and follow-up is determined by a qualitative risk assessment, incorporating tumor number, size, sub-stage and grade. The risk of both tumor recurrence and progression to MIBC rises with the risk category (), with those at the highest risk being most intensively surveilled and having the lowest threshold for radical intervention. summarises the management strategy by risk group, as defined by the latest guidelines from L’Academie franҫaise d’urologie (ccAFU)Citation7.
Figure 1. Simplified schematic of the 2020–2022 ccAFU guidelines for bladder cancer [adapted from Ref. Citation7].
![Figure 1. Simplified schematic of the 2020–2022 ccAFU guidelines for bladder cancer [adapted from Ref. Citation7].](/cms/asset/b571fe13-0c16-4c0d-872c-c371db119a95/ijme_a_2267929_f0001_b.jpg)
Table 1. Risk of recurrence and progression by risk group at baseline [based on data from Ref. Citation8].
Fundamental to the effectiveness of the treatment of NMIBC is the achievement of full resection of the cancer lesions. Incomplete resection is associated with a high risk of disease recurrence and potentially the development of higher grade disease. However, traditional technology using white light illumination for the TURBT procedure is associated with a high level of residual and missed tumors, with as many as 70% of patients having evidence of tumor found within 4 weeks of their index TURBTCitation9. This observation underlies the mandate from the guidelines to carry out a “second look” cystoscopy in high risk patients, but there is also a recommendation to use enhanced optical strategies to maximize the risk of the tumor being missed or inadequately resected. In addition, however enhancement of the diagnostic technique is also recommended.
White-light cystoscopy (WLC) has a sensitivity of 76% for the detection of tumors irrespective of grade and stage, falling to 55–68% for identifying flat lesions and carcinoma in situ (CIS). Whilst the use of digital optical enhancement technology (Narrow-band Imaging; NBI) may improve the sensitivity of the approach, the best evidence base relates to the use of blue-light cystoscopy (BLC) which, when used in combination with a pre-procedural intravesical fluorescent imaging agent (hexaminolevulinate; Hexvix), has been shown to substantially improve the initial tumor detection rate and downstream recurrence-free survivalCitation10. There is also some evidence suggestive that the time to disease progression is extended following the use of BLCCitation10.
Consequently, the French guidelines make strong recommendations that BLC should be used:
For the initial diagnostic TURBT in all but the smallest unifocal tumors
For the second look cystoscopy when cytology and the absence of papillary lesions with WLC suggest the presence of CIS.
For treating recurrent NMIBC in:
All low-risk patients
Small Ta low grade tumors in intermediate-risk patients
Suspicion of CIS in high-risk patients.
The health economic impact of this strategy has been evaluated using a cost-utility analysis, from the perspective of the French healthcare system, and BLC was found to be dominant over WLC – meaning that its use results in better outcomes and lower costs overallCitation11. Despite the existence of a clear clinical and economic evidence base, however, concerns around the potential budgetary impact of the widespread adoption of BLC have led to a degree of reluctance to adopt the technology. In order to address this, we have developed a budget impact model that allows the simulation of the overall costs of implementing a range of strategies involving the use of BLC, which allows the cost consequences of moving towards the recommended management approach to be evaluated by individual organizations.
This paper describes the approach adopted and presents examples of the likely cost outcomes, based on a range of plausible input assumptions.
Methods
A budget impact model analyzing the 3-year cost impact of adopting BLC in NMIBC was developed in Microsoft Excel. The approach adopted in the model was to simulate the costs attributable to primary TURBT and the management of recurrent disease in patients newly diagnosed with NMIBC within the French healthcare system. The perspective of the model is a single hospital or group of hospitals, with cost impacts being calculated based on whether the organization being modeled is a public or private healthcare provider. The basis of the care pathway modeled is the approach recommended in the ccAFU guidelines (), which was used to develop a decision tree structure within the Excel model.
The model was intended to be used as part of an interactive discussion, with the user being prompted to enter a range of parameters in order to ensure that the model results reflected local circumstances. Default values based on literature-based sources and clinical advice were built in to the model, with the user being invited to overwrite these with local data, where available and appropriate. The clinical, organizational and cost parameters included in the model are listed in and , together with an indication of whether these are fixed or user-modifiable inputs.
Table 2. Summary of clinical and organizational parameter inputs in the budget impact model.
Table 3. Summary of cost parameter inputs in the budget impact model.
The specified inputs are used to generate a profile for the cohort of patients entering the care pathway in year 1 of the model. Over a 3-year time horizon, progression to the first disease recurrence is tracked – an event that is assumed to trigger a subsequent TURBT. Recurrence rates are modeled based on an analysis of the baseline risk distribution and annual recurrence rates seen in an analysis of 3-year outcomes from seven EORTC randomized controlled trials carried out in NMIBC patientsCitation8. In years 2 and 3 of the model, two further cohorts of newly diagnosed patients were added to the model, reflecting ongoing disease incidence. The total follow-up duration in the model ranged from 36 months for the year 1 cohort to 12 months for the year 3 cohort.
Costs were captured based on the tariff payment made to the hospital, offset by the expected actual expenditure incurred by the hospital, including the cost of Hexvix in patients undergoing BLCCitation11,Citation12. Unlike some other European countries, the French tariff system does not distinguish between a TURBT carried out using WLC and BLC. Consequently, all patients undergoing a TURBT, regardless of the technology used, will attract the same Diagnosis-Related Group (DRG) payment to the hospital (11C13: Interventions par voie trasurétrale ou transcutanée pour les affectation non lithiasiques). Any budgetary impact offset with BLC use is consequently purely attributable to a reduction in the requirement for subsequent TURBT after the identification of disease recurrence. Costs associated with surveillance flexible cystoscopy are not included within the model, as these would be expected to be the same for all patients in a given risk group – both WLC and BLC.
The actual tariff payment made is modified according to the case complexity and length of stay (see ). For the default analysis, the nationally published distribution of tariff levels have been applied, although the user of the model is able to adjust these to reflect local circumstances, if data are available. Average costs incurred are also applied using national average data, although in this case it is unlikely that the user would have access to alternative figures and the input for this variable is consequently fixed within the model. Full details of the cost parameters applied in the model are listed in .
The final estimation of the budget impact of the use of BLC was calculated by comparing the aggregate costs incurred within an organization over a 3-year period both with and without the use of BLC. Net costs represented the difference between costs incurred by the hospital (procedure +/− Hexvix cost) and the tariff payment received. This result was finally translated into a mean incremental cost per patient entering the model.
Results
The model has been developed as an interactive tool that will output organisation-specific results. For the purposes of illustration, we have analyzed the results for two different theoretical scenarios:
A large public hospital implementing the specific BLC recommendations within the ccAFU guidelines for 300 new patients per year
A small private hospital, using BLC in a more high-risk targeted subgroup from a cohort of 100 new patients per year
Given that this is intended to be a user-modifiable analysis, allowing for local variation in practice, there is considerable scope for users to modify the default values used in the model. For the purposes of this description, in each of the two scenarios the default values listed in and have been used for calculation of the cost consequences. For the exclusively user-specified inputs, the values shown in have been used.
Table 4. User-specified inputs in model scenarios.
shows the outputs from scenario 1, while shows the outputs from scenario 2.
Figure 2. Results – scenario 1 model outputs for large public hospital implementing ccAFU recommendations in full. (A) Costs without BLC. (B) Costs with BLC.
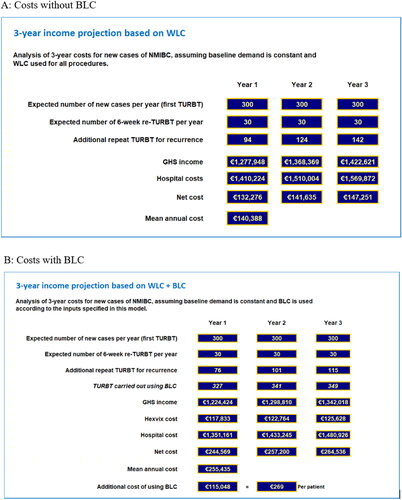
Figure 3. Results – scenario 2 model outputs for small private hospital implementing a high risk-targeted subset of the ccAFU recommendations. (A) Costs without BLC. (B) Costs with BLC.
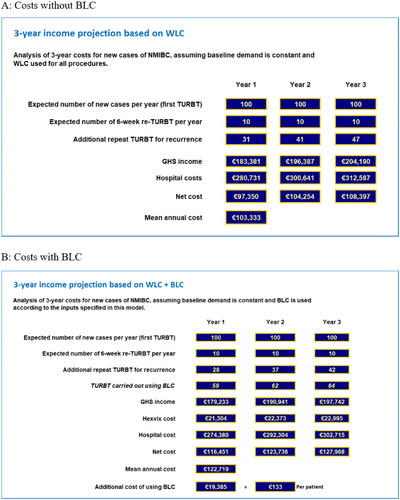
Scenario 1 – a large public hospital implementing the ccAFU recommendations in full – will incur a net mean additional cost of €115.048 per year, equating to €269 per procedure. By contrast in Scenario 2 – a smaller private hospital implementing a more targeted approach – the net mean additional cost is €19.385 per year, equating to €133 per procedure. Although both scenarios yield net additional costs that are substantially lower than the incremental cost of Hexvix, by preferentially targeting the group at higher risk of disease recurrence, scenario 2 yields a net additional cost per procedure approximately half that seen in scenario 1.
Discussion
Given its high incidence and relatively long survival times, overall costs for treatment and long-term surveillance in bladder cancer remain higher than many other cancers. Data from systemic literature reviews have demonstrated that the use of BLC-guided TURBT are associated with improved diagnostic sensitivity and an extended time to first disease recurrence compared with conventional WLC-TURBTCitation10,Citation14,Citation15, a result that has recently been confirmed in an analysis of real-world evidenceCitation16. The use of BLC has been shown to be particularly valuable in the detection of CIS – an area where WLC tends to fall short. In two randomised controlled trials using Hexvix, CIS detection rates vs WLC were 96% vs. 68% and 96% vs. 77%Citation17,Citation18, results confirmed by prospective observational real world evidence, which has shown detection rates of 91% vs. 78%Citation19.
The most recent ccAFU guidelines recommend the use of BLC in a number of different positions in the care pathway for NMIBC. This paper has presented the results of a budget impact model that estimates the financial consequences of implementing this strategy for an individual hospital within the French healthcare system. Although BLC incurs an additional cost of €360 per case for the Hexvix instillation, this cost is partially offset by a reduced requirement for subsequent TURBT, attributable to the anticipated reduction in disease recurrence rates. Based on the two scenarios explored in this paper, full implementation of the ccAFU guideline recommendations would be expected to yield a net cost increase of around €269 per procedure, while a more targeted strategy based on a higher risk subgroup treated in the private sector was shown to yield a net cost differential of €133 per patient. Given that the mean overall cost of care for these patients ranges from €1.991 in the private sector to €3.376 in the public sector, it can be seen that the use of BLC is likely to be associated with an incremental cost of around 5–10% of the index procedural cost.
Implications for practice
The use of BLC rather than WLC for TURBT, especially in high risk patients or in those where CIS is suspected, has achieved wide acceptance in European practice. There remain, however, uncertainties around the net cost impact of this approach that have limited its uptake. In France particularly, where the hospital payment system does not allow for an uplift in the tariff paid for BLC vs WLC, this has meant that, despite the recommendations within the ccAFU, there is currently limited usage of the technique. Our analysis shows that, although the incremental cost of BLC is partially offset by a reduction in early disease recurrence and consequent repeat TURBT, there is nonetheless a 10% increase in cost per patient if the ccAFU guidance on BLC are implemented in full.
Clinical advice, however, is that in practice BLC use is fairly narrowly targeted to the higher risk subgroups where the median time to recurrence is substantially less than for the TURBT population as a wholeCitation8. By modeling this approach, we have demonstrated that the supplementary cost can be brought down substantially. This potentially offers an opportunity to maximize clinical gains, while minimizing the additional expenditure required.
In other European countries the payment structure is different. In the UK, for instance, the hospital tariff for a WLC is £1774, while the tariff for a BLC is £2682. Clearly, in this situation there is no overt budgetary disincentive to the use of BLC, but is worth noting that the technique nonetheless tends to be targeted at the high-risk population, with WLC (with or without digital enhancement) still being used for the lower risk patient groups. Implementation of a similar approach within the French system would be consistent with the guidelines – albeit focussing on a subgroup of patients – while maintaining the additional costs within a manageable level.
Limitations
These estimates are clearly based on a number of underlying assumptions that, although based on published evidence, may not reflect the actual circumstances in an individual hospital. For this reason, tailored local estimates will need to be calculated to ensure applicability to the local care context. Given that the model is based on the individual user determining the input parameters, albeit guided by literature-based default estimates, carrying out traditional deterministic or probabilistic sensitivity analyses was neither possible nor meaningful. The results presented in this report should consequently be considered exemplar scenarios, rather than a definitive base case.
The driver of cost offset in this model is a reduction in the risk of disease recurrence in the group of patients undergoing BLC-assisted TURBT. All the published data for this outcome, however, relate to the time to the first episode of recurrence. We were unable to identify any studies that presented time to second or subsequent recurrence. Any residual benefit of BLC in reducing the risk of downstream recurrences will therefore not have been captured in this analysis, potentially underestimating the total cost savings relating to reduced future event rates.
BLC tends to be used preferentially in patients at high risk of recurrence and progression. For this reason, we tried to obtain estimates of the specific benefit of BLC vs WLC in a high risk subgroup to inform the model. However, the published RCT evidence for Hexvix is based on mixed risk groups and we were unable to extract risk-specific hazard ratios for the high-risk patients alone. We consequently used estimates derived from all risk groups combinedCitation10.
Furthermore, we have ignored any possible effect on disease progression. In a recently published Cochrane review, the authors identified an extended time to disease progression in patients managed with BLC-assisted TURBT (HR = 0.64 l 95% CI: 0.46–0.90). However, two of the six studies included in the analysis used an alternative fluorescent agent − 5-aminolaevulinic acid rather than Hexvix. Additionally, the largest study in the meta-analysis, which contributed 62% of the weighting, only presented data for 9 months follow-up. In order to avoid excessive uncertainty in the model, we did not include a component relating to reduced progression risk. Given the high costs of managing progression to MIBC, this will have potentially had a significant negative impact on the cost offset calculated by the model.
The BIM (Figure S1) focused on direct medical costs incurred in the French healthcare system. It did not consider indirect or societal costs related to the disease itself or the consequences of its treatment.
Finally, there are inherent limitations related to the BLC procedure itself. Studies have shown that WLC and BLC detection rates for NMIBC in low/intermediate risk patients are fairly similar − 78% and 80% respectivelyCitation20, with the greatest differences between the two approaches being seen in high risk patients – particularly those with CISCitation17–19. However, patient-level EORTC risk category data is not readily available for BLC clinical trials leading to the assumption that all patients have an equal likelihood for progression and benefit from BLC. Consequently, recurrence rates for BLC-assisted patients were estimated by applying a single overall hazard ratio estimate to all patient groupsCitation10. Whilst it is unlikely that this approach is entirely correct, it is difficult to say whether the overall effect will favor or detract from the impact of BLC.
BLC requires an extra step of instillation and the patient needs to arrive an hour earlier than for standard WLC, which may render this procedure more time and labor consuming than WLC alone. In the absence of specific resource utilization data, this element was excluded from the modeling. Further research should seek to understand other barriers to the adoption of this technique at both the hospital and provider level.
Conclusion
BLC-assisted TURBT using Hexvix has been shown to be a beneficial intervention for patients with NMIBC. There is clear evidence that tumor detection rates and recurrence-free survival time are both improved vs WLC and there is fair evidence that its use is also associated with an extended time to disease progression. Using a model of patient care that reflects the current recommendations of the ccAFU in France, we have shown that the additional expenditure required to implement BLC-assisted TURBT within individual hospitals is modest and not disproportionate to the overall cost of care for these patients. More nuanced targeting of BLC use has the potential to further improve the budget impact, while future research relating to subsequent event rates and progression risk offer the potential to move towards cost neutrality.
Transparency
Declaration of funding
The article received funding from Photocure GmbH.
Declaration of financial/other relationships
JB Medical Ltd is a market access consultancy and receives funding from the medical device and pharmaceutical industry to provide support and advice in gaining market access. JB Medical has received funding from Photocure for this project and similar projects across Europe.
Reviewer disclosures
A reviewer on this manuscript has disclosed that they are a consultant and on the advisory board for Photocure. The other peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Supplemental Material
Download PNG Image (80.8 KB)Acknowledgements
None stated.
References
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660.
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492.
- WHO, International Agency for Research on Cancer. Factsheet: France (GLOBOCAN 2020). 2021. https://gco.iarc.fr/today/data/factsheets/populations/250-france-fact-sheets.pdf.
- Cancer.Net. Bladder Cancer: statistics. 2023.
- Zhai M, Tang C, Li M, et al. Short term mortality risks among patients with non-metastatic bladder cancer. BMC Cancer. 2020;20(1):1148. doi: 10.1186/s12885-020-07655-x.
- Abdollah F, Gandaglia G, Thuret R, et al. Incidence, survival and mortality rates of stage-specific bladder cancer in United States: a trend analysis. Cancer Epidemiol. 2013;37(3):219–225. doi: 10.1016/j.canep.2013.02.002.
- Rouprêt M, Pignot G, Masson-Lecomte A, et al. Recommandations françaises du comité de cancérologie de l’AFU – actualisation 2020–2022: tumeurs de la vessie. Prog Urol. 2020;30(12S):S78–S135. doi: 10.1016/S1166-7087(20)30751-X.
- Sylvester RJ, van der Meijden A, Oosterlinck W, et al. Predicting recurrence and progression in individual patients with stage ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2,596 patients from seven EORTC trials. Eur Urol. 2006;49(3):466–477. doi: 10.1016/j.eururo.2005.12.031.
- Adiyat KT, Katkoori D, Soloway CT, et al. Complete transurethral resection of bladder tumor": are the guidelines being followed? Urology. 2010;75(2):365–367. doi: 10.1016/j.urology.2009.08.082.
- Maisch P, Koziarz A, Vajgrt J, et al. Blue versus white light for transurethral resection of non-muscle invasive bladder cancer. Cochrane Database Syst Rev. 2021;12(12):CD013776. ArtNoCD013776
- Rouprêt M, Malavaud B, Molinier L, et al. Coût-efficacité de la resection transurétrale de vessie en lumière bleue chez les patients atteints d’un cancer de la vessie non infiltrant en France. Prog Urol. 2015;25(5):256–264. doi: 10.1016/j.purol.2015.01.004.
- Agence Technique de L’Information sur L’Hospitalisation. Tarifs MCO et HAD. https://www.atih.sante.fr/sites/default/files/public/content/1568/tarif_arrete_2023.xlsx.
- ScanSanté. Analyse des coûts. 2019. https://www.scansante.fr/applications/enc-mco.
- Russo G, Sholklapper T, Cocci A, et al. Performance of narrow band imaging (NBI) and photodynamic diagnosis (PDD) fluorescence imaging compared to white light cystoscopy (WLC) in detecting non-muscle invasive bladder cancer: a systematic review and lesion-level diagnostic meta-analysis. Cancers. 2021;13(17):4378. doi: 10.3390/cancers13174378.
- Veeratterapillay R, Gravestock P, Nambiar A, et al. Time to turn on the blue lights: a systematic review and meta-analysis of photodynamic diagnosis for bladder cancer. Eur Urol Open Sci. 2021;31:17–27. doi: 10.1016/j.euros.2021.06.011.
- Das S, Gu L, Eve CT, et al. The impact of blue light cystoscopy use among nonmuscle invasive bladder cancer patients in an equal access setting: implications on recurrence and time to recurrence. Clin Genitourin Cancer. 2023;S1558-7673(23)00098-8. doi: 10.1016/j.clgc.2023.04.011.
- Jocham D, Witjes F, Wagner S, et al. Improved detection and treatment of bladder cancer using hexaminolevulinate imaging: a prospective, phase III multicenter study. J Urol. 2005;174(3):862–866; discussion 866. doi: 10.1097/01.ju.0000169257.19841.2a.
- Schmidbauer J, Witjes F, Schmeller N, et al. Improved detection of urothelial carcinoma in situ with hexaminolevulinate fluorescence cystoscopy. J Urol. 2004;171(1):135–138. doi: 10.1097/01.ju.0000100480.70769.0e.
- Daneshmand S, Bazargani ST, Bivalacqua TJ, et al. Blue light cystoscopy for the diagnosis of bladder cancer: results from the US prospective multicenter registry. Urol Oncol. 2018;36(8):361.e1–361.e6. doi: 10.1016/j.urolonc.2018.04.013.
- Mowatt G, N'Dow J, Vale L, et al. Photodynamic diagnosis of bladder cancer compared with white light cystoscopy: systematic review and meta-analysis. Int J Technol Assess Health Care. 2011;27(1):3–10. doi: 10.1017/S0266462310001364.
