?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Studies found a strong association between HLA-B*13:01 allele and co-trimoxazole-induced severe cutaneous adverse reactions (SCARs). Genetic screening before initiation of co-trimoxazole may decrease the incidence of co-trimoxazole-induced SCARs. This study aims to evaluate the cost-effectiveness of HLA-B*13:01 screening before co-trimoxazole initiation in HIV-infected patients in Thailand. A combination of a decision tree model and a Markov model was used to estimate lifetime costs and outcomes of two strategies including 1) HLA-B*13:01 screening before co-trimoxazole initiation and 2) usual practice from a societal perspective. Alternative drugs are not considered because dapsone (the second-line drug) also presents a genetic risk. Input parameters were obtained from literature, government documents, and part of the TREAT Asia HIV Observational Database (TAHOD). One-way sensitivity analyses and probabilistic analyses were performed to determine robustness of the findings. HLA-B*13:01 screening resulted in 0.0061 quality-adjusted life years (QALYs) loss with an additional cost of 370 THB ($11.84). At the cost-effectiveness threshold of 160,000 THB ($5,112.85), the probability of the genetic screening strategy being cost-effective is 9.54%. This analysis demonstrated that HLA-B*13:01 allele screening before initiation of co-trimoxazole among HIV-infected patients is unlikely to be cost-effective in Thailand. Our findings will help policymakers make an evidence-informed decision making.
Introduction
Severe cutaneous adverse reactions (SCARs) are rare but life-threateningCitation1. SCARs generally include Steven-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), SJS/TEN overlap, drug reaction with eosinophilia, and systemic symptoms (DRESS), and acute generalized exanthematous pustulosis (AGEP)Citation2. These reactions primarily involve skin and may also develop systemic involvement and can lead to hospitalizationCitation3. Mortality rates of SJS, TEN, and DRESS are approximately 5%-12.5%, 50%, and 10%, respectivelyCitation4–6. Moreover, SCARs can result in long-term sequelae in surviving patients. Mucocutaneous and ocular complications are common in SJS/TEN, while DRESS can involve end-organ failure and autoimmune diseaseCitation7,Citation8. Over 80% of SCARs cases are associated with medication useCitation1. A number of studies reveal that genetic factors are associated with the development of SCARsCitation9–13.
Co-trimoxazole, a combination of sulfamethoxazole and trimethoprim, is one of the common causal drugs of SCARsCitation4,Citation14. Co-trimoxazole is the first-line drug for Pneumocystis (carinii) jirovecii pneumonia (PCP) prophylaxis in HIV-infected patients, and is also indicated for the treatment of several infectionsCitation15. In 2020, a study demonstrated that HLA-B*13:01 was significantly associated with co-trimoxazole-induced DRESS in Thai populationCitation16. Additionally, another study reported a strong association between the HLA-B*13:01 allele and co-trimoxazole-induced SCARs in the Asian population as well as in the Thai population, separatelyCitation17.
Genetic screening for some alleles has been recommended in clinical practice to minimize the incidence of SCARsCitation9,Citation18. A screening for HLA-B*13:01 may help prevent co-trimoxazole-induced SCARs and their consequences, mainly since the frequency level of HLA-B*13:10 is high in the Thai population (11.49%)Citation19. Given the increasing role of health economics in supporting policy in allocating limited resources, a cost-effectiveness study is needed to help evidence-informed decision-making, especially in countries like Thailand, where the government is the main payer for the majority of the population in the health systemCitation20–23. This study aims to evaluate the cost-effectiveness of HLA-B*13:01 screening before initiating co-trimoxazole in HIV-infected patients from a societal perspective.
Methods
Overall description
This study evaluated the cost-effectiveness of HLA-B*13:01 screening before co-trimoxazole initiation compared with usual practice, from a societal perspective. The usual practice is defined as the patients receiving co-trimoxazole if they are indicated and stopping the drug if SCARs develop. The most common indication for co-trimoxazole used in Thailand is to prevent PCP in the HIV-infected population. According to the Thailand National Guidelines on HIV/AIDS Diagnosis, Treatment and Prevention 2021/2022Citation15, co-trimoxazole for PCP prevention is prescribed for patients with a CD4 count of fewer than 200 cells/µL until their CD4 count is higher than 200 cells/µL for at least 6 months. The target population of this study is patients diagnosed with HIV with a CD4 count of fewer than 200 cells/µL. A lifetime time horizon was applied to evaluate long-term cost-effectiveness. An annual discount rate of 3% was applied to cost and outcomes. This work is reported in accordance with the Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022)Citation24 and complies with the Guidelines for health technology assessment (HTA) in Thailand (Second edition)Citation25.
Model description
This study utilized the decision tree model followed by the Markov model ( and ). The models were developed in Microsoft Excel (Microsoft, Redmond, WA). A decision tree model was developed to estimate short-term costs and outcomes, including the incidence of SCARs and their consequences in the first 14 days of co-trimoxazole initiation, which would cover the period of SCARs treatment. As demonstrated in the study by Konyoung et al. the average length of stay for the treatment of SCARs was 12.05 days (ranging from 1-108 days)Citation26. We assumed that the period from SCARs occurred to the treatment completed was 14 days. The Markov model was used to evaluate long-term cost-effectiveness. The model was based on a hypothetical cohort of 100,000 patients.
Figure 1. 14-Day decision tree model comparing the strategies of HLA-B*13:01 screening and current practice in HIV-infected patients with a CD4 count of less than 200 cells/µL.
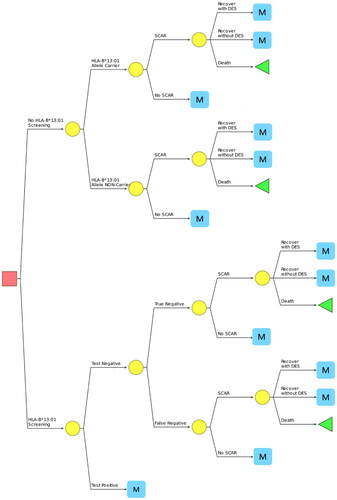
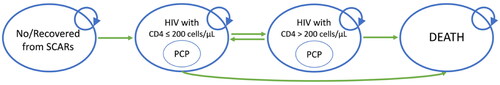
Figure 2. Markov model. The four main health states of the model are represented by the ovals, and transitions between states are represented by arrows. Surviving patients after 14 days of co-trimoxazole initiation entered the “no SCAR” health state.
The analysis began with the targeted population either receiving HLA-B*13:01 genetic screening or starting co-trimoxazole without the genetic screening. Even though dapsone has been recommended as the second-line medication for PCP preventionCitation27, it was found to have a strong association with the development of SCARs in the Thai population carrying HLA-B*13:01Citation28. Therefore, in this model, dapsone was not considered an alternative among those with a positive test of HLA-B*13:01 genetic screening. Another alternative for PCP prevention is pentamidineCitation27, however, it is not available broadly in Thailand. Thus, it seems that no alternative therapy is available for PCP prevention among patients with HLA-B*13:01 positive in Thailand. As a result, in our model, if the genetic screening result was positive, the patients would not receive any medications for PCP prevention. Whereas patients whose screening result was negative would receive co-trimoxazole as indicated. This was based on a consultation with an infectious disease expert in Thailand.
Patients receiving co-trimoxazole may develop SCARs which could either recover or die. Consequently, those who recovered from SCARs may be either fully recovered or recovered with long-term sequelae. Given that the sequelae of SCARs could involve several systems without an explicit patternCitation6, we assume that the sequela for patients who recovered from SCARs was dry eye syndrome (DES), which is a common long-term sequela for patients who have experienced SJS/TENCitation29.
As shown in , all surviving patients entered the “high risk” health state of the Markov model. A patient could have transitioned to another health state every 6-month, which is a cycle length of our model. Health states included “high risk” which is defined by CD4 levels as less than 200 cells/µL, “low risk” which CD4 count of 200 cells/µL and more, and “death.” Alive patients could have PCP infections regardless of their health states. We assume that patients with PCP infection would not have re-infection in the next cycle, and patients changing health state from “high risk” to “low risk” would not be infected in the transition cycle. Patients who had PCP infection received treatment following the Thailand National Guidelines on HIV/AIDS Diagnosis, Treatment and Prevention 2021/2022Citation15. The treatment refers to trimethoprim 15-20 mg/kg/day and sulfamethoxazole 75-100 mg/kg/day in those who are able to use co-trimoxazole. On the other hand, clindamycin 1,200 mg/day in combination with primaquine 30 mg daily for 21 days is prescribed as an alternative treatment for those who are not able to use co-trimoxazole. After receiving the treatment, we assumed that they would either recover or die within one cycle. Full compliance from patients was also assumed.
The primary outcome was lifetime costs, quality-adjusted life-years (QALYs), and incremental cost-effectiveness ratio (ICER) The model started with patients aged 18 years old and continued until patients reached the age of 100 years. The model structures were reviewed and validated by a health economic expert (NC) for the health-economic aspect and an infectious disease specialist for the clinical aspect (SK).
Input parameters
Model input parameters included epidemiology data, test characteristics, probabilities, costs, and utility (). These parameters were obtained from literature, published government document, and hospital databases.
Table 1. Input parameters.
Epidemiology
The incidence of co-trimoxazole-induced SCARs in Thai subjects is 3.14 per 1000 persons based on the previously published study by Limkobpaiboon et al.Citation30 According to a study in Thai individuals, the prevalence of HLA-B*13:01 in the Thai population was 11.49%Citation19. The association of genetics with co-trimoxazole hypersensitivity in Thai subjects was 3.62 (95% CI 2.03, 6.44), obtained from the study by Wang et al.Citation17 The incidences of co-trimoxazole-induced SCARs in persons who are HLA-B*13:01 carriers and non-carriers were calculated based on Eq. (1, 2), using prevalence and association (odds ratio).
Equation 1
Equation 1
Equation 2
Equation 2
Where
is the incidence of co-trimoxazole-induced SCARs in the general population
is the incidence of co-trimoxazole-induced SCARs in those carrying HLA-B*13:01
is the incidence of co-trimoxazole-induced SCARs in those not carrying HLA-B*13:01
P is the prevalence of HLA-B*13:01 in the population
Test characteristics
The HLA-B*13:01 genetic testing was performed using the HLA typing kits based on the polymerase chain reaction with sequence-specific oligonucleotide probes hybridization method coupled with xMAP technology designed to use with the Luminex system. In this study, the Luminex® system was the method for genetic testing because it is the simplest method for the genotyping of HLA alleles. In addition, the cost of testing is relatively low compared to other techniques, which is more suitable for hospital settings. The sensitivity and specificity of the test were 100%Citation31.
Probabilities
We obtained individual patient records of SCARs treatment from Udon Thani Hospital to estimate the probability of death attributable to SCARsCitation26. Ethical approval for the study was provided by the Center of Ethics in Human Research, Khon Kaen University (HE510837). The Center of Ethics in Human Research, Khon Kaen University, waivedCitation32 the consent of all patients to preserve their anonymity. The probabilities of PCP infection in patients who both received and not received co-trimoxazole for PCP prevention in each health state were obtained from a published study conducted in Europe.
We utilized parts of data from the TREAT Asia HIV Observational Database (TAHOD)Citation33, which collected data from HIV-infected patients in multiple centers in various countries. Ethics approval for data access was obtained from the Faculty of Medicine Ramathibodi Hospital (COA. MURA2021/1041). The Human Research Ethics Unit, Faculty of Medicine Ramathibodi Hospital waived for the consent of all patients to preserve their anonymity. We analyze data from Thai patients in Ramathibodi Hospital center in the TAHOD to generate age-specific health state transition probabilities, including the probabilities of “high risk” to “low risk”, and vice versa, and the probabilities of death of each surviving health state. Parametric survival analysis was appliedCitation34. Weibull distribution was assigned as parametric distribution to describe the time-to-eventCitation34.
Costs
According to the guidelines for Health Technology Assessment in Thailand, direct medical costs and direct non-medical costs were includedCitation25. Direct medical costs comprised genetic screening, SCARs and their consequences, medicines, and routine HIV management.
The cost of the HLA-B*13:01 screening was based on the cost of genetic screening by the Department of Medical Sciences, Ministry of Public Health, ThailandCitation35. The cost was 1,000 Thai Baht per test. The costs of medicines used in PCP treatment and prevention were calculated based on the dosage regimens stated in the Thailand National Guidelines on HIV/AIDS Diagnosis, Treatment and Prevention 2021/2022, assuming the patient’s weight of 60 kilogramsCitation15. The medication prices were obtained from the announcement of the national drug cost year 2020 from the Drug and Medical Supply Information Center, Ministry of Public Health, ThailandCitation36. The cost of SCARs management was calculated from the charge of SCAR management using the cost-to-charge ratio of 0.73Citation37. The cost of routine HIV management and DES follow-up were derived from published literature conducted in ThailandCitation38.
Direct non-medical costs for the patients and relatives were calculated based on transport and additional food costs attained from the Standard Cost List for Health Technology Assessment from the Health Intervention and Technology Assessment Program (HITAP)Citation39. The calculation was on the basis that a patient has to visit the hospital 6 times a year or 3 times per cycle.
Costs were presented in Thai Baht (THB). All costs were converted to 2020 value using the consumer price indexCitation40. Money values were converted into USD using the average exchange rate in 2020 of $1, equal to 31.29 THBCitation41.
Utility
The utilities of HIV-infected patients in Thailand were obtained from the survey of the Thai population conducted by the International Health Policy (IHPP) using the EQ-5D Thai versionCitation42. Due to the lack of utility of SCARs patients, we used the utility of SJS/TEN as the representative of SCARsCitation43. We estimated the utility of patients with long-term sequelae using the multiplicative approach by Ara et al. by multiplying the utility of HIV with the utility of DESCitation44–46.
Analytical methods
Base-case analysis
We analyzed costs and quality-adjusted life years (QALYs) over a lifetime horizon and presented them in mean values. The estimated incremental cost-effectiveness ratio (ICER) was calculated using mean costs and QALYs. The cost-effectiveness threshold was 160,000 THB ($5,112) per QALY gainedCitation47. Half-cycle correction was not applied due to the use of a 6-month cycle length, which is associated with minimal effect on biasing the calculation of life expectancy.
Sensitivity analysis
A series of sensitivity analyses, including one-way and probabilistic analyses, were carried out to assess the robustness of the findings. In one-way analysis, we varied one input parameter at a time while holding others constant. In probabilistic analysis, a Monte Carlo simulation was run 1,000 times using Microsoft Excel (Microsoft). Multiple input parameters were varied simultaneously over their feasible ranges. A beta distribution was assigned for probability and utility parameters. A log-normal distribution was used for the association parameter (odds ratio). A gamma distribution was chosen for cost parametersCitation34. The expected net monetary benefit (NMB) was calculated and presented as cost-effectiveness acceptability curves showing the relationship between the probability of favoring each strategy and the level of willingness to pay.
Given that there are a few methods of genetic screening with varied specificity and sensitivity, we also perform a sensitivity analysis where both sensitivity and specificity are 90%.
Scenario analysis
An analysis was performed based on the scenario that pentamidine is broadly available and accessible, patients who are not able to utilize co-trimoxazole will receive pentamidine for PCP prevention and receive clindamycin and primaquine for PCP treatment. The relative risk for pentamidine in comparison with co-trimoxazole for PCP prevention was 1.23Citation48.
Results
Base case analysis
The outcomes of the first 14 days after co-trimoxazole initiation are shown in . The SCAR cases were estimated to be 213.6 and 314 in 100,000 persons in genetic screening strategy and usual practice, where the patients receive co-trimoxazole if they are indicated and stop the drug if SCARs develop, respectively. As a result, deaths from SCARs were also averted in the genetic screening strategy. The estimated 6 SCAR-related deaths in 100,000 persons were expected from the genetic screening strategy, while 8.8 deaths in 100,000 persons from usual practice.
Table 2. Outcomes after 14 days of co-trimoxazole initiation.
After the first 14 days, 88.39% of patients in the genetic screening strategy received co-trimoxazole for PCP prevention as indicated, whereas 99.69% of patients in the usual practice strategy did. The PCP-related outcomes are shown in . In the long-term estimation, 6220 PCP cases per 100,000 persons were estimated in the genetic screening strategy, while 5240 PCP cases per 100,000 persons from usual practice. In addition, the expected deaths from PCP per 100,000 persons was 248 cases in the genetic screening strategy and 209 cases in usual practice.
Table 3. PCP-related outcomes.
The incremental cost-effectiveness analysis is shown in . The lifetime cost of the genetic screening strategy was 1,387,726.25 THB ($44,354.27), while usual practice resulted in a lifetime cost of 1,387,355.88 THB ($44,333.43). Mean quality-adjusted life years (QALYs) were estimated to be 19.3743 and 19.3774 for genetic screening strategy and usual practice, respectively. Compared to current practice, HLA-B*13:01 allele screening before co-trimoxazole initiation resulted in 0.0061 QALYs loss at an additional cost of 370 THB ($11.84) per patient.
Table 4. Base case analysis.
Sensitivity analyses
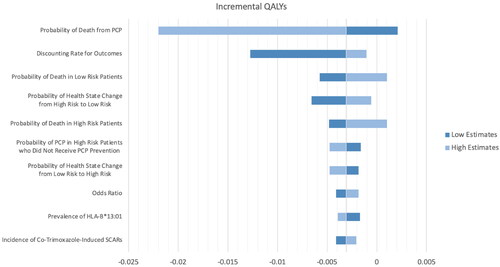
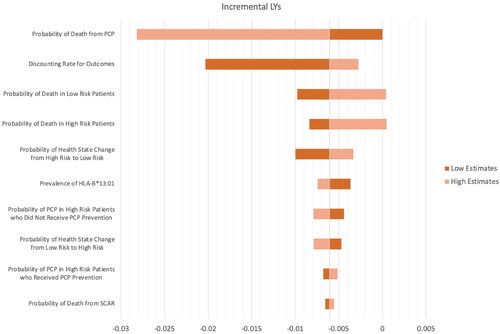
Given the screening strategy resulted in a decrease in QALY, we have depicted sensitivity analyses to see how QALY changes when input parameters change. The results of the one-way sensitivity analyses were presented in the Tornado diagram, as shown in . The one-way sensitivity analysis revealed that the probability of death from PCP was the most influential parameter. Only the probability of death from PCP, the probability of death in patients with a CD4 count of fewer than 200 cells/µL where they have a higher risk of PCP infection (high risk), and the probability of death in patients with a CD4 count of 200 cells/µL and more, (low risk) could result in positive QALY gained. In contrast, most of the parameters resulted in negative QALY gained when the genetic screening strategy was implemented.
Figure 3. Tornado diagram showing a series of one-way sensitivity analyses comparing genetic testing and usual care (QALY).
We also performed a one-way sensitivity analysis over life-years gained, shown in . The most influential parameter remains the probability of death from PCP. While most parameters, in their range, resulted in negative life-year gained, only the probability of death from PCP, the probability of death in “high risk” and “low risk” could result in positive life-year gained, same as QALY gained.
Figure 4. Tornado diagram showing a series of one-way sensitivity analyses comparing genetic testing and usual care (LY).
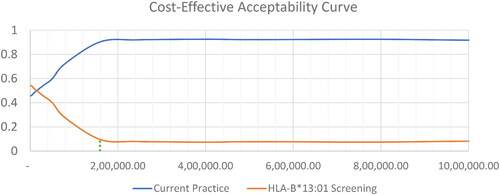
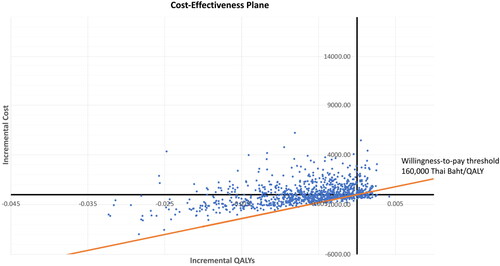
Probabilistic sensitivity analyses demonstrated that the genetic screening strategy, on average, increased both costs and QALYs, but decreases LYs, compared to current practice. At the threshold of 160,000 THB ($5,112.85) per QALY gained, 9.54% of the iterations were cost-effective ( and ).
Figure 5. Cost-effectiveness scatter plot. Each point represents incremental cost and quality-adjusted life year gained (QALYs).
After assigning the sensitivity and specificity of the genetic screening to be 90%, both costs and outcomes are slightly changed. The lifetime costs change from 44,345.36 USD to 44,345.49 USD. The lifetime QALYs gained a change from 19.3743 to 19.3727. Still, it resulted in a decrease in LYs and QALYs, which made the strategy dominated, as shown in Table S3.
Scenario analysis
In the scenario that pentamidine is an alternative for PCP prevention. The lifetime costs were 1,400,716 THB for the genetic screening strategy and 1,387,553 THB for the no genetic screening with pentamidine as alternative PCP prevention. While life-years gained was 22.6767 in the genetic screening test, the life-years gained in no genetic screening with pentamidine as PCP prevention was 22.7014, shown in Table S3.
Discussion
Genetic screening before using certain medicines has been a measure of choice in order to prevent severe cutaneous adverse drug reactionsCitation49,Citation50. Additionally, several cost-effectiveness analyses have demonstrated the cost-effective results of the pharmacogenetic screening of risk alleles because it helps prevent long-term negative consequencesCitation20,Citation21,Citation43,Citation51. Therefore, genetic screening has been implemented for certain medicines, such as HLA-B*15:02 screening for carbamazepine and HLA-B*58:01 for allopurinol. However, this study demonstrates that implementation of HLA-B*13:01 screening before initiation of co-trimoxazole in HIV-infected patients results in a decrease in QALY gained and life-years gained in the long run, in the uncommon context that the second-line medication also presents a similar genetic risk, and another alternative is not broadly available in Thailand.
The study by Wang et al. 2021 demonstrated that the association of co-trimoxazole-induced SCARs and HLA-B*13:01 genetic marker in the Thai population was 3.62 (95% CI 2.03, 6.44)Citation17. Although the association was significant, it was relatively low compared with the other two populations in the study, which are Malaysia and Taiwan populations (12.78 (95% CI 3.13, 52.22) and 8.71 (95% CI 5.67, 6.44), respectively)Citation11. Additionally, it is also relatively low compared with the well-established pharmacogenomic associations such as HLA-B*5801 and allopurinol-induced SJS/TEN (odd ratios (OR) of 348.3 (95% CI 19.2-6,339.9))Citation52, and HLA-B*1502 and carbamazepine-induced SJS/TEN (OR of 54.6 (95% CI 14.62-205.13))Citation53.
According to the significant association of co-trimoxazole-induced SCARs and HLA-B*13:01 genetic marker, genetic screening consequently decreased SCAR cases as well as deaths from SCARs. For this study, there was no alternative medicine for PCP prevention because dapsone, the second-line medication for PCP prevention, also has a strong association with the occurrence of SCARs in those who are HLA-B*13:01 allele carriersCitation27,Citation28. The study by Satapornpong et al. demonstrated an even stronger association between HLA-B*13:01 and dapsone-induced SCARs in the Thai population (OR of 39.00 (7.67-198.21))Citation28, compared with co-trimoxazole-induced SCARs. Moreover, pentamidine, another drug of choice for PCP prevention, is not broadly available in ThailandCitation27. Patients who have tested positive, therefore, would not receive medications for PCP prevention. To this end, fewer patients in the genetic screening strategy received co-trimoxazole for PCP prevention. As a result, greater PCP cases have been observed in the genetic screening strategy. Because PCP can be a life-threatening condition, a decrease in QALY and life-year gained have been observed.
The one-way sensitivity analyses, shown as tornado diagrams, demonstrate that the parameter that is the most influential variable in both incremental QALYs and incremental LYs is the probability of death from PCP, followed by the probabilities of changes in health states. In contrast, the epidemiological parameters, including the association between co-trimoxazole-induced SCARs and HLA-B*13:01 (odds ratio) and prevalence of HLA-B*13:01 in Thai population show minimal influence on the results in terms of both incremental QALYs and incremental LYs. Only three parameters can provide positive incremental QALYs in their possible range when varied one parameter at a time. They are the probability of death from PCP, the probability of death in “low-risk” patients, and the probability of death in “high-risk” patients. It could be considered that the parameters that have an impact on the incremental QALYs the most are the parameters related to “death” because they cause differences in the number of patients between two groups in the hypothetical cohorts, especially for their LYs gained. Our analysis showed that even if the prevalence of gene is 100% or the cost of genetic testing becomes zero, the cost remained negative. This is a unique situation where the break-even point for genetic test price or the gene prevalence threshold cannot be determined as the intervention will not result in cost-neutrality.
This study obtained real-world data from HIV-infected patients collected in Ramathibodi Hospital. The data included patient demographic, CD4 count levels, death status, and cause of death. The country-specific health state transition probabilities were applied in our model, resulting in more reliable findings than using only data from the literature. Several model parameters were country-specific. However, there was limited data regarding PCP infection and deaths from PCP in HIV-infected patients in Thailand. The data was then obtained from the literature, where PCP prophylactic agents are more varied, including co-trimoxazole (86.9%), nebulized pentamidine (6.5%), and pyrimethamine-sulfadoxine (0.5%), which have differences in efficacyCitation54–56.
In addition, we have the assumption that deserves further discussion. We assumed that the long-term sequela of SCARs was DES because the sequelae of SCARs do not occur in an explicit pattern. This possibly resulted in the underestimated benefits of the screening since sequelae of SCARs could be more severe, require more health care resources, and affect the patient’s quality of life. Furthermore, we assumed full compliance with co-trimoxazole use for PCP prevention. However, adverse effects of co-trimoxazole are not uncommon. Intolerance of the drug could occur, and could ultimately result in discontinuationCitation57. Because co-trimoxazole serves as preventive medicine, compliance might affect the effectiveness of the medicine. Nevertheless, we did not incorporate compliance into our analyses. The prophylactic effect of co-trimoxazole might be higher than in the real world.
Although our analyses demonstrate reduced QALYs and LYs gained after the implementation of the HLA-B*13:01 genetic screening in the specified setting and population, the value of genetic screening is not limited to such conditions. Previous studies suggest that HLA-B*13:01 is also associated with severe hypersensitivity in other medicines, e.g. dapsone, which is the alternative drugCitation28. To this end, genetic screening may avoid not only hypersensitivity from a single drug but also from other drugs that are associated with hypersensitivity in patients carrying the same genetic marker. This may also be a point to consider for future decision-making.
Because of the relatively low association of co-trimoxazole-induced SCARs and the HLA-B*13:01 allele, it costs the opportunity to receive a prophylactic agent for PCP in those carrying HLA-B*13:01 but not hypersensitive to the drug. Currently, patch testing could be an intervention to help confirm the hypersensitivity to a medicine. Even though the sensitivity to the agent is relatively low compared to the standard methods, it is safe and shows high specificityCitation58–60. If the allele is detected in the genetic screening, patch testing can be utilized to confirm the hypersensitivity. In case adverse drug reactions are not observed, physicians could consider prescribing the medicine further to keep the patients from losing an opportunity to save their lives.
In addition to the hypersensitivity issue, this study also reveals an important point worth discussing. As demonstrated in our study, there is a case where patients are at risk for severe hypersensitivity due to both the first-line and the second-line drugs while other alternatives are not at hand. This group of patients will have to live with the risk of a serious condition that can be prevented. The accessibility of alternative drugs to be used in a fatal condition deserve more attention, especially in the case that severe hypersensitivity has frequently occurred, like co-trimoxazole. Although an alternative medication may have a lower potency compared to the first-line drug, it is still important to those who are contraindicated to the first-line drug and could help save lives in such conditions. The scenario analysis demonstrated in Table S3 that if the HLA-B*13:01 screening is implemented with pentamidine provided, the overall QALYs and LYs gained increased compared to the usual practice. In this case, we recommend policymakers consider including pentamidine to be accessible for those who are not able to use co-trimoxazole and dapsone.
There is a recently published study focusing on multiple-pharmacogenes testing for the prevention of adverse drug reactions in people living with HIVCitation61. The study considered HLA-B*13:01 allele screening for the prevention of DRESS as a part of their study. The results consistently show that the genetic screening strategy decreases adverse event cases. The study found that single and multiple pharmacogenomic testing strategies were likely cost-effective under the Thai willingness to pay. The results were not consistent with our study. This is because the scope of the study is different. Their study considered multiple pharmacogenetic screenings. Additionally, they focus only on the adverse event outcomes, whereas our study also captured the consequences after the patients are not able to receive the prevention medication. Our study will be complementary evidence highlighting the concern regarding the risk of PCP when patients do not receive the prevention drug.
We believe that our analysis will be a case study for further genetic screening consideration. A long-term outcome should be estimated to capture the actual effect of the screening before making a decision. Although our findings show that the HLA-B*13:01 screening before co-trimoxazole imitation for PCP prevention in HIV-infected patients is not cost-effective, our study has pointed out issues to further be discussed and built on to fill the gap in the healthcare system.
Conclusion
Our cost-effectiveness demonstrated that HLA-B*13:01 screening before initiation of co-trimoxazole for PCP prevention in HIV-infected patients in Thailand can reduce SCARs cases. But it is unlikely to be cost-effective in the current context because the number of PCP cases increased. In addition to the cost-effectiveness findings, alternative medication is still inaccessible. We recommend policymakers consider providing pentamidine, as an alternative drug, to be available and accessible.
Transparency
Author contributions
W.K., N. N., S.K., C.S., W.T., and N.C. contributed to research design, N.N. and S.K. contributed to data acquisition, W.K. and N.C. contributed to statistical analysis, W.K. drafted the manuscript, N. N., S.K., C.S., W.T., and N.C. critically revised the manuscript. All authors have read and approved the final version of the manuscript.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplemental Material
Download MS Word (30.6 KB)Declaration of financial/other relationship
The authors declare no competing interests.
Data availability statement
Data could be available upon request to the corresponding author
Additional information
Funding
References
- Roujeau JC, Stern RS. Severe adverse cutaneous reactions to drugs. N Engl J Med. 1994;331(19):1272–1285. doi: 10.1056/NEJM199411103311906.
- Adler NR, Aung AK, Ergen EN, et al. Recent advances in the understanding of severe cutaneous adverse reactions. Br J Dermatol. 2017;177(5):1234–1247. doi: 10.1111/bjd.15423.
- Cho Y-T, Chu C-Y. Treatments for severe cutaneous adverse reactions. J Immunol Res. 2017;2017:1503709–1503709. doi: 10.1155/2017/1503709.
- Roujeau JC, Kelly JP, Naldi L, et al. Medication use and the risk of Stevens-Johnson syndrome or toxic epidermal necrolysis. N Engl J Med. 1995;333(24):1600–1607. doi: 10.1056/NEJM199512143332404.
- Kardaun SH, Sekula P, Valeyrie-Allanore L, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. Results from the prospective RegiSCAR study. Br J Dermatol. 2013;169(5):1071–1080. doi: 10.1111/bjd.12501.
- Cacoub P, Musette P, Descamps V, et al. The DRESS syndrome: a literature review. Am J Med. 2011;124(7):588–597. doi: 10.1016/j.amjmed.2011.01.017.
- Chen YC, Chang CY, Cho YT, et al. Long-term sequelae of drug reaction with eosinophilia and systemic symptoms: a retrospective cohort study from Taiwan. J Am Acad Dermatol. 2013;68(3):459–465. doi: 10.1016/j.jaad.2012.08.009.
- Lee HY, Walsh SA, Creamer D. Long-term complications of Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN): the spectrum of chronic problems in patients who survive an episode of SJS/TEN necessitates multidisciplinary follow-up. Br J Dermatol. 2017;177(4):924–935. doi: 10.1111/bjd.15360.
- Chung WH, Wang CW, Dao RL. Severe cutaneous adverse drug reactions. J Dermatol. 2016;43(7):758–766. doi: 10.1111/1346-8138.13430.
- Mullan KA, Anderson A, Illing PT, et al. HLA-associated antiepileptic drug-induced cutaneous adverse reactions. HLA. 2019;93(6):417–435. doi: 10.1111/tan.13530.
- Hung SI, Chung WH, Liou LB, et al. HLA-B*5801 allele as a genetic marker for severe cutaneous adverse reactions caused by allopurinol. Proc Natl Acad Sci U S A. 2005;102(11):4134–4139. doi: 10.1073/pnas.0409500102.
- Yang SC, Chen CB, Lin MY, et al. Genetics of severe cutaneous adverse reactions. Front Med (Lausanne). 2021;8:652091. doi: 10.3389/fmed.2021.652091.
- Chen CB, Hsiao YH, Wu T, et al. Risk and association of HLA with oxcarbazepine-induced cutaneous adverse reactions in asians. Neurology. 2017;88(1):78–86. doi: 10.1212/WNL.0000000000003453.
- Mockenhaupt M, Viboud C, Dunant A, et al. Stevens-Johnson syndrome and toxic epidermal necrolysis: assessment of medication risks with emphasis on recently marketed drugs. The EuroSCAR-study. J Invest Dermatol. 2008;128(1):35–44. doi: 10.1038/sj.jid.5701033.
- Bureau of AIDS Tuberculosis and Sexually Transmitted Infection. Thailand national guidelines on HIV/AIDS diagnosis. Treatment and Prevention 2021/2022. 2022.
- Sukasem C, Pratoomwun J, Satapornpong P, et al. Genetic association of Co-Trimoxazole-Induced severe cutaneous adverse reactions is Phenotype-Specific: HLA class I genotypes and haplotypes. Clin Pharmacol Ther. 2020;108(5):1078–1089. doi: 10.1002/cpt.1915.
- Wang CW, Tassaneeyakul W, Chen CB, et al. Whole genome sequencing identifies genetic variants associated with co-trimoxazole hypersensitivity in asians. J Allergy Clin Immunol. 2021;147(4):1402–1412. doi: 10.1016/j.jaci.2020.08.003.
- Tempark T, John S, Rerknimitr P, et al. Drug-Induced severe cutaneous adverse reactions: insights into clinical presentation, immunopathogenesis, diagnostic methods, treatment, and pharmacogenomics [review. Front Pharmacol. 2022;13:832048. doi: 10.3389/fphar.2022.832048.
- Satapornpong P, Jinda P, Jantararoungtong T, et al. Genetic diversity of HLA class I and class II alleles in thai populations: contribution to Genotype-Guided therapeutics. Front Pharmacol. 2020;11:78. doi: 10.3389/fphar.2020.00078.
- Saokaew S, Tassaneeyakul W, Maenthaisong R, et al. Cost-effectiveness analysis of HLA-B*5801 testing in preventing allopurinol-induced SJS/TEN in thai population. PLoS One. 2014;9(4):e94294. doi: 10.1371/journal.pone.0094294.
- Rattanavipapong W, Koopitakkajorn T, Praditsitthikorn N, et al. Economic evaluation of HLA-B*15:02 screening for carbamazepine-induced severe adverse drug reactions in Thailand. Epilepsia. 2013;54(9):1628–1638. doi: 10.1111/epi.12325.
- Leelahavarong P, Doungthipsirikul S, Kumluang S, et al. Health technology assessment in Thailand: institutionalization and contribution to healthcare decision making: review of literature. Int J Technol Assess Health Care. 2019;35(6):467–473. doi: 10.1017/S0266462319000321.
- Tantivess S. Policy making and roles of health technology assessment. J Med Assoc Thai. 2008;91 Suppl 2(Suppl 2):S88–S99.
- Husereau D, Drummond M, Augustovski F, et al. Consolidated health economic evaluation reporting standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. Value Health. 2022;25(1):3–9. doi: 10.1016/j.jval.2021.11.1351.
- Chaikledkaew U, Kittrongsiri K. Guidelines for health technology assessment in Thailand (second edition)–the development process. J Med Assoc Thai. 2014;97(Suppl 5):S4–S9.
- Konyoung P, Nakkam N, Khawsuk H, et al. Severe cutaneous adverse drug reactions: a study of 219 patients from udon thani hospital. Thai J Pharmacol. 2020;42(1):5–19.
- Panel on Opportunistic Infections in Adults and Adolescents with HIV. Guidelines for the prevention and treatment of opportunistic infections in adults and adolescents with HIV: recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. 2022. Available from: http://aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf.
- Satapornpong P, Pratoomwun J, Rerknimitr P, et al. HLA-B*13: 01 is a predictive marker of Dapsone-Induced severe cutaneous adverse reactions in thai patients. Front Immunol. 2021;12:661135. doi: 10.3389/fimmu.2021.661135.
- Yip LW, Thong BY, Lim J, et al. Ocular manifestations and complications of Stevens-Johnson syndrome and toxic epidermal necrolysis: an asian series. Allergy. 2007;62(5):527–531. doi: 10.1111/j.1398-9995.2006.01295.x.
- Limkobpaiboon S, Dhana N, Jongjarearnprasert K. Prevalence and mortality rate of severe cutaneous adverse reactions at siriraj hospital. Chula Med J. 2010;54(5):468–477.
- Immucor Transplant Diagnostic Inc. Lifecodes®hla-sso typing kits. [product insert]. 2015.
- Roux A, Canet E, Valade S, et al. Pneumocystis jirovecii pneumonia in patients with or without AIDS, France. Emerg Infect Dis. 2014;20(9):1490–1497. doi: 10.3201/eid2009.131668.
- Jung IY, Rupasinghe D, Woolley I, et al. Trends in mortality among ART-treated HIV-infected adults in the Asia-Pacific region between 1999 and 2017: results from the TREAT asia HIV observational database (TAHOD) and Australian HIV observational database (AHOD) of IeDEA Asia-Pacific. J Int AIDS Soc. 2019;22(1):e25219.
- Briggs A, Sculpher M, Claxton K. Decision modelling for health economic evaluation. New York (NY): Oxford University Press; 2006.
- Department of Medical Sciences MoPH. Service fee for analysis and service. 2023. [cited 2023 June 23]. Available from: https://www3.dmsc.moph.go.th.
- Drug and Medical Supply Information Center. Announcement of national drug cost year. 2020. Available from: http://dmsic.moph.go.th/index/dataservice/97/0.
- Ministry of Public Health Network of Unit Cost. Unit cost manual for hospital. Nonthaburi, Thailand: MUCC; 2011.
- Boettiger DC, Newall AT, Chattranukulchai P, et al. Statins for atherosclerotic cardiovascular disease prevention in people living with HIV in Thailand: a cost-effectiveness analysis. J Int AIDS Soc. 2020;23 Suppl 1(Suppl 1):e25494.
- Riewpaiboon A. Standard cost lists for health economic evaluation in Thailand. J Med Assoc Thai. 2014;97 Suppl 5(Suppl 5):S127–S34.
- World Bank. Consumer Price Index 2020. 2021. Available from: https://data.worldbank.org/indicator/FP.CPI.TOTL.
- World Bank. Official Exchange Rate 2021. 2021. Available from: https://data.worldbank.org/indicator/PA.NUS.FCRF?locations=TH.
- T. W. Health-Related quality of life survey using EQ-5D in 5 diseases: HIV/AIDS, diabetes mellitus, liver cancer, stroke, and injury International Health Policy Program-IHPP; 2016.
- Dong D, Sung C, Finkelstein EA. Cost-effectiveness of HLA-B*1502 genotyping in adult patients with newly diagnosed epilepsy in Singapore. Neurology. 2012;79(12):1259–1267. doi: 10.1212/WNL.0b013e31826aac73.
- Ara R, Brazier J. Comparing EQ-5D scores for comorbid health conditions estimated using 5 different methods. Med Care. 2012;50(5):452–459. doi: 10.1097/MLR.0b013e318234a04a.
- Buchholz P, Steeds CS, Stern LS, et al. Utility assessment to measure the impact of dry eye disease. Ocul Surf. 2006;4(3):155–161. doi: 10.1016/s1542-0124(12)70043-5.
- Schiffman RM, Walt JG, Jacobsen G, et al. Utility assessment among patients with dry eye disease. Ophthalmology. 2003;110(7):1412–1419. doi: 10.1016/S0161-6420(03)00462-7.
- Thavorncharoensap M, Teerawattananon Y, Natanant S, et al. Estimating the willingness to pay for a quality-adjusted life year in Thailand: does the context of health gain matter? Clinicoecon Outcomes Res. 2013;5:29–36. doi: 10.2147/CEOR.S38062.
- Mallolas J, Zamora L, Gatell JM, et al. Primary prophylaxis for Pneumocystis carinii pneumonia: a randomized trial comparing cotrimoxazole, aerosolized pentamidine and dapsone plus pyrimethamine. Aids. 1993;7(1):59–64. doi: 10.1097/00002030-199301000-00009.
- Chang CJ, Chen CB, Hung SI, et al. Pharmacogenetic testing for prevention of severe cutaneous adverse drug reactions. Front Pharmacol. 2020;11:969. doi: 10.3389/fphar.2020.00969.
- Fernando SL, Broadfoot AJ. Prevention of severe cutaneous adverse drug reactions: the emerging value of pharmacogenetic screening. CMAJ. 2010;182(5):476–480. doi: 10.1503/cmaj.090401.
- Sukri A, Salleh MZ, Masimirembwa C, et al. A systematic review on the cost effectiveness of pharmacogenomics in developing countries: implementation challenges. Pharmacogenomics J. 2022;22(3):147–159. doi: 10.1038/s41397-022-00272-w.
- Tassaneeyakul W, Jantararoungtong T, Chen P, et al. Strong association between HLA-B*5801 and allopurinol-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in a thai population. Pharmacogenet Genomics. 2009;19(9):704–709. doi: 10.1097/FPC.0b013e328330a3b8.
- Tassaneeyakul W, Tiamkao S, Jantararoungtong T, et al. Association between HLA-B*1502 and carbamazepine-induced severe cutaneous adverse drug reactions in a thai population. Epilepsia. 2010;51(5):926–930. doi: 10.1111/j.1528-1167.2010.02533.x.
- Schürmann D, Bergmann F, Albrecht H, et al. Twice-weekly pyrimethamine-sulfadoxine effectively prevents Pneumocystis carinii pneumonia relapse and toxoplasmic encephalitis in patients with AIDS. J Infect. 2001;42(1):8–15. doi: 10.1053/jinf.2000.0772.
- Schürmann D, Bergmann F, Albrecht H, et al. Effectiveness of twice-weekly pyrimethamine-sulfadoxine as primary prophylaxis of Pneumocystis carinii pneumonia and toxoplasmic encephalitis in patients with advanced HIV infection. Eur J Clin Microbiol Infect Dis. 2002;21(5):353–361.
- Mocroft A, Reiss P, Kirk O, et al. Is it safe to discontinue primary Pneumocystis jiroveci pneumonia prophylaxis in patients with virologically suppressed HIV infection and a CD4 cell count <200 cells/microL? Clin Infect Dis. 2010;51(5):611–619.
- Weyant RB, Kabbani D, Doucette K, et al. Pneumocystis jirovecii: a review with a focus on prevention and treatment. Expert Opin Pharmacother. 2021;22(12):1579–1592. doi: 10.1080/14656566.2021.1915989.
- Pinho A, Coutinho I, Gameiro A, et al. Patch testing - a valuable tool for investigating non-immediate cutaneous adverse drug reactions to antibiotics. J Eur Acad Dermatol Venereol. 2017;31(2):280–287. doi: 10.1111/jdv.13796.
- Friedmann PS, Ardern-Jones M. Patch testing in drug allergy. Curr Opin Allergy Clin Immunol. 2010;10(4):291–296. doi: 10.1097/ACI.0b013e32833aa54d.
- Romano A, Viola M, Gaeta F, et al. Patch testing in non-immediate drug eruptions. Allergy Asthma Clin Immunol. 2008;4(2):66–74.
- Turongkaravee S, Praditsitthikorn N, Ngamprasertchai T, et al. Economic evaluation of Multiple-Pharmacogenes testing for the prevention of adverse drug reactions in people living with HIV. Clinicoecon Outcomes Res. 2022;14:447–463. doi: 10.2147/CEOR.S366906.