Abstract
Aims
To investigate the prevalence, treatment status, and effect of anemia on medical costs, quality of life (QOL), and productivity loss in Japan.
Methods
This cross-sectional study used a database containing claims, health check-ups, and questionnaire data. Adults with hemoglobin data at 2020 check-ups were included. QOL and productivity loss were evaluated using EuroQol 5-Dimension (EQ-5D) and Work Productivity and Activity Impairment questionnaire data available for a subset of the population. Nationwide anemia prevalence, including both diagnosed as having anemia and undiagnosed but with low hemoglobin levels, were estimated. Treatment status was described by hemoglobin levels. Differences in medical costs, QOL, and productivity loss were compared between individuals with and without anemia. Subgroup analyses were performed using the Charlson Comorbidity Index (CCI).
Results
The study population included 554,798 individuals. Anemia prevalence was estimated at 15.1% with 55.3% undiagnosed. In patients with anemia, 85.3% were untreated; 79.5% of the treated patients received only oral iron drugs. In patients with anemia, monthly medical costs were ¥17,766 higher, EQ-5D score was 0.0118 lower, and productivity loss was 2.6% higher than in those without anemia. The trends were consistent even in limited patients with CCI = 0. Nationwide annual excess medical costs, deficit QOL, and productivity loss in patients with anemia were estimated at ¥3.32 trillion, 138,000 quality-adjusted life-years, and ¥1.13 trillion, respectively.
Limitations
As the study population only included individuals who underwent health check-ups, they may be healthier than general population. Whether the differences in medical costs, QoL, and productivity loss are caused by anemia or other underlying differences in patient characteristics is unclear, given the likelihood of residual confounding.
Conclusions
The results suggest that more than half of patients with anemia were undiagnosed and untreated. Patients with anemia had higher medical costs, lower QOL, and greater productivity loss than those without anemia.
Introduction
Anemia is recognized as a major global health issue. One of the six global nutrition targets of the World Health Organization’s (WHO) for 2025 is to reduce anemia in women of reproductive age by 50%Citation1. Anemia causes various symptoms, including fatigue, headache, paleness, and dyspnea on exertion, as well as a reduction in physical capacityCitation1,Citation2. Further, recent studies have revealed an association between anemia and depressionCitation3,Citation4. As a result, it can be suggested that anemia may affect various demographic groups. Preterm birth, low birth weight, and maternal, perinatal, and neonatal mortality are associated with anemia during pregnancyCitation5. An association has been reported between iron deficiency anemia and child development, including poor cognitive and motor developmentCitation6, although causality has not been clearly establishedCitation7. In terms of work productivity, a review study showed a longitudinal relationship between moderate-to-severe iron deficiency anemia and a decrease in physical work capacity, leading to a decrease in economic productivityCitation8. Anemia has been associated with increased risks of various diseases and health outcomes in older adults, including frailtyCitation9, fallsCitation10, worsening cognitive abilityCitation11,Citation12, depressionCitation12, impaired executive functionCitation13, hospitalizationCitation14, and deathCitation15.
Although anemia is primarily a health issue in developing countriesCitation16,Citation17, it also affects people in developed countriesCitation18. Nutritional deficiencies, particularly iron deficiency, are the most common causes of anemiaCitation1,Citation2,Citation19. In comparison with other developed countries, Japan appears to have a relatively high prevalence of anemia. According to a WHO report, the prevalence of anemia (defined as hemoglobin [Hb] < 12 g/dL for non-pregnant women and <11 g/dL for pregnant women) among women of reproductive age (15–49 yr) was estimated to be approximately 22% in 2011Citation20. According to this report, Japan’s prevalence is in the second most significant category (moderate public health problem: 20%–39% of prevalence) of four public health problem categories. The prevalence is higher than that in other developed countries, such as France, Germany, the United Kingdom, and the United States, and is comparable with that in China and Singapore. The prevalence in Japanese older adults is also higher than in other developed countriesCitation21. Anemia (defined as Hb of <13 g/dL in men and <12 g/dL in women) is reported to be 17.1% in those aged ≥65 yr based on the National Health and Nutrition Survey dataset between 2010 and 2015Citation22 and 22.3% in those aged 69–91 yr based on blood tests on community-dwelling participants in an observational studyCitation23.
For patients with anemia, there are some guidelines, such as those focusing on treatment with iron preparationsCitation24 and those for renal anemia in chronic kidney diseaseCitation25. However, no general treatment guidelines are available in Japan, which can lead to anemia being overlooked and undertreated. Iron supplementation is generally used to treat anemia. Oral iron tablets are primarily used, and intravenous (IV) iron infusions are used when rapid iron delivery is required or when oral iron treatment is ineffective or cannot be used. In Japan, three medications are available for IV iron infusions: saccharated ferric oxide, ferric carboxymaltose, and ferric derisomaltose. Blood transfusions are also used to treat anemia, particularly severe anemiaCitation26 and chronic anemia caused by blood disorders or chronic hemorrhagic anemiaCitation27.
Given the reported relatively high prevalence of anemia in Japan and the problems caused by the disease, the magnitude of the effects should be quantified to understand the impact on patients and society. However, it does not appear that the impact on patients and potential patients as well as society has been fully reported. In terms of anemia diagnosis and treatment, one study reported that less than half of older adults admitted to a rural community hospital with low Hb values (<11 g/dL) were not diagnosed with anemiaCitation28. Treatment status has only been reported in patients with specific health contexts, such as patients with non-dialysis-dependent chronic kidney disease (CKD)Citation29, and not in all patients with anemia. To address the issue of anemia, it is important to understand the current status of diagnosis and treatment. Thus, using real-world data in Japan, this study investigated the prevalence of anemia, including those who were diagnosed with anemia and those who were not diagnosed but had a low Hb level, the treatment status of patients with anemia, and medical costs, quality of life (QOL) based on the EuroQol 5-Dimension (EQ-5D), and productivity loss based on the Work Productivity and Activity Impairment (WPAI), in patients with anemia. We believe that this study provides information that has not been fully investigated in Japan.
Methods
Study design and data source
This was a claims-based cross-sectional study that used descriptive statistics to analyze the data (between June 2018 and May 2021) from a database provided by DeSC Healthcare, Inc. (Tokyo, Japan)Citation30–33. The database contained claims data from approximately 5 million individuals who were members of various types of health insurers, including health insurance societies, national health insurance, and the medical care system for the elderly, and who lived throughout Japan. The database also contained health check-up examination data from a subset of individuals who had the check-ups. Furthermore, EQ-5D and WPAI (2020 only) questionnaire data were available from individuals who had voluntarily enrolled in an online health management application offered by their health insurance associations.
Analyses were conducted on data generated in 2019 or 2020. The study year was defined as the year in which the data used for each analysis were generated. Only 2020 data were analyzed for treatment status and productivity loss.
Study population and patients
Individuals who had Hb values in their health check-up examination data in 2019 or 2020 and were aged ≥20 yr in each study year comprised the study population. Individuals who had EQ-5D data (in 2019 or 2020) or WPAI data (in 2020) were included in the analysis of QOL or productivity loss, respectively.
Patients with anemia included diagnosed patients and undiagnosed low Hb patients. Diagnosed patients were those who had at least one record of anemia diagnosis in each study year. Undiagnosed low Hb patients were those who had no record of anemia diagnosis but had low Hb levels in each year. Anemia diagnosis was defined as one of the following International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD-10) codesCitation34: D50–D53 (nutritional anemias), D55–D59 (hemolytic anemias), D60–D64 (aplastic and other anemias and other bone marrow failure syndromes), or O99.0 (anemia complicating pregnancy, childbirth, and the puerperium). Undiagnosed low Hb patients were those without anemia diagnosis and with a low Hb level in each study year. Herein, Hb levels of ≤13 g/dL for men or ≤12 g/dL for womenCitation35,Citation36 were defined as low. The same thresholds were applied to all age groups, including older adults, according to the WHO definitionCitation37. The same standard (≤12 g/dL) was applied to pregnant women because the majority of them could not be identified in the database. This is because pregnancy and childbirth-related medical expenses are covered in other ways due to the nature of Japanese health insurance system, and their information is not recorded in claims.
Outcomes and analyses
The prevalence of anemia, treatment status, medical costs, QOL, and productivity loss in patients with anemia were estimated in this study. The medical costs include payer expenditures and patient out-of-pocket costs. Excess medical costs in patients with anemia were determined by calculating the difference between the medical costs of patients with and without anemia. QOL deficit or productivity loss in patients with anemia was determined by calculating the difference in QOL or productivity loss between patients with and without low Hb. We assumed that QOL or productivity might not be impaired in diagnosed patients without low Hb levels due to treatment after diagnosis, so the comparison was performed between patients with low Hb levels and those without. Charlson Comorbidity Index (CCI) scoreCitation38,Citation39 was calculated using the diagnosis records from each study year. We summarized the patients background data as mean (standard derivation [SD]) for continuous variables and number (%) for categorical variables.
The prevalence of anemia was estimated by demographic group (combination of sex and age every 5 yr) in each year and for the entire adult population in 2020 following the steps outlined below.
Patients with anemia (diagnosed and undiagnosed low Hb patients) were identified from the study population.
For each demographic group, weight was calculated by dividing the number of people in the countryCitation40 by the number of individuals in the study population.
The nationwide number of patients with anemia for each demographic group was calculated by multiplying the number of patients in the study population with the weight.
Prevalence was calculated by dividing the above-mentioned nationwide number of patients by the total population for each demographic group and the entire adult population.
The prevalence of each diagnosed and undiagnosed (with low Hb levels but undiagnosed) anemia was also estimated. The percentage of patients with low Hb levels by sex and age group was compared with that calculated based on the most recently published survey (as of December 2022), including 1,015 men and 1,405 women conducted in Japan in 2019Citation41.
The treatment status was described by Hb levels as combinations of treatments: blood transfusion and iron prescription (oral iron and IV iron infusion). Patients who received a blood transfusion were defined as those who had a medical procedure record code K920-2 (blood transfusion management fee). Patients who underwent iron prescriptions were defined based on the Anatomic Classification Code by EPHMRACitation42. Among the drugs coded as B3A1 (plain iron), IV iron drugs available in Japan (saccharated ferric oxide and ferric carboxymaltose until December 2022) were identified using the drug code, and the other drugs were identified as oral iron drugs. At each Hb level, the nationwide percentage of patients was calculated by treatment combination, including no treatment. The number of individuals taking each combination of treatments was counted by Hb level and then weighted based on the number of individuals in the database and nationwide individuals in each demographic group.
The mean medical costs (in Japanese yen, ¥) per patient per month (PPPM) or per member per month (PMPM) for patients with anemia (diagnosed and undiagnosed low Hb patients) and individuals without anemia were calculated for each demographic group. These medical costs in each study year were calculated for each group as (weighted medical costs)/(weighted member months) using the weight developed to estimate the prevalence described above. The member months refer to the total number of months that all patients or individuals in each group were in the database during each study year. For each demographic group, excess medical costs were calculated as (medical costs PPPM for patients with anemia) − (medical costs PMPM for individuals without anemia), and then, those in all patients were estimated by weighting excess medical costs by demographic group by the estimated total member months for patients with anemia. Excess medical costs were also estimated by categorizing the patients into three groups based on CCI score: CCI = 0, 1, and >1. The estimated excess medical costs PPPM in all patients or those by CCI levels were multiplied by the estimated total member months for patients with anemia to obtain the total excess medical costs in patients with anemia nationwide.
The mean EQ-5D score (utility value based on the five dimensions of health status) for patients with and without low Hb was calculated using data from the EQ-5D-5L questionnaire. The deficit QOL was calculated as (mean EQ-5D score in individuals without low Hb) − (mean EQ-5D score in patients with low Hb). The deficit QOL was calculated by categorizing the patients into the three above-mentioned groups based on CCI score. Furthermore, a quality-adjusted life-year (QALY) loss was calculated nationwide by multiplying the estimated deficit QOL in 2020 by all patients or by CCI class by the estimated nationwide number of patients with low Hb.
The mean productivity loss (% overall work impairment) for patients with and without low Hb was calculated using data from the WPAI questionnaire. The excess productivity loss was calculated as (mean productivity loss in patients with low Hb) − (mean productivity loss for individuals without low Hb). Annual productivity loss was calculated as a monetary value using the average wage for all industries, ages, and both sexes (¥307,700/month; $1.00 corresponded to approximately ¥133.44 as of January 2023) from the “2020 Basic Survey on Wage Structure”Citation43. The estimated annual productivity loss in 2020 for all patients or by CCI class was multiplied by the estimated nationwide number of people with low Hb value.
To assess the validity of the analyses for excess medical costs, deficit QOL, and excess productivity loss in 2020, a subanalysis using a multivariable linear regression model was performed. Explanatory variables in the model included sex, age, categorized CCI score (CCI1 for CCI = 1 and CCI2 for CCI >1), and presence of anemia for excess medical costs, and age, categorized CCI score, and presence of low Hb for deficit QOL and productivity loss. A weighted regression model was used for the excess medical costs, using the weight developed to estimate the prevalence described above.
Ethics statement
Ethical approval and informed consent were not required because this was a non-interventional study that used anonymized, routinely collected secondary data in accordance with the Ethical Guidelines for Medical and Health Research Involving Human Subjects by the Ministry of Education, Culture, Sports, Science and Technology and the Ministry of Health, Labour and WelfareCitation44.
Results
Population identification and background information
From the database including 4,953,671 individuals, we identified 554,798 individuals as the study population in 2020. The percentage of women was 48.5%, mean (SD) age was 63.4 (16.2) years, and mean (SD) CCI score was 1.1 (1.6) (). There were 19,344 and 11,748 individuals in the study population who had EQ-5D and WPAI data, respectively (). The study population in 2019 was 598,993 individuals (), with a similar proportion of women, mean age, and CCI score to those in 2020 (Table S1).
Figure 1. Flow diagram to identify the population in 2020 (A) and 2019 (B) for the analyses. Abbreviations: EQ-5D, EuroQol 5-Dimension; Hb, Hemoglobin; QOL, Quality of life; WPAI, Work productivity and activity impairment.
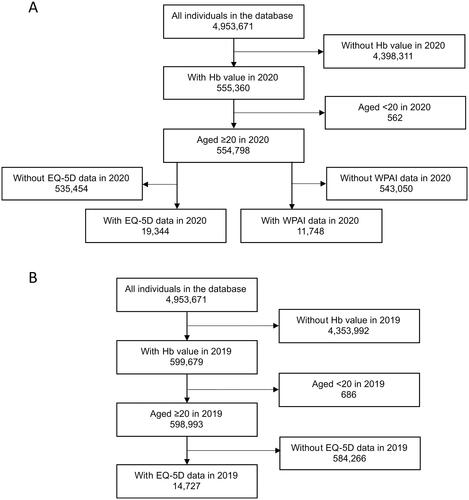
Table 1. Background information on study population in 2020.
Prevalence of anemia
In 2020, the database identified 90,953 patients with anemia, including 40,677 diagnosed patients and 50,276 undiagnosed low Hb patients (). The total number of patients and the prevalence of anemia were estimated to be approximately 15.9 million and 15.1%, respectively (), of the total nationwide population, including 6.9% of diagnosed patients and 8.3% of undiagnosed low Hb patients. Up to the age of 64 yr, women had a higher prevalence than men; then it became similar at ages 65–74 yr, and men had a higher prevalence than women aged 75 yr or older (). The prevalence was similar to that estimated based on the 2019 data (Table S2).
Table 2. Prevalence of anemia in 2020.
The percentage of patients with low Hb was similar to that based on the Japanese survey in 2019 in most sex and age groups (Table S3).
Treatment status in patients with anemia
Among individuals with Hb levels of ≤12 g/dL, 85.3% did not receive any treatment. Although the percentage decreased as the Hb level decreased, more than half of patients with each Hb level between 8 and 12 g/dL were untreated, ranging from 53.5% to 92.2% (). The percentage of those who did not receive any treatment was 44.8% and 44.2% in those with an Hb levels of 6–7 and 7–8 g/dL, respectively. Oral iron was the most common treatment type with 88.8% of individuals with Hb levels of ≤12 g/dL who received any treatment. Of those who received any treatment, 79.5% received oral iron only. Individuals who took IV iron were less than half of all individuals who took any treatments in all Hb levels with a peak of 6–7 g/dL (34.3% of all individuals with any treatments). Individuals who received blood transfusions had a peak Hb level of 6–7 g/dL, accounting for 22.7% of those with any treatment. Those who received blood transfusions without iron treatment accounted for 4.0% of those with any treatment among those with Hb levels of ≤12 g/dL.
Table 3. Treatment status by Hb levels in 2020.
Medical costs in patients with anemia
In 2020, diagnosed patients had the highest mean medical costs (PPPM or PMPM), followed by undiagnosed low Hb patients and individuals without anemia in each demographic group (). The excess medical costs were calculated to be ¥17,766 PPPM. The amount of excess medical costs in the CCI = 0 group was ¥4,529 PPPM, which increased as the score increased, ¥8,956 PPPM in CCI = 1, and ¥27,327 PPPM in CCI >1 (Table S4). The amount was similar in 2019 and 2020 (Figure S1 and Table S5).
Figure 2. Estimated monthly medical costs for population and patients with anemia in 2020. Excess medical costs were calculated as (medical costs PPPM for patients with anemia) − (medical costs PMPM for individuals without anemia) by demographic group, and those in all patients were estimated by weighting excess medical costs by demographic group by the estimated total member months for patients with anemia. The shade of the cells represents the medical costs (PMPM/PPPM) by age groups for each group based on anemia status by sex from low (light) to high (dark). Abbreviations. PMPM, Per member per month; PPPM, Per patient per month.
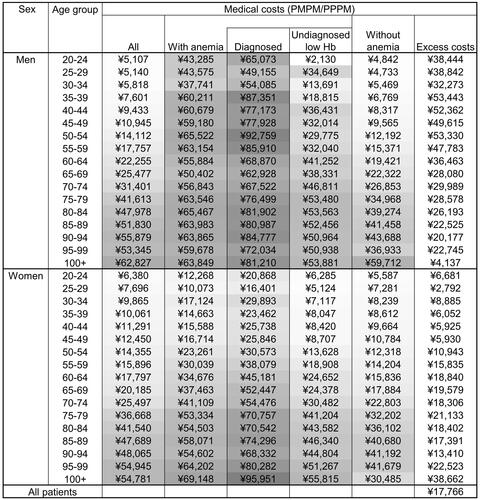
The nationwide annual excess medical costs in patients with anemia were estimated to be ¥3.32 trillion based on the estimated excess medical costs PPPM in all patients or ¥0.85 trillion based on CCI = 0.
The regression model estimated ¥14,641 PPPM in excess medical costs ( and S6).
Table 4. Estimation of medical costs (PMPM/PPPM) in 2020 by a regression model.
QOL in patients with anemia
In 2020, the mean EQ-5D scores for the with low Hb group and the without low Hb group were 0.9075 and 0.9194, respectively (), and the deficit QOL was 0.0118. By CCI, the mean EQ-5D scores in the with low Hb group were lower than in the without low Hb group, and the deficit QOL in CCI = 0 was 0.0084. QOL was also found to be lower in 2019 (Table S7).
Table 5. QOL in 2020.
The estimated nationwide deficit QOL associated with patients with low Hb in 2020 (11.7 million) based on the deficit QOL in 2020 was 138,000 QALY based on the deficit QOL in all patients or 99,000 QALY based on CCI = 0.
The regression model estimated the QOL deficit in individuals with low Hb to be 0.0111 ( and S8A).
Table 6. Estimation of QOL (A) and productivity loss (B) in 2020 by a regression model.
Productivity loss in patients with anemia
The mean productivity loss was higher in the with low Hb group (19.9%) than in the without low Hb group (17.3%); the excess productivity loss was 2.6% (). Higher productivity loss in the low Hb group was also found by CCI score, with the excess productivity loss being 1.67% in CCI = 0. Annual productivity loss was estimated to be ¥96,702 or ¥61,786, ¥171,207, and ¥157,042 in CCI = 0, 1, and >1, respectively, based on the mean productivity loss in all patients or by CCI level. The nationwide annual productivity loss of all patients was estimated to be ¥1.13 trillion or ¥0.72 trillion in CCI = 0.
Table 7. Excess productivity loss in 2020.
The regression model estimated a 2.3% excess productivity loss in individuals with low Hb ( and S8B).
Discussion
Using nationwide real-world data from over 500,000 study populations and 90,000 patients with anemia, this study examined anemia in Japanese adults, including prevalence, treatment status, medical costs, QOL, and productivity loss. The real-world data included health insurance claims data, health check-up data, and survey data. To the best of our knowledge, this is the first study to comprehensively examine anemia in Japan. The prevalence of anemia was estimated to be 15.1%, with approximately 15.9 million patients suffering from anemia nationwide. Many patients with anemia were untreated, including more than half of those with Hb levels of 8–12 g/dL and more than 40% of those with Hb levels of 6–8 g/dL. The majority of the treated patients were treated with oral iron drugs, with IV iron infusions being used by less than half of the treated patients. Patients with anemia were found to have higher medical costs, lower QOL, and higher productivity loss than those without anemia.
Here, we calculated the prevalence in some sex and age groups based on the prevalence by sex and age group () to compare with previous reports. It should be noted that the prevalence of anemia in this study may be higher than that in previous studies because of differences in the definition of anemia. In our study, both diagnosed patients and undiagnosed low Hb patients were included, whereas in most previous studies, patients were defined by Hb level only. The prevalence in women aged 20–49 yr was 21.8%, which was similar to that among women of reproductive age (15–49 yr), 22%, in a WHO reportCitation20. The prevalence in individuals aged ≥65 yr was 23.5%, which was higher than that reported in a Japanese study using the National Health and Nutrition Survey dataset, 17.1%. That study did not adjust for sex and age distribution, which may also contribute to the difference in the prevalence between studies. The prevalence in individuals aged ≥65 yr in our study was similar to that in another Japanese study including community-dwelling participants aged 69–91 yr, 22.3%Citation23. It should be noted that the percentage of patients with low Hb levels was comparable with that in a published survey in JapanCitation41. We found that the prevalence in women was higher than that in men up to 64 yr, it became comparable between 65 and 74 yr, and reversed at the age of 75 yr or older. A similar difference in the prevalence between sexes by age was also found in previous reports in JapanCitation45 and the United StatesCitation46.
This study found that more than half of the patients with anemia, defined by anemia diagnosis or low Hb level, went undiagnosed. That is, a certain number of patients with low Hb levels considered anemia were not diagnosed and were not treated for anemia. One possible underlying reason for the underdiagnosis could be the lack of treatment knowledge among physicians, resulting in inadequate treatment of individuals with low Hb levels. Physicians may disregard anemia because it does not directly cause death or because they are not anemia specialists. Furthermore, the perception of low Hb levels can vary among physicians, similar to the perceptions of the Hb levels requiring intervention. The reason behind the underdiagnosis of anemia from patient’s perspective can be the fact that if the Hb level is found to be low, the individual may disregard it and not seek medical attention. Some of these individuals may not experience any symptoms as a result of anemiaCitation29, or if they do, they may not recognize that the symptoms are a result of anemia. Nevertheless, anemia develops in patients with certain diseases, such as CKD, chronic heart failureCitation47, and some types of cancerCitation48–51; i.e. anemia would be an indicator of these diseases. Therefore, the identification of anemia may be useful for physicians to identify and manage these underlying diseases.
Because diagnosis is usually made for medical intervention for the disease, underdiagnosis may be associated with under treatment. Indeed, the possibility of under treatment was raised—more than half of individuals with low Hb levels did not receive any treatment. Oral iron drugs were commonly used in the treatments, and IV iron infusions were used for approximately one third or less of the patients who were treated. A Japanese study of 156 patients aged >65 yr with anemia (defined as Hb <11.0 g/dL) admitted to a rural community hospital in 2020 found that 40.4% of patients had anemia recorded in their medical records by the attending physicians within 1 week after admissionCitation28. Among the factors evaluated in that study, which included albumin value, BMI, CCI ≥5, dysphagia, and functional independence measure, only age was found to be significantly associated with the record of anemia. The authors proposed that ageism, which is an age-based stereotype, prejudice, and discrimination, may exist in recognizing anemia by the physicians and emphasized the importance of providing appropriate medical care to patients to improve their prognosis. Regarding IV iron infusion treatment, factors including adverse events and number of required infusions to achieve treatment effect affected patients’ preferencesCitation52. Moreover, in a Japanese study that used a hospital-based claims database to report on the treatment status of anemia in patients with non-dialysis-dependent CKD, a delay in initiation of erythropoiesis-stimulating agent (ESA) treatment, which is recommended in these patients when Hb levels fall below 10 g/dL, was reported in patients with low Hb levelsCitation29. The authors hypothesized that this was due to a lack of anemia symptoms and the burden of the doctor’s visit for ESA injections. These explanations may apply to under treatment in general and the low percentage of patients with IV iron infusions in this study.
According to this study, patients with anemia had higher medical costs, lower QOL, and higher productivity loss than those without anemia. To consider clinical background in patients with and without anemia, we examined these outcomes by CCI score and performed a regression analysis with demographic data and CCI score as explanatory variables. As a result, even patients with a CCI score of 0 have a substantial impact on these outcomes (). We confirmed that the regression analysis yielded a similar range of values to the mean values of all patients. Therefore, we believe that this study may have provided some useful information for assessing the impact of anemia. Excess medical costs were estimated to be ¥3.32 trillion for all patients and ¥0.85 trillion if we use the excess medical costs associated with CCI = 0. Compared with Japan’s national health expenditure of approximately ¥42.97 trillion in 2020Citation53, this amount does not appear to be negligible. Nevertheless, there may be a residual confounding even for those with a CCI of 0.
Table 8. Estimated excess medical costs, deficit QOL, and excess productivity loss in patients with anemia nationwide in 2020.
The global, regional, and national burden of anemia has been reported as years lived with disabilityCitation16,Citation17. However, little research has been conducted on the cost effect of anemia. Higher medical costs in patients with anemia than in those without anemia were reported in a study conducted in the United States in 2015Citation54. In the study, after adjusting for potential confounders, the average annual medical costs per patient for patients with anemia were more than double that of patients without anemia.
The deficit in QOL in patients with anemia shown in our study is consistent with previous studies. A Korean study reported the impact of anemia on QOL according to the severity level of anemia, which were 0.802, 0.596, and 0.416 for mild, moderate, and severe anemia, respectively, using the EQ-5D scoreCitation55. An impact of anemia on QOL was also reported in individuals with comorbidities. For example, a Spanish study revealed that the EQ-5D scores of patients with cancer with and without anemia were 0.65 and 0.73, respectivelyCitation56. In addition to its effect on QOL, differences in the effect of anemia in various countries have been reported in patients with CKD. The differences in mean EQ-5D scores between patients with Hb levels of >12 g/dL who presented with CKD (stage 3a) and those with Hb levels of 10–12 or <10 g/dL were 0.06 or 0.08 in Europe, 0.01 or 0.10 in the United States, and 0.02 or 0.02 in China, respectivelyCitation57. Compared with the effect on QOL in another disease in Japanese patients, the deficit in QOL of patients with low Hb levels and those without low Hb levels (approximately 0.01) was similar to that of patients with type 2 diabetes with fair or poor Hb A1c levels (6.5%–7.9% or ≥8.0%) and those with excellent Hb A1c levels (≤5.7%), with EQ-5D scores of 0.86 or 0.85 versus 0.87Citation58.
Further, Hb levels were reportedly associated with productivity loss in patients with CKD. The productivity loss was 19.5%, 26.9%, and 30.6% in patients with CKD stage 3a with Hb levels of >12, 10–12, and <10 g/dL, respectively, in a multi-country studyCitation57. The excess productivity loss in patients with anemia in this study was comparable to that associated with cervical cancer, breast cancer, and endometriosis as gynecological diseases among working women in Japan in fiscal 2015; the productivity loss was 5.8% and ¥0.89 trillion based on women’s average wageCitation59.
This study has several limitations. First, the characteristics of the database may affect the generalizability of this study. This study included people enrolled in various types of health insurance, including health insurance societies, national health insurance, and the medical care system for the elderly. However, the distribution of people by insurance type did not reflect the actual distribution nationwide, despite the fact that the distribution of people by sex and age was adjusted when estimating the prevalence of anemia and excess costs in patients. Additionally, all results were analyzed only for those who underwent health check-ups. These individuals may be healthier than the general Japanese population, which may lead to selection bias. Furthermore, data from the EQ-5D and WPAI questionnaires were collected from a limited number of individuals who used an online health management application. These individuals may be younger, healthier, and more health conscious than the general Japanese population, which may also weaken the generalizability of the results. Second, because the majority of the pregnant women were unable to be identified in the database, the same Hb levels (≤12 g/dL) were used to define patients with anemia for all women. Therefore, the number of patients in the childbearing age group may be overestimated. Third, the Hb value may contain measurement error, although the error is random error and may not substantially affect the results. Fourth, because diagnosed patients were defined based on the diagnosis on the claims, the accuracy of recording the diagnosis affects the patient identification reliability. It should be noted that it is unclear whether the diagnosis was made on the basis of Hb levels or not due to lack of information in the database. In addition, although diagnosed patients included those without low Hb levels, it is unclear whether the Hb levels of these patients normalized after receiving appropriate treatment. Fifth, the difference between patients with and without anemia or those with and without low Hb levels was evaluated in terms of excess costs, deficit QOL, and excess productivity loss in patients with anemia. However, it does not imply a causal relation with anemia. That is, whether the differences are caused by anemia or other underlying differences in patient populations is unclear, given the likelihood of residual confounding. The residual confounding may appear although we calculated these outcomes by CCI score to eliminate the effects of comorbidities. Finally, we examined the treatment status of anemia based on the information in the claims data. Given the universal health insurance coverage in Japan, all prescriptions covered by health insurance could be captured in the database. However, the use of over-the-counter oral iron supplements and dietary supplements was excluded.
Conclusions
Patients with anemia had higher medical costs, lower QOL, and greater productivity loss than those without anemia, indicating that the presence of anemia should not be overlooked. Despite the substantial impact on patients and society, the study shows that anemia may be underdiagnosed and undertreated in Japan. This situation may be related to the lack of recognition of anemia as a major health issue, general treatment guidelines for anemia, and education and awareness of anemia among physicians in Japan. The comprehensive situation of anemia nationwide illustrated in this study may be useful when considering medical policies for patients with anemia.
Transparency
Declaration of financial/other interests
TT, KI, and CH are employed by Milliman Inc., which has received consultancy fees from Nippon Shinyaku Co., Ltd. YY has received consultancy fees from Nippon Shinyaku Co., Ltd inside the submitted work, and received personal fees from Sun Pharmaceutical Industries Ltd., Asahi Kasei Pharma Corporation, Toray Medical Co., Ltd., Ono Pharmaceutical Co., Ltd., MSD K.K., IQVIA Solutions Japan K.K., and Pfizer Japan Inc., outside the submitted work. MO, AS, YS, NH, and HN are employed by Nippon Shinyaku Co., Ltd. JT has received consultancy fees from Nippon Shinyaku Co., Ltd inside the submitted work.
Author contributions
All authors contributed to the conception and design. KI and CH contributed to data analysis. All authors contributed to interpretation of the data. TT drafted the paper, and all authors revised it critically for intellectual content. All authors were involved in the final approval of the version to be published and agreed to accountable for all aspects of the work.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplemental Material
Download PDF (313.1 KB)Acknowledgements
The authors would like to thank Enago (www.enago.jp) for the English language review.
Additional information
Funding
References
- Global nutrition targets 2025: anaemia policy brief. Policy brief. Geneva: World Health Organization; 2014.
- Iron deficiency anaemia: assessment, prevention, and control. A guide for programme managers. Geneva: World Health Organization; 2001.
- Vulser H, Wiernik E, Hoertel N, et al. Association between depression and anemia in otherwise healthy adults. Acta Psychiatr Scand. 2016;134(2):150–160. doi: 10.1111/acps.12595.
- Hidese S, Saito K, Asano S, et al. Association between iron-deficiency anemia and depression: a web-based japanese investigation. Psychiatry Clin Neurosci. 2018;72(7):513–521. doi: 10.1111/pcn.12656.
- Black RE, Victora CG, Walker SP, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. 2013;382(9890):427–451. doi: 10.1016/S0140-6736(13)60937-X.
- Beard JL. Why iron deficiency is important in infant development. J Nutr. 2008;138(12):2534–2536. doi: 10.1093/jn/138.12.2534.
- McCann JC, Ames BN. An overview of evidence for a causal relation between iron deficiency during development and deficits in cognitive or behavioral function. Am J Clin Nutr. 2007;85(4):931–945. doi: 10.1093/ajcn/85.4.931.
- Haas JD, Brownlie T. Iron deficiency and reduced work capacity: a critical review of the research to determine a causal relationship. J Nutr. 2001;131(2s-2):676S–690S. doi: 10.1093/jn/131.2.676S.
- Ng TP, Feng L, Nyunt MSZ, et al. Frailty in older persons: multisystem risk factors and the frailty risk index (FRI). J Am Med Dir Assoc. 2014;15(9):635–642. doi: 10.1016/j.jamda.2014.03.008.
- Penninx BWJH, Pluijm SMF, Lips P, et al. Late-life anemia is associated with increased risk of recurrent falls. J Am Geriatr Soc. 2005;53(12):2106–2111. doi: 10.1111/j.1532-5415.2005.00491.x.
- Hong CH, Falvey C, Harris TB, et al. Anemia and risk of dementia in older adults: findings from the health ABC study. Neurology. 2013;81(6):528–533. doi: 10.1212/WNL.0b013e31829e701d.
- Palapar L, Kerse N, Rolleston A, et al. Anaemia and physical and mental health in the very old: an individual participant data meta-analysis of four longitudinal studies of ageing. Age Ageing. 2021;50(1):113–119. doi: 10.1093/ageing/afaa178.
- Chaves PHM, Carlson MC, Ferrucci L, et al. Association between mild anemia and executive function impairment in community-dwelling older women: the women’s health and aging study II. J Am Geriatr Soc. 2006;54(9):1429–1435. doi: 10.1111/j.1532-5415.2006.00863.x.
- Penninx BW, Pahor M, Woodman RC, et al. Anemia in old age is associated with increased mortality and hospitalization. J Gerontol A Biol Sci Med Sci. 2006;61(5):474–479. doi: 10.1093/gerona/61.5.474.
- Patel KV. Epidemiology of anemia in older adults. Semin Hematol. 2008;45(4):210–217. doi: 10.1053/j.seminhematol.2008.06.006.
- Kassebaum NJ, GBD 2013 Anemia Collaborators. The global burden of anemia. Hematol Oncol Clin North Am. 2016;30(2):247–308. doi: 10.1016/j.hoc.2015.11.002.
- Safiri S, Kolahi AA, Noori M, et al. Burden of anemia and its underlying causes in 204 countries and territories, 1990–2019: results from the global burden of disease study 2019. J Hematol Oncol. 2021;14(1):185. doi: 10.1186/s13045-021-01202-2.
- Global nutrition targets 2025: policy brief series. Policy brief. Geneva: World Health Organization; 2014.
- Nutritional anaemias: tools for effective prevention and control. Geneva: World Health Organization; 2017.
- The global prevalence of anaemia in 2011. Geneva: World Health Organization; 2015.
- Imai E, Nakade M. Fish and meat intakes and prevalence of anemia among the Japanese elderly. Asia Pac J Clin Nutr. 2019;28(2):276–284.
- Kito A, Imai E. The association with dietary patterns and risk of anemia in Japanese elderly. J Nutr Sci Vitaminol (Tokyo). 2020;66(1):32–40. doi: 10.3177/jnsv.66.32.
- Noma T, Kabayama M, Gondo Y, et al. Association of anemia and SRH in older people: the SONIC study. Geriatr Gerontol Int. 2020;20(7):720–726. doi: 10.1111/ggi.13945.
- Society TJB. Guidelines for the treatment of anemia by proper use of iron preparations., Revised 3rd Edition. Sapporo, Japan: Kyobun-sha; 2015.
- Yamamoto H, Nishi S, Tomo T, et al. Japanese society for dialysis therapy: guidelines for renal anemia in chronic kidney disease. Ren Replace Ther. 2015;2017;3,36.
- Carson JL, Brittenham GM. How I treat anemia with red blood cell transfusion and iron. Blood. 2023;142(9):777–785.
- Guidelines for use of blood products. Revised Edition [Internet]. Tokyo (Japan): Ministry of Health, Labour and Welfare; 2005 [cited 2023 Apr 10]. Available from https://www.mhlw.go.jp/new-info/kobetu/iyaku/kenketsugo/5tekisei3b.html. Japanese.
- Amano S, Ohta R, Sano C. Recognition of anemia in elderly people in a rural community hospital. Int J Environ Res Public Health. 2021;18(21):11179. doi: 10.3390/ijerph182111179.
- Kokado Y, Ishii M, Ueta K, et al. Characteristics of Japanese patients with non-dialysis-dependent chronic kidney disease initiating treatment for anemia: a retrospective real-world database study. Curr Med Res Opin. 2022;38(12):2175–2182. doi: 10.1080/03007995.2022.2125256.
- Hashimoto Y, Okada A, Matsui H, et al. Recent trends in anti-vascular endothelial growth factor intravitreal injections: a large claims database study in Japan. Jpn J Ophthalmol. 2023;67(1):109–118. doi: 10.1007/s10384-022-00969-2.
- Sakai F, Hirata K, Igarashi H, et al. A study to investigate the prevalence of headache disorders and migraine among people registered in a health insurance association in Japan. J Headache Pain. 2022;23(1):70. doi: 10.1186/s10194-022-01439-3.
- Satoh M, Murakami T, Obara T, et al. Time-series analysis of blood pressure changes after the guideline update in 2019 and the coronavirus disease pandemic in 2020 using Japanese longitudinal data. Hypertens Res. 2022;45(9):1408–1417. doi: 10.1038/s41440-022-00961-w.
- Shigemi D, Okada A, Yasunaga H. Postoperative adverse events and re-treatment among patients who have undergone laparoscopic and robotic sacrocolpopexy for pelvic organ prolapse in Japan. Int J Gynaecol Obstet. 2023;161(1):114–119. doi: 10.1002/ijgo.14497.
- Statistical classification of diseases and cause of death 2013[Internet.]. Tokyo (Japan): Ministry of Health, Labour and Welfare; [cited 2023 Apr 10]. Available from: http://www.mhlw.go.jp/toukei/sippei/.
- Nutritional anaemias. Report of a WHO scientific group. World Health Organ Tech Rep Ser. 1968;405:5–37.
- Kitamura K. Guidelines for laboratory tests JSLM 2021. Tokyo: Japanese Society of Laboratory Medicine; 2021. Chapter 36, Anemia. [cited 2023 Apr 10]. Available from https://www.jslm.org/books/guideline/36.pdf. Japanese.
- Girelli D, Marchi G, Camaschella C. Anemia in the elderly. Hemasphere. 2018;2(3):e40. doi: 10.1097/HS9.0000000000000040.
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8.
- Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83.
- Population Estimates. #3. Population and Percentage distribution by Age (5-Year Age Group) and Sex - Total population, October 1, Each Year [Internet]. Tokyo (Japan): Ministry of Internal Affairs and Communications; 2021 [cited 2023 Apr 10]. Available from: https://www.e-stat.go.jp/en/stat-search/files?page=1&layout=datalist&toukei=00200524&tstat=000000090001&cycle=7&year=20200&month=0&tclass1=000001011679&stat_infid=000032153671&tclass2val=0.
- The National Health and Nutrition Survey in Japan in 2019. [Internet]. Tokyo (Japan): Ministry of Health, Labour and Welfare; 2020. Table 24-1. [cited 2023 Apr 10]. Available from: https://www.e-stat.go.jp/stat-search/files?page=1&layout=datalist&toukei=00450171&tstat=000001041744&cycle=7&year=20190&month=0&tclass1=000001148507&tclass2val=0. Japanese.
- Anatomical Classification [Internet]. Basel: The European Pharmaceutical Market Research Association; [cited 2023 Jan 11,]. Available from: https://www.ephmra.org/anatomical-classification.
- Japanese Basic Survey on Wage Structure on 2020. [Internet]. Tokyo (Japan): Ministry of Health, Labour and Welfare; 2021 [cited 2023 Apr 20]. Available from: https://www.mhlw.go.jp/toukei/itiran/roudou/chingin/kouzou/z2020/dl/13.pdf. Japanese.
- Ethical guidelines for life science and medical research involving human subjects. Tokyo: Ministry of Education, Sports, Science and Technology; Ministry of Health, Labour and Welfare; 2021, partial revision in 2022.
- GBD 2021 Anaemia Collaborators. Prevalence, years lived with disability, and trends in anaemia burden by severity and cause, 1990–2021: findings from the global burden of disease study 2021. Lancet Haematol. 2023;10(9):e713–e734. doi: 10.1016/S2352-3026(23)00160-6.
- Guralnik JM, Eisenstaedt RS, Ferrucci L, et al. Prevalence of anemia in persons 65 years and older in the United States: evidence for a high rate of unexplained anemia. Blood. 2004;104(8):2263–2268. doi: 10.1182/blood-2004-05-1812.
- De Franceschi L, Iolascon A, Taher A, et al. Clinical management of iron deficiency anemia in adults: systemic review on advances in diagnosis and treatment. Eur J Intern Med. 2017;42:16–23. doi: 10.1016/j.ejim.2017.04.018.
- Zhang Y, Chen Y, Chen D, et al. Impact of preoperative anemia on relapse and survival in breast cancer patients. BMC Cancer. 2014;14(1):844. doi: 10.1186/1471-2407-14-844.
- Vayrynen JP, Tuomisto A, Vayrynen SA, et al. Preoperative anemia in colorectal cancer: relationships with tumor characteristics, systemic inflammation, and survival. Sci Rep. 2018;8(1):1126. doi: 10.1038/s41598-018-19572-y.
- Cross AJ, Wooldrage K, Robbins EC, et al. Whole-colon investigation vs. flexible sigmoidoscopy for suspected colorectal cancer based on presenting symptoms and signs: a multicentre cohort study. Br J Cancer. 2019;120(2):154–164. doi: 10.1038/s41416-018-0335-z.
- Boennelykke A, Jensen H, Ostgard LSG, et al. Cancer risk in persons with new-onset anaemia: a population-based cohort study in Denmark. BMC Cancer. 2022;22(1):805. doi: 10.1186/s12885-022-09912-7.
- Takeshima T, Ha C, Iwasaki K. Estimation of the utilities of attributes of intravenous iron infusion treatment for patients with iron-deficiency anemia: a conjoint analysis in Japan. J Med Econ. 2023;26(1):84–94. doi: 10.1080/13696998.2022.2158661.
- Overview of National Health Care Expenditures in 2020. [Internet]. Tokyo (Japan): Ministry of Health, Labour and Welfare; 2022 [cited 2023 Apr 10]. Available from: https://www.mhlw.go.jp/toukei/saikin/hw/k-iryohi/20/dl/data.pdf. Japanese.
- Nissenson AR, Wade S, Goodnough T, et al. Economic burden of anemia in an insured population. J Manag Care Pharm. 2005;11(7):565–574. doi: 10.18553/jmcp.2005.11.7.565.
- Ock M, Jo MW, Gong YH, et al. Estimating the severity distribution of disease in South Korea using EQ-5D-3L: a cross-sectional study. BMC Public Health. 2016;16(1):234. doi: 10.1186/s12889-016-2904-5.
- Barca-Hernando M, Muñoz-Martin AJ, Rios-Herranz E, et al. Case-control analysis of the impact of anemia on quality of life in patients with cancer: a Qca study analysis. Cancers. 2021;13(11):2517. doi: 10.3390/cancers13112517.
- van Haalen H, Jackson J, Spinowitz B, et al. Impact of chronic kidney disease and anemia on health-related quality of life and work productivity: analysis of multinational real-world data. BMC Nephrol. 2020;21(1):88. doi: 10.1186/s12882-020-01746-4.
- Sakamaki H, Ikeda S, Ikegami N, et al. Measurement of HRQL using EQ-5D in patients with type 2 diabetes mellitus in Japan. Value Health. 2006;9(1):47–53. doi: 10.1111/j.1524-4733.2006.00080.x.
- Findings of Research on Health Promotion and Working Women. [Internet]. Tokyo (Japan): Health and Global Policy Institute; 2016 [cited 2023 Apr 10]. Available from: https://hgpi.org/en/lecture/475.html.
