Abstract
Aims
Health care providers (HCPs) treating multiple sclerosis (MS) in clinical practice have numerous disease-modifying therapies (DMTs) to consider when evaluating treatment options. This study assessed the treatment preferences of HCPs in the United States, both direct (explicit) and derived (explicit and implicit), when selecting MS DMTs based on clinical and logistical treatment attributes.
Materials and methods
A 45-minute web-enabled questionnaire was administered to HCPs who manage patients with MS to assess the importance of treatment attributes. HCPs were recruited through an online panel. This study examined treatment attributes relevant to treatment decisions in MS, with a focus on the burden to HCPs and their staff, as well as HCP attitudes toward various aspects of MS care such as diagnosis, treatment prioritization, and ease of initiating or switching DMTs. The study also employed a discrete choice experiment (DCE) to assess direct and derived treatment preferences.
Results
The study recruited 145 HCPs. Direct assessments (a score of greater than 7.0 was considered important) suggested that safety (mean importance rating = 7.8/9) and relative risk reduction in relapses (7.6/9) and disability progression (7.5/9) were most important when selecting DMTs. In contrast, derived importance from the DCE (higher points corresponding to greater importance) suggested that logistical attributes such as dose frequency (mean relative attribute importance = 17.5%), dose titration (10.3%), formulation (9.4%), and volume of calls (9.1%) were important considerations, along with efficacy (16.5%), safety (9.8%), and gastrointestinal tolerability (9.4%).
Limitations
This study may have been subject to selection bias due to the application of eligibility criteria, the convenient sampling recruitment methodology, and recruitment of HCPs with internet access.
Conclusion
In the direct assessment, clinical attributes were chosen as the most important treatment attributes by HCPs. However, in the DCE, derived treatment decisions rated logistical attributes as also being as important in treatment choice.
PLAIN LANGUAGE SUMMARY
In this study, researchers aimed to understand what multiple sclerosis (MS) neurologists, nurse practitioners, and physician assistants think is most important when choosing medicines for their patients. They surveyed 145 health care providers (HCPs) in the United States for this study. The HCPs reported that safety and reducing the risk of relapses and disability were most important when selecting medicines. Additionally, the researchers used a method called a discrete choice experiment to determine the relative importance of medication characteristics to HCPs. They found that additional factors, such as how often the medicine needs to be taken, how it is given, and how easy it is to use, were also very important. The study may not represent the opinions of all HCPs due to the number of participants and participation criteria.
Introduction
Multiple sclerosis (MS) is a chronic, demyelinating, inflammatory disease with a heterogenous disease courseCitation1–9. The prevalence of MS in the United States of America (US) in 2010 was estimated to be around 309.2 per 100,000 persons, with the highest prevalence in the 55–64 year age groupCitation10. Health care providers (HCPs) make individualized treatment decisions based on the patient’s clinical profile and patient preferences. HCP preferences in the current MS treatment landscape have not been extensively evaluated and understanding HCP preferences may inform the clinical or logistical attributes important for MS treatment decision making.
Several disease modifying therapies (DMTs) have come to market in the past two decades including interferon beta, glatiramer acetate, teriflunomide, dimethyl fumarate, diroximel fumarate, natalizumab, fingolimod, alemtuzumab, mitoxantrone, cladribine, ocrelizumab, ofatumumab, ublituximab, siponimod, ozanimod, and ponesimodCitation11–16. The utilization of the proper agent for a given patient or patient population is becoming more complex as new mechanisms, routes of administration (ROAs), safety, and efficacy profiles are developedCitation17. Prior studies have consistently demonstrated the importance of certain clinical attributes driving treatment preferences among HCPs and patients with MS. These attributes include reducing disease progression, minimizing side effects, and considering the frequency and method of administrationCitation18–25. HCPs have traditionally considered these clinical and safety related factors when making treatment choices. However, there is a growing recognition of non-clinical factors that also play a significant role in decision making among HCPsCitation26. HCPs are likely to take into consideration both clinical (e.g. safety, efficacy) and logistical (e.g. patient monitoring, burden to patient, caregiver, or HCP, administrative challenges for clinical staff) treatment attributes when choosing treatments for their MS patientsCitation27–30. This research aims to understand the relative impact that clinical and logistical attributes have on HCP decisions when making treatment choices for their MS patients.
The main objective of this study was to quantitatively assess the importance of clinical and logistical treatment attributes in selection of DMTs for MS by HCPs. The degree that each studied treatment attribute impacted HCP decisions when selecting treatments for their MS patients was assessed to generate comparative evidence differentiating MS therapies. Importance was assessed via two methods – direct assessment via importance rating questions and derived assessment via a discrete choice experiment (DCE, ). DCEs are often used in healthcare research to quantify the value respondents place on specific characteristics or attributes of disease interventionsCitation31,Citation32. These treatment attributes can have different levels that represent the possible options or choices for each attribute. A DCE study design involves the development of a grid or table of attributes and their respective levels to create hypothetical product profiles, and then subsequently testing these profiles with respondents. This study also aimed to examine the treatment-related burden of MS therapies across different burden attributes from the perspective of HCPs and their staff. In addition, we sought to quantify the relative importance of each attribute and identify what truly drives treatment selection.
Table 1. Discrete choice experiment design grid.
Methods
Study overview
A web-based, cross-sectional quantitative survey was conducted among US HCPs who treated and managed patients with MS. Importantly, this study used various treatment attributes that may be used in treatment decisions, particularly focusing on the burden to HCPs and their staff, as well as HCP attitudes toward the current state of MS care, including diagnosis, treatment prioritization, and ease of initiating or switching DMTs.
In addition, the study included a DCE that was used to quantify the preferences of HCPs and perceived burden for currently available therapies. The DCE methodology is a well-established approach used to understand individual preferences by presenting respondents with a series of hypothetical choices based on product attributes/profilesCitation33. Treatment attributes were developed based on 12 DMTs approved by the US Food and Drug Administration (FDA) that represented DMTs administered orally (dimethyl fumarate, teriflunomide, diroximel fumarate, fingolimod, ozanimod, siponimod, and cladribine), via injection (ofatumumab, glatiramer acetate, and interferons) or via infusion (ocrelizumab, natalizumab)Citation34–45. HCPs participating in the study were presented with various hypothetical scenarios that included varying combinations of these treatment attributes. By statistically analyzing the choices made by HCPs across these scenarios, study researchers were able to quantify and assess HCP preferences of different available therapies. All study materials were reviewed and approved by a central institutional review board (IRB) in the US (Advarra).
This study was conducted in accordance with the protocol, the current version of the Declaration of HelsinkiCitation46, and applicable national and local requirements for good pharmacovigilance practices. This study recruited HCPs in the US and included both neurologists and advanced practice clinicians (APCs, such as nurse practitioners or physician assistants) that treat MS patients across community and academic settings.
Web-based questionnaire
The web-based questionnaire was approximately 45 min in length and was developed following good research practices. Respondents for this study answered initial screening questions to help establish their eligibility to participate in the study. Eligible respondents received the complete questionnaire immediately following screening. Consent was obtained from all respondents prior to entry into the web-based questionnaire. The questionnaire included the current diagnosis and management approach to MS, an MS DMT DCE, a deep dive into logistical burden, and clinigraphics, demographics, and the impact of the COVID-19 pandemic.
Three cognitive interviews were conducted with eligible respondents, during which the moderator hosted the web-enabled questionnaire on their computer and shared their screen with the HCPs via screensharing software. These interviews focused on subjective validation of the instrument – to ensure that all instrument sections were capturing intended data, the instrument was appropriately timed, and questions were appropriately detailed and clear for HCPs to answer accurately without the need for external assistance.
The following attributes included in the direct assessment of treatment importance were measured on a 9-point scale, where 1 was “not at all important” and 9 was “extremely important”: safety; relative risk reduction (RRR) in relapses; RRR in disability progression; ROA; ability for self-administration; minimal time required for treatment initiation; gastrointestinal (GI) tolerability; dosing frequency; minimal patient monitoring required during treatment; mechanism of action (MOA); impact on vaccinations or vaccination status; resulted in minimal volume of patient phone calls to clinic; and dose titration. Attributes were aligned based on secondary research to attributes of current DMTsCitation34–45.
Discrete choice experiment
Lighthouse Studio 9.8.0 (a Sawtooth software) was used to generate an orthogonal design for the DCE. Such a design ensures minimal correlation between the treatment attributes when presented as hypothetical treatment profiles. Attributes from the DCE included: safety; efficacy; formulation; time required for treatment initiation; GI tolerability profile; dosing frequency; patient monitoring; patient population; vaccination recommendation; volume of patient phone calls to clinic; and dose titration. A 14-choice-set experimental design with two hypothetical treatment profiles in each choice-set was generated using an 11-attribute grid with 2–3 levels per attribute. The design had two-way frequencies between each attribute level greater than 50 and standard errors lower than 0.05. Respondents were also offered a “neither therapy” option. The responses were used to assess the strength of preference for each attribute and levelCitation31,Citation32. Respondents were asked to make four decisions for each choice set:
Question #1: most preferred when selecting a DMT for your eligible MS patients.
Question #2: least burdensome to the respondent’s MS patients.
Question #3: therapy in which the respondent thought their MS patients would be most adherent.
Question #4: least burdensome to the respondent, their staff, or their practice.
Study recruitment and selection of study population
HCPs were recruited for participation in the study using convenience sampling from an online panel that was representative of the US HCP population. Those who qualified based on the eligibility criteria were allowed to participate in the study. Respondents needed to complete screening and fulfill the following criteria to qualify for participation in the study:
Inclusion criteria
Respondent must have been board eligible (if physician) and licensed or accredited by a board (if APC) in neurology with the following positions:
Neurologists.
APCs (nurse practitioners [NP], physician assistants [PA]) in neurology.
In practice post-residency for 3–35 years, inclusive.
Had spent at least 70% of their professional time providing direct patient care.
Treated at least 25 unique MS patients in an average month.
Treated at least five unique MS patients who were currently on DMTs in an average month.
Exclusion criteria
Been a participant in market research, health economic and outcomes research, or general pharmaceutical research in the last 30 days.
A current employee of or had an affiliation with the following:
Pharmaceutical manufacturer or biotech.
Contract research organization.
Market research or advertising firm.
FDA or government agency.
The study fielded for 5 weeks in February–March 2022 until the target sample size was reached. Respondents were enrolled in the study for up to 45 min or the time that it took to complete the web-based questionnaire. No additional follow-up was needed.
Data analysis
Statistical analyses were conducted in Lighthouse Studio 9.8.0 (a Sawtooth software) and Q Research Software 5.6. Descriptive statistics (means, medians, maximum or minimum ranges, and frequencies) were used to describe the recruited sample for the initial topic areas covered by the study instrument. A hierarchical Bayesian random-effects-only model was used on the data captured in the DCE to compute mean relative attribute importance scores for each treatment attribute and mean relative preference weights for each attribute level. Data were reported as mean (standard deviation; SD) relative attribute importance and mean (SD) relative preference weights.
Means for the product attribute ratings obtained via direct assessments and mean relative importance scores obtained from the DCE were plotted on a chart to help reveal potential differences between direct (explicit) and derived (explicit and implicit) importance of MS DMT treatment attributes.
Results
Study population – Demographics
Data was collected on 151 HCPs. A total of six respondents were removed during and after fieldwork due to poor data quality (e.g. completion of the survey in a time well below the mean length of interview, ratings with absolutely no differentiation despite different products and attributes being evaluated). A final sample of 145 HCPs participated in this study, including 100 neurologists and 45 APCs (n = 30 NPs and n = 15 PAs) (). The attributes and levels do not indicate any ranking or prioritization from a study design standpoint and were only used to create the hypothetical treatment profiles for each choice-set. Mean years in practice was 11.6 years for APCs and 18.6 years for neurologists and mean years prescribing DMTs was 7.7 years for APCs and 17.2 years for neurologists. Gender differences were observed in the study population, with 74% of neurologists identifying as male while 26% were female and 24% of APCs identifying as males while 76% were female. Data on type of practice (community hospital, private practice, academic hospital), staff assisting in reimbursement, and average patient load was also collected and is summarized in .
Table 2. Demographics of respondents.
Direct assessment of treatment preferences
For direct assessments, a score of greater than 7.0 was considered important for DMT choices by HCPs. Direct assessments indicated that safety (mean importance rating = 7.8/9) and efficacy (defined as the combined “RRR in relapse” [7.6/9] and “RRR in disability progression” [7.5/9]) were the top attributes impacting overall treatment selection ( and ). Compared to safety and efficacy, HCPs considered attributes such as minimal patient monitoring required during treatment (6.5/9), results in minimal volume of patient phone calls to the clinic (5.9/9), minimal time required for initiation (6.5/9), and dose titration (5.8/9) as less important ().
Figure 1. Direct assessment of product attribute importance by HCPs rating on a 9-point scale.
Abbreviations. APC, Advanced Practice Clinicians; RRR, Relative Risk Reduction; ROA, Route of Administration; MOA, Mechanism of Action.
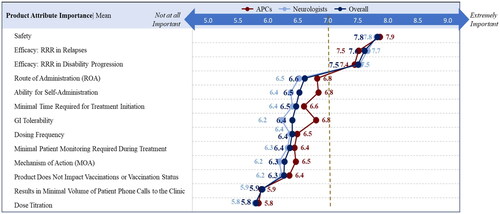
Table 3. Mean product attribute importance that HCPs considered for all MS patients.
Derived treatment preferences via discrete choice experiment (DCE)
For the DCE, scores higher than 9.09 were considered important for DMT choices by HCPs. As there were a total of 11 attributes and 100 points for the DCE score, attributes would all have a score of 9.09 if they were considered equally important by HCPs. Therefore, attributes with a score higher than 9.09 can be considered important for therapy preference. Derived importance from the DCE of overall preferred DMT for their eligible MS patients (Question #1) demonstrated that logistical attributes such as dosing frequency (mean [SD] relative attribute importance = 17.5% [6.7]), dose titration (10.3% [5.2]), volume of patient calls to the clinic (9.1% [4.7]), and formulation (9.4% [4.8]) were also important when selecting DMTs, along with efficacy (16.5% [8.9]), safety (9.8% [4.5]), and GI tolerability profile (9.4% [6.1]) ().
Figure 2. Derived assessment of DCE Question #1 Output: Mean [SD] overall therapy preference of relative attribute importance.
Note: Question #1: most preferred when selecting a DMT for your eligible MS patients.
Abbreviation. GI, Gastrointestinal.
![Figure 2. Derived assessment of DCE Question #1 Output: Mean [SD] overall therapy preference of relative attribute importance.Note: Question #1: most preferred when selecting a DMT for your eligible MS patients.Abbreviation. GI, Gastrointestinal.](/cms/asset/9bd07540-1dea-44da-a6e9-99e318966b6b/ijme_a_2279883_f0002_b.jpg)
When asked to indicate their preference for a therapy that is least burdensome to patients (Question #2), HCPs indicated that attributes such as dosing frequency (27.4% [8.5]) and formulation (16.0% [6.2]) were important when choosing a DMT ( and Supplementary Table S1). Similarly, HCPs, when asked for preference for a therapy to which patients would adhere the most (Question #3), indicated that attributes such as dosing frequency (22.0% [10.0]), formulation (18.2% [3.6]), and dose titration (12.3% [6.8]) were important when choosing a DMT ( and Supplementary Table S2). Derived analysis from the DCE asking HCPs for preference for a therapy least burdensome to their staff and themselves (Question #4) indicated that attributes such as volume of patient phone calls to the clinic (19.5% [11.8]) and dosing frequency (15.1% [8.0]) were important ( and Supplementary Table S3).
Figure 3. Derived assessment of DCE Question #2 Output: Mean [SD] relative attribute importance for least burdensome therapy to patients.
Note: Question #2: least burdensome to the respondent’s MS patients.
Abbreviation. GI, Gastrointestinal.
![Figure 3. Derived assessment of DCE Question #2 Output: Mean [SD] relative attribute importance for least burdensome therapy to patients.Note: Question #2: least burdensome to the respondent’s MS patients.Abbreviation. GI, Gastrointestinal.](/cms/asset/cdc71ee2-addf-4e9f-8c36-9e5a591a4746/ijme_a_2279883_f0003_b.jpg)
Figure 4. Derived assessment of DCE Question #3 Output: Mean [SD] relative attribute importance for therapy with the best adherence.
Note: Question #3: therapy in which the respondent thought their MS patients would be most adherent.
Abbreviation. GI, Gastrointestinal.
![Figure 4. Derived assessment of DCE Question #3 Output: Mean [SD] relative attribute importance for therapy with the best adherence.Note: Question #3: therapy in which the respondent thought their MS patients would be most adherent.Abbreviation. GI, Gastrointestinal.](/cms/asset/99ca3b53-1bd6-4bc0-99ed-3b52ab1f6e27/ijme_a_2279883_f0004_b.jpg)
Figure 5. Derived assessment of DCE Question #4 Output: Mean [SD] relative attribute importance for least burdensome therapy to HCP and staff.
Note: Question #4: least burdensome to the respondent, their staff, or their practice.
Abbreviation. GI, Gastrointestinal.
![Figure 5. Derived assessment of DCE Question #4 Output: Mean [SD] relative attribute importance for least burdensome therapy to HCP and staff.Note: Question #4: least burdensome to the respondent, their staff, or their practice.Abbreviation. GI, Gastrointestinal.](/cms/asset/ad002f06-7f54-40cd-85eb-168f7323de03/ijme_a_2279883_f0005_b.jpg)
Mean relative preference weights for Question #1 suggested that HCPs preferred treatments that required less frequent dosing (every 6 months), allowed flexibility of dose titration (varies from 1 to 4 weeks), resulted in a low volume of patient phone calls to the clinic, were a patient-administered oral capsule, and had better efficacy, safety, and tolerability profiles ( and ). HCPs preferred comparable treatments with regards to the most important attributes driving decisions on Questions #2 (Supplementary Table S1), #3 (Supplementary Table S2), and #4 (Supplementary Table S3).
Figure 6. Derived assessment of DCE Output Question #1: Mean relative preference weights.
Note: Question #1: most preferred when selecting a DMT for your eligible MS patients.
Abbreviations. Mos. = Months; RRR = Relative Risk Reduction; BB = Black Box; PML = Progressive Multifocal Leukoencephalopathy; SC = subcutaneous; IV = intravenous; min = minute; hr = hour; Hep B = Hepatitis B; RRMS = Relapsing Remitting Multiple Sclerosis; PPMS = Primary Progressive Multiple Sclerosis; GI = Gastrointestinal.
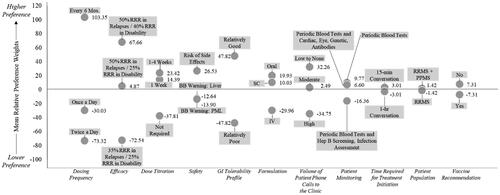
Table 4. HCPs overall preference for DMTs for their Eligible MS patients – Derived analysis from DCE Question #1.
Comparing direct and derived assessment of treatment preferences
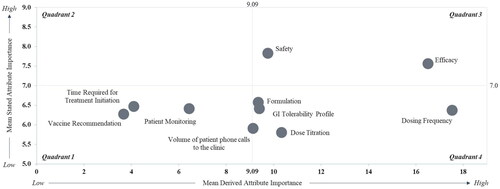
An analysis comparing direct versus derived attribute preference highlighted dosing frequency, formulation, GI tolerability profile, volume of patient phone calls to the clinic, and dose titration as relatively less important via direct (Quadrant 3) analysis; in contrast, these factors were shown to be important via derived (Quadrant 4) analysis () and are therefore implicit drivers of treatment preference.
Figure 7. Direct versus derived rated importance by DCE relative importance scores.
Note: Direct assessments are plotted on the y-axis and derived assessments are plotted on the x-axis; the mean stated preference threshold of >7.0 = high score on a 9-point scale is based on accepted convention.
Abbreviation. GI, Gastrointestinal.
Discussion
When HCPs were asked which attributes were important when choosing DMTs via direct assessment, clinical attributes such as efficacy (including RRR in relapse and RRR in disability progression) and safety were rated the highest. However, the DCE results demonstrated that, while efficacy and safety were still important, real world logistical attributes such as dosing frequency, dose titration, volume of patient phone calls to the clinic, and formulation were also important when choosing a DMT for MS. This DCE helps shed light on these logistical attributes as implicit drivers of DMT preference. A previous DCE study examining patient treatment preferences reported that patients considered dosing regimen (including frequency and ROA) as equally important attributes to safety and efficacyCitation47. This DCE helps validate findings from prior research from the HCP perspective to provide a holistic view of treatment preferences of two key stakeholders in the literature.
Perceptual decisions are biased by the cost to actCitation48. Humans make decisions based on the amount of effort required for an outcome. Decisions on which DMTs are prescribed are partly based on the level of burden for patients, caregivers, HCPs, and practice staff. Treatment burden, or the workload and effect on patient functioning and wellbeing for patients with chronic diseases, can cause a decrease in adherence to treatmentsCitation49,Citation50. Additionally, studies have demonstrated that 16% to 24% of an HCP’s time is spent on administrative duties related to patient care, and that these affected their career satisfaction as well as their ability to deliver high-quality careCitation51,Citation52. It is no surprise then that logistical attributes linked to level of burden such as dosing frequency, minimal time required for treatment initiation (i.e. dose titration), ability for self-administration (i.e. route of administration), and minimal patient monitoring required during treatment (i.e. lower volume of patient phone calls to the clinic) featured largely in importance for HCPs when considering which DMTs to prescribe to patients. While it is crucial to emphasize that all DMTs necessitate monitoring to validate treatment decisions and make necessary adjustments as required, HCPs generally prefer DMTs that require fewer periodic tests. Furthermore, results presented here suggest that HCPs preferred treatments that require less frequent dosing, such as ocrelizumab or natalizumab with dosing of every 6 months or every 4 weeks. Some DMTs had high mean relative preference weights in multiple attribute categories (e.g. diroximel fumarate); mean relative preference weights associated with diroximel fumarate were preferred for nine of the 11 attributes tested. Other DMTs such as ozanimod and dimethyl fumarate also had high relative preference weights in categories such as ROA, efficacy, safety, and tolerabilityCitation44,Citation45.
Previous DCE studies conducted among MS HCPs have examined only clinical attributes such as safety, efficacy, or administration of therapyCitation18,Citation20–22,Citation24,Citation53. This study will be additive to the literature on MS DMT preference as it looks at the treatment selection holistically from both clinical and logistical perspectives. Scientific publications highlighting the role of logistical process attributes in driving HCP treatment preference, in concert with clinical efficacy outcomes and safety profiles, will help drive medical education and highlight the myriad factors HCPs need to consider in their real-world clinical practice. This is critical in the crowded MS DMT landscape, as HCPs need to weigh multiple options while determining optimal treatments for their patients.
This study suggests that the thoughtful selection of an appropriate therapy from a range of good therapy options weighs both clinical and logistical attributes. An oral product with a strong efficacy and good safety profile that also has a favorable dose titration, a good GI tolerability profile, and an oral formulation can reduce burden to both HCPs and patients, and both the patient and HCP aspects of therapy burden could be addressed.
Limitations of research methods
As the eligibility criteria required physicians to treat at least 25 unique MS patients in an average month and at least five unique MS patients who were receiving DMTs in an average month, the study may have been subject to selection bias. As HCPs were required to provide their retrospective use of MS products in a portion of the study instrument, respondents could have been subject to recall bias; however, given the reliance on the DCE for the primary objectives of the study, the impact of such a recall bias on the data captured should have been at a minimum. Recruitment was conducted with a focus on ensuring that the respondent sample was broadly representative of the US HCP population; however, selection bias could have played a role in recruitment. While the DCE study design and analysis were quite complex, they were still limited to a finite number of treatment attributes and may not have been fully reflective of all possible factors that HCPs consider in treatment selection. Additionally, interpretation of the results of this study was constrained by the levels of each attribute that were represented in the survey. Although every effort was made to ensure that these attribute levels reflect the ranges present for clinically available treatments, these values may not reflect the real-world process involved in clinical decision-making. The impact of the COVID-19 pandemic between 2019 and 2022 may have altered treatment choices and approaches to MS management, with effects that may only be known in the next few years. This study was limited to individuals with internet access, and therefore the results can only be generalized to this population.
Conclusions
This study demonstrates that the decision process used by HCPs in the selection of an appropriate DMT for MS patients involves consideration of a wide range of clinical and logistical attributes. In addition to clinical attributes like efficacy and safety, HCPs aim to minimize potential real-world logistical burdens on their patients and practice (e.g. dosing frequency, formulation, GI tolerability, dose titration, and volume of patient calls to the clinic). Based on the attribute levels preferred by MS DMT treaters sampled in this study, diroximel fumarate may be a compelling treatment option for HCPs.
Transparency
Declaration of funding
This study was funded by Biogen, Cambridge, MA, USA.
Declaration of financial/other relationships
DB, consultant, and speaker bureaus for Biogen, EMD-Serono, Horizon, Janssen, Bristol Myers-Squibb, Sanofi Genzyme and Genentech; MA, consultant for Biogen and TG Therapeutics; MB, consultant for Biogen and Novartis; AG, consultant for Biogen, Novartis, Sanofi Genzyme, EMD Serono and Jansen. AA, BO, and NH are employees of Trinity Life Sciences. NH and AA hold equity in Trinity Life Sciences. JBL and SLS are employees of and hold stock or stock options in Biogen. CG and FB were employees of and held stock options in Biogen at the time of the study.
Author contributions
All authors contributed to the study concept and design. Study material preparation, data collection, analyses, and manuscript development were conducted by BO, AA, and NH. FB, CG, JBL, and SLS provided overall strategic guidance, reviewed materials, and critically revised the manuscript. DB, MA, MB, and AG provided expert guidance on designing the DCE grid and reviewed the work. All authors read and approved the final published version.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplemental Material
Download ()Acknowledgements
Iona Bartek, of Seshet Scribes LLC, provided medical writing services funded by Biogen. Data analysis support was provided by Josiah Edelblut, Harrison Malec, and Sarah Schneider from Trinity Life Sciences.
References
- Dobson R, Giovannoni G. Multiple sclerosis—a review. Eur J Neurol. 2019;26(1):27–40. doi: 10.1111/ene.13819.
- Duquette P, Pleines J, Girard M, et al. The increased susceptibility of women to multiple sclerosis. Can J Neurol Sci. 1992;19(4):466–471. doi: 10.1111/ene.13819.
- Goldenberg MM. Multiple sclerosis review. P T. 2012;37(3):175–184.
- Harbo HF, Gold R, Tintore M. Sex and gender issues in multiple sclerosis. Ther Adv Neurol Disord. 2013;6(4):237–248. doi: 10.1177/1756285613488434.
- Kister I, Bacon TE, Chamot E, et al. Natural history of multiple sclerosis symptoms. Int J MS Care. 2013;15(3):146–158. doi: 10.7224/1537-2073.2012-053.
- Lunde HMB, Assmus J, Myhr KM, et al. Survival and cause of death in multiple sclerosis: a 60-year longitudinal population study. J Neurol Neurosurg Psychiatry. 2017;88(8):621–625. doi: 10.1136/jnnp-2016-315238.
- Poser S, Kurtzke JF, Poser W, et al. Survival in multiple sclerosis. J Clin Epidemiol. 1989;42(2):159–168. doi: 10.1016/0895-4356(89)90089-9.
- Steinman L. Multiple sclerosis: a coordinated immunological attack against myelin in the central nervous system. Cell. 1996;85(3):299–302. doi: 10.1016/s0092-8674(00)81107-1.
- Grytten Torkildsen N, Lie SA, Aarseth JH, et al. Survival and cause of death in multiple sclerosis: results from a 50-year follow-up in Western Norway. Mult Scler. 2008;14(9):1191–1198. doi: 10.1177/1352458508093890.
- Wallin MT, Culpepper WJ, Campbell JD, et al. The prevalence of MS in the United States: a population-based estimate using health claims data. Neurology. 2019;92(10):e1029–e1040. doi: 10.1212/WNL.0000000000007035.
- Torkildsen O, Myhr KM, Bo L. Disease-modifying treatments for multiple sclerosis—a review of approved medications. Eur J Neurol. 2016;23(Suppl 1):18–27. doi: 10.1111/ene.12883.
- Claflin SB, Tan B, Taylor BV. The long-term effects of disease modifying therapies on disability in people living with multiple sclerosis: a systematic review and meta-analysis. Mult Scler Relat Disord. 2019;36:101374. doi: 10.1016/j.msard.2019.08.016.
- Ostrovsky L. Mavenclad (cladribine) first short-course oral therapy approved for relapsing-remitting multiple sclerosis and active secondary progressive disease; 2019. Available from: https://www.ahdbonline.com/issues/2019/2019-payers-guide-mid-year-addendum/2807-mavenclad-cladribine-first-short-course-oral-therapy-approved-for-relapsing-remitting-multiple-sclerosis-and-active-secondary-progressive-disease
- Steinman L, Fox E, Hartung HP, et al. Ublituximab versus teriflunomide in relapsing multiple sclerosis. N Engl J Med. 2022;387(8):704–714. doi: 10.1056/NEJMoa2201904.
- Travers BS, Tsang BK, Barton JL. Multiple sclerosis: diagnosis, disease-modifying therapy and prognosis. Aust J Gen Pract. 2022;51(4):199–206. doi: 10.31128/AJGP-07-21-6103.
- D’Ambrosio D, Freedman MS, Prinz J. Ponesimod, a selective S1P1 receptor modulator: a potential treatment for multiple sclerosis and other immune-mediated diseases. Ther Adv Chronic Dis. 2016;7(1):18–33. doi: 10.1177/2040622315617354.
- Ghezzi A. European and American guidelines for multiple sclerosis treatment. Neurol Ther. 2018;7(2):189–194. doi: 10.1007/s40120-018-0112-1.
- Garcia-Dominguez JM, Munoz D, Comellas M, et al. Patient preferences for treatment of multiple sclerosis with disease-modifying therapies: a discrete choice experiment. Patient Prefer Adherence. 2016;10:1945–1956. doi: 10.2147/PPA.S114619.
- Hincapie AL, Penm J, Burns CF. Factors associated with patient preferences for disease-modifying therapies in multiple sclerosis. J Manag Care Spec Pharm. 2017;23(8):822–830. doi: 10.18553/jmcp.2017.23.8.822.
- Jonker MF, Donkers B, Goossens LMA, et al. Summarizing patient preferences for the competitive landscape of multiple sclerosis treatment options. Med Decis Making. 2020;40(2):198–211. doi: 10.1177/0272989X19897944.
- Kumar J, Cambron-Mellott MJ, Tencer T, et al. Patient and neurologist preferences in the United States for relapsing-remitting multiple sclerosis treatments: findings from a discrete choice experiment. Patient Prefer Adherence. 2021;15:1515–1527. doi: 10.2147/PPA.S306498.
- Mansfield C, Thomas N, Gebben D, et al. Preferences for multiple sclerosis treatments: using a discrete-choice experiment to examine differences across subgroups of US patients. Int J MS Care. 2017;19(4):172–183. doi: 10.7224/1537-2073.2016-039.
- Mansfield CA, Thomas N, Gebben D. US RRMS patient preferences for multiple sclerosis treatments: an online survey. Value Health. 2015;18(7):A761. doi: 10.1016/j.jval.2015.09.2486.
- Poulos C, Wakeford C, Kinter E, et al. Patient and physician preferences for multiple sclerosis treatments in Germany: a discrete-choice experiment study. Mult Scler J Exp Transl Clin. 2020;6(1):2055217320910778. doi: 10.1177/2055217320910778.
- Tatlock S, Sully K, Batish A, et al. Individual differences in the patient experience of relapsing multiple sclerosis (RMS): a multi-country qualitative exploration of drivers of treatment preferences among people living with RMS. Patient. 2023;16(4):345–357. doi: 10.1007/s40271-023-00617-y.
- Hajjaj FM, Salek MS, Basra MK, et al. Non-clinical influences on clinical decision-making: a major challenge to evidence-based practice. J R Soc Med. 2010;103(5):178–187. doi: 10.1258/jrsm.2010.100104.
- Dyrbye LN, Shanafelt TD. Physician burnout: a potential threat to successful health care reform. JAMA. 2011;305(19):2009–2010. doi: 10.1001/jama.2011.652.
- Fleming ML, Driver L, Sansgiry SS, et al. Drug enforcement administration rescheduling of hydrocodone combination products is associated with changes in physician pain management prescribing preferences. J Pain Palliat Care Pharmacother. 2019;33(1-2):22–31. doi: 10.1080/15360288.2019.1615027.
- Stockl KM, Hughes TE, Jarrar MA, et al. Physician perceptions of the use of medications for attention deficit hyperactivity disorder. J Manag Care Pharm. 2003;9(5):416–423. doi: 10.18553/jmcp.2003.9.5.416.
- White J, Teoh S, Vindici J, et al. The burden of treatment monitoring for patients and physicians in multiple sclerosis (MS) in Europe and the United States. Multiple Sclerosis J. 2019;25:321–321.
- Bridges JF, Hauber AB, Marshall D, et al. Conjoint analysis applications in health–a checklist: a report of the ISPOR Good Research Practices for Conjoint Analysis Task Force. Value Health. 2011;14(4):403–413. doi: 10.1016/j.jval.2010.11.013.
- Hauber AB, Gonzalez JM, Groothuis-Oudshoorn CG, et al. Statistical methods for the analysis of discrete choice experiments: a report of the ISPOR Conjoint Analysis Good Research Practices Task Force. Value Health. 2016;19(4):300–315. doi: 10.1016/j.jval.2016.04.004.
- Wang Y, Wang Z, Wang Z, et al. Application of discrete choice experiment in health care: a bibliometric analysis. Front Public Health. 2021;9:673698. doi: 10.3389/fpubh.2021.673698.
- Aubagio. Aubagio (teriflunomide) product information; 2023. Available from: https://www.aubagio.com/
- Copaxone. Copaxone (glatiramer acetate) product information; 2023. Available from: https://www.copaxonehcp.com
- Gilenya. Gilenya (fingolimod) product information; 2022. Available from: https://www.gilenya.com/
- Interferons. Interferons product information; 2021. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/103628s5266lbl.pdf
- Kesimpta. Kesimpta (ofatumumab) product information; 2022. Available from: https://www.kesimptahcp.com
- Mavenclad. Mavenclad (cladribine) product information; 2022. Available from: https://www.mavenclad.com
- Mayzent. Mayzent (Siponimod) product information; 2023. Available from: https://www.mayzent.com/
- Ocrevus. Ocrevus (ocrelizumab) product information; 2023. Available from: https://www.ocrevus-hcp.com/
- Tecfidera. Tecfidera (dimethyl fumarate) product information; 2023. Available from: https://www.tecfiderahcp.com/
- Tysabri. Tysabri (natalizumab) product information; 2023. Available from: https://www.tysabri.com
- Zeposia. Zeposia (ozanimod) product information; 2023. Available from: https://www.zeposia.com
- Vumerity. Vumerity (diroximel fumarate) product information; 2023. Available from: https://www.vumerity.com
- World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. doi: 10.1001/jama.2013.281053.
- Bauer BB, Devonshire V, Charbonne A, et al. An international discrete choice experiment assessing patients’ preferences for disease-modifying therapy attributes in multiple sclerosis. Neurodegener Dis Manag. 2020;10(6):369–382. doi: 10.2217/nmt-2020-0034.
- Hagura N, Haggard P, Diedrichsen J. Perceptual decisions are biased by the cost to act. eLife. 2017;6:e18422. doi: 10.7554/eLife.18422.
- Dobler CC, Harb N, Maguire CA, et al. Treatment burden should be included in clinical practice guidelines. BMJ. 2018;363:k4065. doi: 10.1136/bmj.k4065.
- Heckman BW, Mathew AR, Carpenter MJ. Treatment burden and treatment fatigue as barriers to health. Curr Opin Psychol. 2015;5:31–36. doi: 10.1016/j.copsyc.2015.03.004.
- Rao SK, Kimball AB, Lehrhoff SR, et al. The impact of administrative burden on academic physicians: results of a hospital-wide physician survey. Acad Med. 2017;92(2):237–243. doi: 10.1097/ACM.0000000000001461.
- Woolhandler S, Himmelstein DU. Administrative work consumes one-sixth of U.S. physicians’ working hours and lowers their career satisfaction. Int J Health Serv. 2014;44(4):635–642. doi: 10.2190/HS.44.4.a.
- Tencer TA-OX, Will OA-O, Kumar JA-O, et al. Patient and neurologist preferences in the UK for relapsing-remitting multiple sclerosis treatments: findings from a discrete choice experiment. Curr Med Res Opin. 2021;37(9):1589–1598. doi: 10.1080/03007995.2021.1940911.
