Abstract
Objective
To assess the public health impact and cost effectiveness of gender-neutral vaccination (GNV) versus female-only vaccination (FOV) with human papillomavirus (HPV) vaccination in Japan.
Methods
We modeled the public health impact and cost effectiveness of GNV versus FOV to prevent HPV-associated diseases in Japan over the next 100 years. We used one-way sensitivity analyses to examine the impact of varying key model input parameters and conducted scenario analyses to explore the effects of varying the vaccination coverage rate (VCR) of each cohort.
Results
In the base-case analysis, GNV averted additional cancer cases (17,228 female/6,033 male) and deaths (1,892 female/1,849 male) compared to FOV. When all HPV-associated diseases were considered, GNV had an incremental cost-effectiveness ratio of ¥4,732,320 (US$35,987)/quality-adjusted life year gained compared to FOV. The model was most sensitive to the discount rate and the disutility associated with HPV-related diseases. GNV had greater relative public health benefits when the female VCR was lower and was cost effective at a female VCR of 30%.
Conclusions
Immediate implementation of GNV would reduce the disease burden and mortality associated with HPV in Japan, and would be cost effective compared to FOV if the female VCR remains low (30%).
PLAIN LANGUAGE SUMMARY
Human papillomavirus (HPV) is a common sexually transmitted infection and, in Japan, the prevalence of HPV infection and the incidence of its associated diseases are high among both men and women. In the present manuscript we modeled the public health impact and cost effectiveness of gender-neutral vaccination versus female-only vaccination to prevent HPV-associated diseases in Japan over the next 100 years and found that immediate implementation of a gender-neutral vaccination strategy would reduce the burden and mortality associated with HPV in Japan.
Introduction
Human papillomavirus (HPV) is a common sexually transmitted infection that can cause cancers, anogenital warts (AGWs), and recurrent respiratory papillomatosis (RRP) [Citation1,Citation2]. In Japan the prevalence of HPV infection and the incidence of its associated diseases are high among both men and women [Citation3–7]. Three HPV vaccines have been licensed for use in girls in Japan: a bivalent vaccine (2vHPV, approved in 2009) that protects against high-risk oncogenic strains HPV 16 and 18; a quadrivalent vaccine (4vHPV, 2011 [and later expanded to include boys in 2021 [Citation8]]) that protects against HPV 16 and 18 in addition to AGW-associated strains HPV 6 and 11; and a nonavalent vaccine (9vHPV, 2020) that includes strains HPV 6, 11, 16, and 18, as well as oncogenic strains 31, 33, 45, 52, and 58.
Government-funded HPV vaccination began in Japan in 2010, with a primary vaccination cohort of girls 12–16 years of age [Citation8–11]. However, the Ministry of Health, Labor, and Welfare suspended the governmental recommendation for routine HPV vaccinations in June 2013, in response to media reports of adverse events following HPV vaccination [Citation12–14]. Although the Vaccine Adverse Reactions Review Committee investigated these events and reported in 2014 that there was no evidence of a causal link to HPV vaccination, the governmental recommendation remained suspended until April 2022 [Citation13,Citation15–17]. Vaccination against HPV remained available in Japan in the years during which the governmental recommendation was suspended, but uptake declined to <1% [Citation13,Citation18–20].
It is estimated that the reduction in the Japanese HPV vaccination coverage rate (VCR) between 2013 and 2019 will eventually result in an additional 24,600–27,300 cases of and 5,000–5,700 deaths from cervical cancer, compared to a scenario in which coverage remained at ∼70% during the same period [Citation21]. There is already evidence of increases in the rates of HPV infection and abnormal cervical cytology results among young Japanese women [Citation22,Citation23]. Given the extended period with very low vaccination rates, concerns are growing regarding how Japan can “fill the void” [Citation24]. Many other countries strengthened their own HPV vaccination strategies during the period in which the governmental recommendation was suspended in Japan, providing tangible examples of updated best practices that could be implemented in Japan [Citation25]. Suggestions for strengthening HPV prevention efforts in Japan include switching to vaccines that protect against more strains of HPV, offering catch-up vaccination, and expanding vaccination to additional groups, starting with boys [Citation24,Citation26–28]. Some of these suggestions have already been implemented. From the initial resumption of the government’s recommendation for HPV vaccination for girls, the national immunization program recommended 3 doses of 4vHPV. Since April 2023, however, the national immunization program has recommended 2 doses of 9vHPV for girls 12–14 years of age and 3 doses of 9vHPV for girls 15–16 years of age. A catch-up vaccination initiative running from April 2022 until March 2025 has also been added for girls and women 17–26 years of age, with an initial recommendation of 3 doses of 4vHPV and then a switch in March 2023 to 3 doses of 9vHPV [Citation16,Citation17,Citation24]. However, the national immunization program has not yet recommended vaccination of boys in a gender-neutral vaccination (GNV) strategy. Modeling studies set in developed countries have shown that GNV can be a cost-effective strategy, especially when the female VCR is low [Citation29–31]. Hence, the objective of this modeling analysis was to assess the public health impact and cost effectiveness of GNV versus female-only vaccination (FOV) in the Japan.
Methods
Study design and vaccination strategies
This was a cost-effectiveness modeling study that used Japan-specific input data on population demographics, sexual activity and behavior, clinical and vaccine parameters, and health care costs to compare the public health outcomes and cost effectiveness of different FOV and GNV HPV vaccination strategies in Japan from the payer’s perspective. In line with many studies from other countries, these outcomes were assessed over a 100-year time horizon [Citation31]. The base-case analysis compared FOV (using 9vHPV) to GNV (using 9vHPV for girls and 4vHPV for boys) with a VCR of 30% for the primary female cohort, 15% for the catch-up female cohort, and 15% for males. These parameters were selected as realistic average VCRs for Japan given the low starting point following the long suspension of an active recommendation, the lack of school-based vaccination programs that have achieved higher VCRs in other countries, and the fact that Japan has one of the lowest rates of vaccine confidence in the world and high rates of parental vaccine hesitancy, particularly for HPV vaccination [Citation32,Citation33]. We also modeled optimistic scenarios with higher primary female and male cohort VCRs and a range of catch-up cohort VCRs. The Japanese Yen was adjusted to US dollars using the annual exchange rate provided by the Organisation for Economic Co-operation in Development (131.5 Japanese Yen = 1 USD) [Citation34].
Model structure and parameters
Model overview
The model and the input parameters used for this study were essentially the same as those in our previous analysis of the cost effectiveness of FOV using 9vHPV in Japan [Citation35]. The data used to generate the model’s parameters have been described in detail by Insinga et al. [Citation36] and a comprehensive technical report of the current iteration of the model is attached here as an Appendix. Briefly, we adapted published, validated deterministic dynamic transmission metapopulation modeling approaches [Citation37,Citation38] to the Japanese context by incorporating publicly available Japan-specific input data [Citation35]. The model comprises separate disease transmission models for distinct demographic groups segmented by age, sex, and sexual activity. For each of these groups, the model estimates the rate of transmission of each of the HPV strains covered by 9vHPV and the rate of development of associated diseases (cervical, vaginal, and vulvar pre-cancers/cancers in women; penile pre-cancer/cancer in men; and anal pre-cancer/cancer, oropharyngeal cancer, AGWs, and RRP in men and women). Vaccine efficacy is modeled separately for transient infection, persistent infection, and HPV-associated disease, and varies by disease site, HPV strain, and sex [Citation35]. Specifically, vaccine efficacy against transient infection is modeled as the extent of the reduction in the probability of infection among vaccinated populations; vaccine efficacy against persistent infections as the extent of the reduction in the proportion of breakthrough infections that become persistent; and vaccine efficacy against HPV-related pre-cancers as the extent of the reduction in the rate of progression of persistent breakthrough infections to precancerous lesions. All values were extracted from clinical trials that included the relevant endpoints.
Modifications to the published model parameters
A number of modifications and updates were made to the published model [Citation35]. The percentages of oropharyngeal cancers attributable to each 9vHPV viral strain were updated using newly published data (Supplementary Table 1) [Citation39]. These attribution data were then used to generate new model calibration targets using the same oropharyngeal cancer incidence data from the National Cancer Registry as in our previous analysis [Citation35,Citation40]. Model calibration was conducted using Bayesian history matching, as before (Supplementary Figure 1) [Citation35]. The set of oropharyngeal cancer parameters with the best fit was selected for use in the base-case analysis (Supplementary Table 2). These parameters overestimated the incidence of oropharyngeal cancer among younger individuals, particularly males; however, in a modified base-case analysis in which we excluded oropharyngeal cancer among men <60 years of age, we determined that the impact of this model fit resulted in a small (2.2%) increase in the incremental cost-effectiveness ratio (ICER) of GNV versus FOV and did not affect the cost-effectiveness conclusion of the analysis. The costs associated with screening for cervical cancer and with treating all HPV-associated diseases were also updated, based on the 2022 National Health Insurance fee schedule (Supplementary Table 3) [Citation41].
Analyses
Model outputs
The public health outcomes estimated by the model comprised the annual incidence and cumulative cases of and deaths from diseases attributable to the strains of HPV covered by 9vHPV, from 2024 to 2124. The model also estimated health economic outcomes for the same period, namely the cost per capita associated with HPV vaccination and with screening for and treating 9vHPV-strain-attributable diseases, as well as the quality-adjusted life years (QALYs) per capita lost due to these diseases. These measures were used to estimate the ICER of GNV versus FOV.
Base-case analysis
In the base-case FOV strategy, vaccination of the primary cohort (girls 12–16 years of age) began in April 2022 using 4vHPV, transitioning to 9vHPV from April 2023 onward. Vaccination coverage among this cohort was increased over 5 years in a linear fashion from 10% to the target rate of 30%; the target rate was then maintained for the entire modeled period. Catch-up vaccination of girls and women 17–26 years of age was included from April 2022 to March 2025 with an assumed VCR that increased in a linear fashion to 15% by the end of the initiative. The base-case GNV strategy added vaccination of boys 12–16 years of age beginning in April 2024 and increasing linearly over 5 years to a target VCR of 15%. Since 9vHPV is not yet approved for boys and men in Japan, the base-case analysis assumed that male vaccination would exclusively use 4vHPV. It was assumed that from April 2023 onward girls <15 years of age would receive 2 doses of vaccine; all other individuals were assumed to receive 3 vaccine doses.
Sensitivity analyses
We conducted one-way sensitivity analyses to determine how the ICER in the GNV base-case (VCR of 30% for the primary female cohort, 15% for the catch-up female cohort, and 15% for males using 9vHPV for girls and 4vHPV for boys) was affected by varying the following input parameters: discount rate (0–5%), disutility for HPV-associated disease (±20%), disutility for survivors of HPV-associated cancers (set to 0), utility among the population without HPV-associated disease (±20%), vaccine price (±20%), disease treatment costs (±20%), duration of vaccine-mediated protection (set to 20 years), and 10 parameter combinations drawn from the posterior distributions of the calibrated parameters (e.g. HPV transmission/seroconversion/infection persistence probability for each cancer site), to understand how the ICER depends on uncertainty in the model parameters [Citation35]. Each analysis was conducted independently, with all other variables set to their base-case values.
Scenario analyses
Several scenario analyses were also included to explore the impact of the assumptions made regarding VCR. We included scenarios in which the female VCR was increased linearly to 50% or 70% over 5 years, compared to 30% in the base case. For each primary female cohort VCR, we also explored the impact of varying the VCRs of the female catch-up and the male cohorts.
Results
Public health outcomes
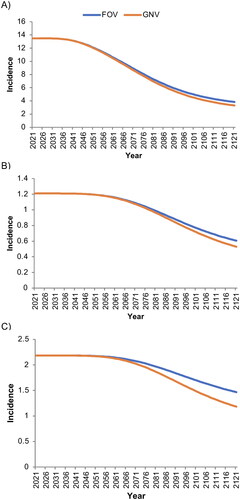
In the base-case analysis, the projected annual incidence of cervical cancer attributable to 9vHPV strains- decreased more rapidly and to a greater extent with GNV than with FOV, from 13.48/100,000 woman-years in 2024 to 3.30/100,000 with GNV and 3.84/100,000 with FOV in 2124 (). Similar trends were observed for the projected incidence of all other 9vHPV-strain-attributable cancers in women, which decreased from 1.21/100,000 in 2024 to 0.53/100,000 with GNV and 0.61/100,000 with FOV in 2124 (), and for the incidence of all 9vHPV-strain-attributable cancers in men, which decreased from 2.18/100,000 man-years in 2024 to 1.18/100,000 with GNV and 1.47/100,000 with FOV in 2124 (). The estimated incidences of these cancers had not yet reached a stable minimum at the end of the modeled period.
Figure 1. Base-case estimated incidence per 100,000 person-years of A) cervical cancer, B) all other cancers (female), and C) all cancers (male) associated with human papillomavirus strains covered by the nonavalent vaccine.
‘Male’ and ‘female’ refer to sex assigned at birth. FOV, female-only vaccination with coverage rates of 30% among the primary cohort and 15% among the catch-up cohort; GNV, gender-neutral vaccination with coverage rates of 30% among the primary female cohort and 15% among the female catch-up and male cohorts.
The estimated incidences of 9vHPV-strain-attributable AGWs and RRP in both sexes also decreased more rapidly and reached a minimum at a lower incidence with GNV than with FOV (). In the base-case analysis, the use of GNV versus FOV over a 100 year time period was projected to avert a total of 17,228 cases of and 1,892 deaths from 9vHPV-strain-attributable cancers (primarily cervical cancers) among women, and 6,033 cases of and 1,849 deaths from 9vHPV-strain-attributable cancers (primarily oropharyngeal cancers) among men (). In addition, GNV was projected to avert >28,000 cases of 9vHPV-strain-attributable CIN1, >94,000 of CIN2–3, >170,000 of AGWs, and 266 cases of/161 deaths from RRP among women (). Among men, GNV averted an estimated 382 cases of/17 deaths from 9vHPV-strain-attributable RRP as well as >460,000 cases of AGWs ().
Figure 2. Base-case estimated incidence per 100,000 person-years of A) anogenital warts (female), B) anogenital warts (male), C) Juvenile-Onset recurrent respiratory papillomatosis (female), and D) Juvenile-Onset recurrent respiratory papillomatosis (male) associated with human papillomavirus strains covered by the nonavalent vaccine.
‘Male’ and ‘female’ refer to sex assigned at birth. FOV, female-only vaccination with coverage rates of 30% among the primary cohort and 15% among the catch-up cohort; GNV, gender-neutral vaccination with coverage rates of 30% among the primary female cohort and 15% among the female catch-up and male cohorts.
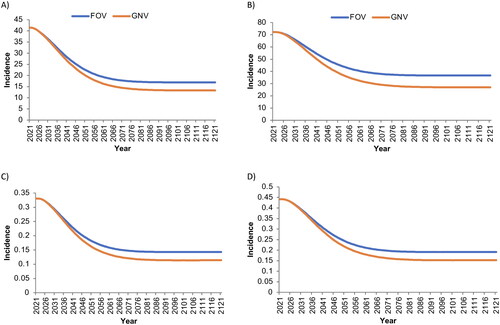
Table 1. Cases and deaths averted in the base-case analysis for gender-neutral instead of female-only human papillomavirus vaccination strategies over 100 years in Japan.
Cost-effectiveness outcomes
In the base-case analysis, the per-capita cost of vaccination was estimated to be ¥3,888 (US$30) for FOV and ¥5,796 (US$44) for GNV (). The overall costs of HPV vaccination, cervical cancer screening, and treatment of diseases associated with strains of HPV covered by 9vHPV were ¥37,223 (US$283) per capita for FOV and ¥38,923 (US$296) per capita for GNV (). Female-only vaccination was associated with 0.0330498607 QALYs lost per capita from these diseases, compared to 0.0326906479 QALYs lost per capita for GNV (). Gender-neutral vaccination was thus associated with ¥1,700 (US$13) additional costs and 0.0003592128 QALYs gained per capita compared to FOV, for an ICER of ¥4,732,320 (US$35,987)/QALY gained.
Table 2. Base-case health care costs and quality-adjusted life years lost for gender-neutral versus female-only vaccination strategies over 100 years in Japan.
Sensitivity and scenario analyses
In one-way sensitivity analyses, the model’s cost-effectiveness outcomes were most sensitive to variation in the discount rate and the disutility associated with HPV-related disease ( and ). An overview of public health and cost-effectiveness outcomes in scenarios with varying VCRs is presented in . When the VCR of the primary female cohort was set to 30%, GNV strategies averted more cases of and from 1,825 to 3,761 deaths from 9vHPV-strain-attributable diseases in females and males compared to FOV, and had cost-effectiveness ratios largely below a threshold of ¥5 M (US$38,023)/QALY (). Among the modeled scenarios with these parameters, GNV generally averted more associated disease cases and was more cost effective compared to FOV when the VCR of the catch-up cohort was set at 30% rather than 50% or 70%. The comparative public health benefits and cost effectiveness of GNV versus FOV were generally the least favorable in scenarios in which the VCR of the primary female cohort was set to 70%. The ICER values of GNV versus FOV were below a threshold of ¥5 M (US$38,023)/QALY only if the costs of treating all 9vHPV-strain-attributable diseases were included in the analysis (Supplementary Table 4).
Figure 3. Tornado diagram illustrating how varying key model parameters affects the incremental cost-effectiveness ratio in the base case.
ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life years.
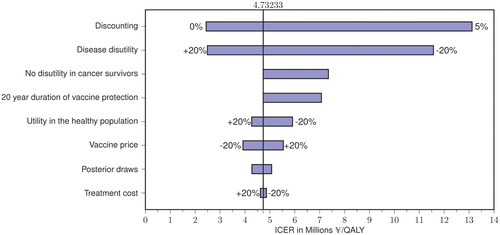
Table 3. One-way sensitivity analyses to determine how key model parameters affect the incremental cost-effectiveness ratio of gender-neutral versus female-only vaccination over 100 years in the base case.
Table 4. Public health and cost-effectiveness outcomes for gender-neutral versus female-only human papillomavirus vaccination strategies over 100 years in scenario analyses with varying female, catch-up, and male vaccination coverage rates.
Discussion
In our base-case analysis—GNV using 9vHPV for girls and 4vHPV for boys, with a VCR of 30% for the primary female cohort and 15% for the catch-up female cohort and 15% for the male cohort—GNV would be a cost-effective strategy in Japan when assessed over a 100-year time horizon. The ICER of GNV versus FOV was ¥4.7 M (US$35,741) per QALY gained, which is below the commonly used Japanese cost-effectiveness threshold of ¥5 M (US$38,023) [Citation42]. As in our prior analysis of the cost effectiveness of FOV with 9vHPV in Japan, the model was most sensitive to the discount rate and the disutility associated with HPV-related disease [Citation35]. In scenario analyses that explored the impact of varying the VCR, GNV averted additional cases of and deaths from HPV-related diseases compared to FOV in all scenarios, but was cost effective (i.e. had an ICER of <¥5 M [US$38,023]) only in scenarios in which the VCR of the primary female cohort was set to 30% as opposed to 50% or 70%.
Our findings are in line with modeling studies from other developed countries that have predicted GNV to be a cost-effective strategy compared to FOV, especially when the female VCR is low, as it currently is in Japan [Citation29–31]. Our model also predicted that implementing GNV would confer substantial public health benefits compared to FOV. Japanese men would primarily benefit from GNV through the prevention of oropharyngeal cancers, almost half of which are attributable to HPV infections [Citation39]. A substantial number of additional 9vHPV-strain-attributable male anal cancers, AGWs, and other diseases would also be prevented by implementing GNV. GNV was also predicted to substantially reduce the 9vHPV-strain-attributable disease burden and mortality among Japanese women, particularly for cervical cancer. Indeed, implementing GNV in place of FOV would prevent almost 3 times as many female as male cancer cases (17,228 versus 6,033), although the number of cancer deaths averted was similar between the sexes (1,892 female and 1,849 male). In contrast, implementing GNV would avert more than double the number of AGWs among men compared to women.
In the one-way sensitivity analyses, the cost effectiveness of GNV with 9vHPV was most sensitive to variation in the discount rate and the disutility associated with HPV-related disease. Similarly, in an analysis of the cost effectiveness of GNV with 9vHPV compared with 4vHPV in the UK, disease utilities, the discount rate, and vaccine efficacy were the most influential parameters [Citation43]. Here, in the scenario analysis, the ICER value for GNV with 9vHPV increased when the VCR was raised from the base-case value of 30% to 50% or 70%, with all ICER values exceeding the ¥5 M (US$38,023) threshold at a VCR of 70%. Similarly, in a modeling study set in Hong Kong, compared to a FOV strategy, ICER values for GNV with 9vHPV increased 2- to 3-fold in scenarios where the VCR was raised from 30% to 60% or 80% [Citation44]. (Vaccination coverage rates below 75% were not considered in the UK study [Citation43].) Finally, in a modeling study of GNV with 9vHPV set in France, the results were influenced most by a higher discount rate and a shorter duration of protection; increasing the VCR from 26% to 80% resulted in an approximate doubling of the ICER value compared with FOV [Citation45].
The justification for GNV includes epidemiological, economic, social, and ethical considerations. Men and boys benefit indirectly from vaccination of girls (and vice versa) via the reduced population prevalence of HPV infections. The drastic decrease in the female VCR in Japan caused by the suspension of the governmental recommendation has greatly reduced the extent of this indirect protection for males. This is a concern because, excluding cervical cancer, there is a higher crude incidence of HPV-associated cancers among Japanese men than Japanese women, especially for oropharyngeal cancers [Citation6]. Direct protection of boys and men via GNV is a common and successful strategy for the prevention of HPV-associated diseases in many countries, and the World Health Organization invites the vaccination of boys as a secondary target if it is “feasible and affordable, and does not divert resources from vaccination of the primary target population or effective cervical cancer screening programmes” [Citation46]. GNV can accelerate the eradication of pathogenic HPV strains from the population, protect men who have sex with men (who only marginally benefit from FOV), and promote “the moral norm of a shared responsibility in all adolescents regardless of gender, while reducing misconceptions of girls as having principal responsibility for HPV transmission and prevention” [Citation29–31,Citation47–51].
As with all modeling studies, our analyses are subject to inherent limitations. First, a number of assumptions were made regarding the model’s input parameters, which might impact the accuracy of our estimated public health and cost-effectiveness outcomes. The parameters were based on comprehensive analysis of published epidemiological data; nevertheless, numerical exploration of the high-dimensional parameter space is difficult, and the uncertainty ranges used in our sensitivity analyses are unlikely to be a perfect representation of the plausible parameters for Japan. These assumptions are likely to have greater impacts later in the modeled period. A 100-year time horizon is standard in studies of this kind due to the young age of the primary HPV vaccination cohort, the long (often several decades) lag time before the development of HPV-related diseases, particularly cancers, and the potential for long-term health impacts and costs as a result of these diseases. However, the length of the modeled period increases the likelihood that input parameters, including the VCR, will change over time. For example, it is difficult to predict trends in sexual behavior; the assumption that current behavior would persist throughout the 100-year modeled period could therefore introduce significant errors. We ran a separate sensitivity analysis with a 50-year time horizon and found that the ICER in the base-case analysis increased from ¥4,732,320 (US$35,987)/QALY gained to ¥10,184,538 (US$77,449)/QALY gained compared to the FOV strategy. This highlights that, even with discounting, the majority of the value of vaccination is realized as vaccinated adolescents age into the highest risk age groups for HPV-related disease (≥50 years of age). We also assumed that the incidence of HPV-associated cancers was stable at the beginning of the modeled period. However, there is evidence for an increasing incidence of cervical cancer in Japan and of HPV-associated cancers among men globally [Citation52–54]. Increasing incidences of HPV-associated cancers in Japan at the start of the modeled period could result in our model underestimating the public health benefits and the cost effectiveness of GNV versus FOV. We also assumed that all members of the target age group for catch-up vaccination were unvaccinated, although a proportion of women in this age group would have been vaccinated prior to the cessation of the government recommendation in 2013. This assumption would not affect the analyses related to routine primary vaccination but might overestimate the cost effectiveness of catch-up vaccination strategies.
The probability and timing of potential future programmatic changes to Japanese preventive health care are also unknown and could not be accounted for in the model. Most notably, improvements to current cervical cancer screening technology could affect the model’s cervical cancer-related outcomes. Expansion of the regulatory approval of 9vHPV to include boys, and the implementation of this vaccine for boys in Japan’s national immunization program, would also affect the model’s results. Implementing 9vHPV for boys would be expected to increase the public health benefits of GNV versus FOV, and could potentially remain a cost-effective strategy (as it has in other developed countries [Citation29–31]) due to the similar costs of 3 doses of 4vHPV versus 2 doses of 9vHPV. We did not include this scenario analysis in the current study as the potential timing of 9vHPV approval for boys in Japan is not known. Finally, only direct costs from the payer’s perspective were included in the model; societal costs such as lost economic activity were not considered.
In conclusion, the recent reinstatement of the governmental recommendation for HPV vaccination of girls was an important step for public health in Japan. However, the current vaccination rate remains low among girls and women. In response, implementing gender-neutral HPV vaccination has been suggested as a means to improve protection against HPV-related diseases for both sexes. Our study adds to the current body of evidence supporting GNV [Citation47–51] by estimating the additional diseases and deaths prevented and the cost effectiveness of implementing GNV in Japan in place of the current FOV strategy. We found that compared to FOV, GNV would avert additional cases of and deaths from HPV-related diseases among men and women at all VCRs, and was a cost-effective strategy in Japan at a female VCR of 30%. The sooner that boys are included in Japan’s HPV vaccination program, the sooner the benefits outlined in this work can be realized. Forgoing or delaying the implementation of GNV would miss an important generational opportunity to strengthen Japan’s strategy for the prevention of HPV-related cancers and other diseases.
Transparency
Declaration of financial/other relationships
CP, KT, YN, XY, YTC, and MA are current employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, and may hold equity interest in Merck & Co., Inc., Rahway, NJ, USA.
Author contributions
All authors have substantially contributed to the conception and design of the article, interpreting the relevant literature and were involved in writing the article or revised it for intellectual content.
Reviewer disclosures
Peer reviewers on this manuscript have received an honorarium from JME for their review work but have no other relevant financial relationships to disclose.
Supplemental Material
Download MS Word (139.2 KB)Supplemental Material
Download MS Word (8.5 MB)Acknowledgements
The authors thank Cath Ennis, in collaboration with ScribCo for medical writing assistance.
Additional information
Funding
References
- de Martel C, Plummer M, Vignat J, et al. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer. 2017;141(4):664–670. doi:10.1002/ijc.30716.
- Forman D, de Martel C, Lacey CJ, et al. Global burden of human papillomavirus and related diseases. Vaccine. 2012;30(Suppl 5):F12–23. doi:10.1016/j.vaccine.2012.07.055.
- Kitamura T, Suzuki M, Shigehara K, et al. Prevalence and risk factors of human papillomavirus infection among Japanese female people: a nationwide epidemiological survey by self-sampling. Asian Pac J Cancer Prev. 2021;22(6):1843–1849. doi:10.31557/APJCP.2021.22.6.1843.
- Palmer M, Katanoda K, Saito E, et al. Genotype prevalence and age distribution of human papillomavirus from infection to cervical cancer in Japanese women: a systematic review and meta-analysis. Vaccine. 2022;40(41):5971–5996. doi:10.1016/j.vaccine.2022.07.052.
- Matsuzawa Y, Kitamura T, Suzuki M, et al. Prevalence, genotype distribution, and predictors against HPV infections targeted by 2-, 4-, 9-Valent HPV vaccines among Japanese males. Vaccines (Basel). 2020;8(2):221. doi:10.3390/vaccines8020221.
- ICO/IARC Information Centre on HPV and Cancer. Japan: human papillomavirus and related cancers, fact sheet 2023: HPV Information Centre; 2023 [updated March 10, 2023; cited 2023 March 30]. Available from: https://hpvcentre.net/statistics/reports/JPN_FS.pdf.
- Kawado M, Hashimoto S, Ohta A, et al. Estimating nationwide cases of sexually transmitted diseases in 2015 from sentinel surveillance data in Japan. BMC Infect Dis. 2020;20(1):77. doi:10.1186/s12879-020-4801-x.
- Ujiie M, Kitano T, Tsuzuki S. Changing trends in HPV vaccination in Japan. Hum Vaccine Immunother. 2022;18(1):1–3. doi:10.1080/21645515.2021.1986333.
- Yamamoto N, Mori R, Jacklin P, et al. Introducing HPV vaccine and scaling up screening procedures to prevent deaths from cervical cancer in Japan: a cost-effectiveness analysis. BJOG. 2012;119(2):177–186. doi:10.1111/j.1471-0528.2011.03036.x.
- Konno R, Sasagawa T, Fukuda T, et al. Cost-effectiveness analysis of prophylactic cervical cancer vaccination in Japanese women. Int J Gynecol Cancer. 2010;20(3):385–392. doi:10.1111/IGC.0b013e3181d189b8.
- Yamabe K, Singhal PK, Abe M, et al. The cost-effectiveness analysis of a quadrivalent human papillomavirus vaccine (6/11/16/18) for females in Japan. Value Health Reg Issues. 2013;2(1):92–97. doi:10.1016/j.vhri.2013.02.001.
- Gilmour S, Kanda M, Kusumi E, et al. HPV vaccination programme in Japan. Lancet. 2013;382(9894):768. doi:10.1016/S0140-6736(13)61831-0.
- Hanley SJ, Yoshioka E, Ito Y, et al. HPV vaccination crisis in Japan. Lancet. 2015;385(9987):2571. doi:10.1016/S0140-6736(15)61152-7.
- The Ministry of Health Labour and Welfare. 2013. Available from: https://www.mhlw.go.jp/bunya/kenkou/kekkaku-kansenshou28/pdf/kankoku_h25_6_01.pdf.
- Saitoh A, Okabe N. Recent progress and concerns regarding the Japanese immunization program: addressing the "vaccine gap”. Vaccine. 2014;32(34):4253–4258. doi:10.1016/j.vaccine.2014.06.022.
- Haruyama R, Obara H, Fujita N. Japan resumes active recommendations of HPV vaccine after 8.5 years of suspension. Lancet Oncol. 2022;23(2):197–198. doi:10.1016/S1470-2045(22)00002-X.
- Ujiie M. Resumption of active recommendation of the human papillomavirus vaccine in Japan and future challenges for the national immunization program. Hum Vaccin Immunother. 2022;18(6):2090777. doi:10.1080/21645515.2022.2090777.
- Ueda Y, Enomoto T, Sekine M, et al. Japan’s failure to vaccinate girls against human papillomavirus. Am J Obstet Gynecol. 2015;212(3):405–406. doi:10.1016/j.ajog.2014.11.037.
- Sekine M, Kudo R, Adachi S, et al. Japanese crisis of HPV vaccination. Int J Pathol Clin Res. 2016;2(2):039. doi:10.23937/2469-5807/1510039.
- Nakagawa S, Ueda Y, Yagi A, et al. Corrected human papillomavirus vaccination rates for each birth fiscal year in Japan. Cancer Sci. 2020;111(6):2156–2162. doi:10.1111/cas.14406.
- Simms KT, Hanley SJB, Smith MA, et al. Impact of HPV vaccine hesitancy on cervical cancer in Japan: a modelling study. Lancet Public Health. 2020;5(4):e223–e234. doi:10.1016/S2468-2667(20)30010-4.
- Sekine M, Yamaguchi M, Kudo R, et al. Suspension of proactive recommendations for HPV vaccination has led to a significant increase in HPV infection rates in young Japanese women: real-world data. Lancet Reg Health West Pac. 2021;16:100300. doi:10.1016/j.lanwpc.2021.100300.
- Yagi A, Ueda Y, Ikeda S, et al. The looming health hazard: a wave of HPV-related cancers in Japan is becoming a reality due to the continued suspension of the governmental recommendation of HPV vaccine. Lancet Reg Health West Pac. 2022;18:100327. doi:10.1016/j.lanwpc.2021.100327.
- Yagi A, Ueda Y, Nakagawa S, et al. Can Catch-Up vaccinations fill the void left by suspension of the governmental recommendation of HPV vaccine in Japan? Vaccines (Basel). 2022;10(9):1455. doi:10.3390/vaccines10091455.
- Markowitz LE, Unger ER. Human papillomavirus vaccination. N Engl J Med. 2023;388(19):1790–1798. doi:10.1056/NEJMcp2108502.
- Mikamo H, Yamagishi Y, Murata S, et al. Efficacy, safety, and immunogenicity of a quadrivalent HPV vaccine in Japanese men: a randomized, phase 3, placebo-controlled study. Vaccine. 2019;37(12):1651–1658. doi:10.1016/j.vaccine.2019.01.069.
- Ueda Y, Yagi A, Ikeda S, et al. Beyond resumption of the Japanese government’s recommendation of the HPV vaccine. Lancet Oncol. 2018;19(12):1563–1564. doi:10.1016/S1470-2045(18)30573-4.
- Yagi A, Ueda Y, Nakagawa S, et al. Potential for cervical cancer incidence and death resulting from Japan’s current policy of prolonged suspension of its governmental recommendation of the HPV vaccine. Sci Rep. 2020;10(1):15945. doi:10.1038/s41598-020-73106-z.
- Brisson M, Van de Velde N, Boily MC. Economic evaluation of human papillomavirus vaccination in developed countries. Public Health Genomics. 2009;12(5–6):343–351. doi:10.1159/000214924.
- Canfell K, Chesson H, Kulasingam SL, et al. Modeling preventative strategies against human papillomavirus-related disease in developed countries. Vaccine. 2012;30(5):F157–67. doi:10.1016/j.vaccine.2012.06.091.
- Ng SS, Hutubessy R, Chaiyakunapruk N. Systematic review of cost-effectiveness studies of human papillomavirus (HPV) vaccination: 9-Valent vaccine, gender-neutral and multiple age cohort vaccination. Vaccine. 2018;36(19):2529–2544. doi:10.1016/j.vaccine.2018.03.024.
- de Figueiredo A, Simas C, Karafillakis E, et al. Mapping global trends in vaccine confidence and investigating barriers to vaccine uptake: a large-scale retrospective temporal modelling study. Lancet. 2020;396(10255):898–908. doi:10.1016/S0140-6736(20)31558-0.
- Lelliott M, Sahker E, Poudyal H. A review of parental vaccine hesitancy for human papillomavirus in Japan. J Clin Med. 2023;12(5):2004. doi:10.3390/jcm12052004.
- Organisation for Economic Co-operation and Development. Exchange rates [cited 2023 November 1]. Available from: https://data.oecd.org/conversion/exchange-rates.htm.
- Cody P, Tobe K, Abe M, et al. Public health impact and cost effectiveness of routine and catch-up vaccination of girls and women with a nine-valent HPV vaccine in Japan: a model-based study. BMC Infect Dis. 2021;21(1):11. doi:10.1186/s12879-020-05632-0.
- Insinga RP, Dasbach EJ, Elbasha EH. Epidemiologic natural history and clinical management of human papillomavirus (HPV) disease: a critical and systematic review of the literature in the development of an HPV dynamic transmission model. BMC Infect Dis. 2009;9(1):119. doi:10.1186/1471-2334-9-119.
- Elbasha EH, Dasbach EJ. Impact of vaccinating boys and men against HPV in the United States. Vaccine. 2010;28(42):6858–6867. doi:10.1016/j.vaccine.2010.08.030.
- Elbasha EH, Dasbach EJ, Insinga RP. Model for assessing human papillomavirus vaccination strategies. Emerg Infect Dis. 2007;13(1):28–41. doi:10.3201/eid1301.060438.
- The BROADEN study: burden of human papillomavirus-related head and neck cancers in Japan. Public health oral: epidemiology and risk factors. In: 35th International Papillomavirus Conference; Washington DC, 2023.
- National Cancer Registry. Cancer Statistics in Japan [cited 2023 April 1]. Available from: https://ganjoho.jp/reg_stat/statistics/data/dl/en.html.
- Shirobon Net. 2022 Medical Fee Score Table [cited 2023 October 2]. Available from: https://shirobon.net/medicalfee/latest/.
- Shiroiwa T, Igarashi A, Fukuda T, et al. WTP for a QALY and health states: more money for severer health states? Cost Eff Resour Alloc. 2013;11(1):22. doi:10.1186/1478-7547-11-22.
- Lin F, Kwong WJ, Shi S, et al. Assessing the health and economic outcomes of a 9-Valent HPV vaccination program in the United Kingdom. J Health Econ Outcomes Res. 2022;9(1):33289–33150. doi:10.26469/jheor.2022.33289.
- Cheung TH, Cheng SSY, Hsu D, et al. Health impact and cost-effectiveness of implementing gender-neutral vaccination with the 9-valent HPV vaccine in Hong Kong. Hum Vaccin Immunother. 2023;19(2):2184605. doi:10.1080/21645515.2023.2184605.
- Majed L, Bresse X, El Mouaddin N, et al. Public health impact and cost-effectiveness of a nine-valent gender-neutral HPV vaccination program in France. Vaccine. 2021;39(2):438–446. doi:10.1016/j.vaccine.2020.10.089.
- World Health Organization. Human papillomavirus vaccines: WHO position paper. Weekly Epidemiological Record. 2022;50(97):645–672.
- Chow EPF, Tabrizi SN, Fairley CK, et al. Prevalence of human papillomavirus in young men who have sex with men after the implementation of gender-neutral HPV vaccination: a repeated cross-sectional study. Lancet Infect Dis. 2021;21(10):1448–1457. doi:10.1016/S1473-3099(20)30687-3.
- Elfstrom KM, Lazzarato F, Franceschi S, et al. Human papillomavirus vaccination of boys and extended catch-up vaccination: effects on the resilience of programs. J Infect Dis. 2016;213(2):199–205. doi:10.1093/infdis/jiv368.
- Lehtinen M, Gray P, Louvanto K, et al. In 30 years, gender-neutral vaccination eradicates oncogenic human papillomavirus (HPV) types while screening eliminates HPV-associated cancers. Expert Rev Vaccines. 2022;21(6):735–738. doi:10.1080/14760584.2022.2064279.
- Sundaram N, Voo TC, Tam CC. Adolescent HPV vaccination: empowerment, equity and ethics. Hum Vaccin Immunother. 2020;16(8):1835–1840. doi:10.1080/21645515.2019.1697596.
- Vanska S, Luostarinen T, Baussano I, et al. Vaccination with moderate coverage eradicates oncogenic human papillomaviruses if a Gender-Neutral strategy is applied. J Infect Dis. 2020;222(6):948–956. doi:10.1093/infdis/jiaa099.
- Chaturvedi AK, Anderson WF, Lortet-Tieulent J, et al. Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. J Clin Oncol. 2013;31(36):4550–4559. doi:10.1200/JCO.2013.50.3870.
- Utada M, Chernyavskiy P, Lee WJ, et al. Increasing risk of uterine cervical cancer among young Japanese women: comparison of incidence trends in Japan, South Korea and Japanese-Americans between 1985 and 2012. Int J Cancer. 2019;144(9):2144–2152. doi:10.1002/ijc.32014.
- Varga S, Wang X, Luttropp K, et al. Global burden of HPV-related cancers in men: a systematic literature review. J Clin Oncol. 2019;37(15_suppl):e13108–e13108. doi:10.1200/JCO.2019.37.15_suppl.e13108.
- Union for International Cancer Control. TNM classification of malignant tumours. 8th ed. In: Wittekind C, Brierley JD, Gospodarowicz MK, editors. Wiley; 2017.
