Abstract
Aim
Evaluate the cost utility of menopausal hormone therapy for women in China.
Materials and methods
A bespoke Markov cost utility model was developed to evaluate a cohort of symptomatic perimenopausal women (>45 years) with intact uterus in China in accordance with China’s Pharmacoeconomic guideline. Short (5-year) and long (10-year) treatment durations were evaluated over a lifetime model time horizon with 12-month cycle duration. Societal and healthcare payer perspectives were evaluated in the context of a primary care provider/prescriber, outpatient setting with inpatient care for patients with chronic conditions. Disease risk and mortality parameters were derived from focused literature searches, and China Diagnosis-related Group cost data was included. Comprehensive scenario, univariate and probabilistic sensitivity analysis were undertaken along with independent validation. This is the first model to include MHT-related disease risks.
Results
According to base case results, the total cost for MHT was 22,516$ (150,106¥) and total quality adjusted life years 12.32 versus total cost of no MHT 30,824$ (205,495¥) and total quality adjusted life years 11.16 resulting in a dominant incremental cost effectiveness ratio of −7,184$ (−47,898¥) per QALY. Results hold true over a range of univariate deterministic sensitivity and scenario analyses. Probabilistic analysis showed a 91% probability of being cost effective at a willingness to pay threshold of three times Gross Domestic Product per capita in China.
Conclusion
Contingent on the structure and assumptions of the model, combination of estradiol plus dydrogesterone MHT is potentially cost saving in symptomatic women over the age of 45 years in China.
PLAIN LANGUAGE SUMMARY
Menopausal hormone therapy is publicly funded in many countries to alleviate symptoms of menopause; however, uptake has been comparatively slow in China. This has implications for the estimated 168 million menopausal-aged women. This analysis is the first to evaluate the cost effectiveness of menopausal hormone therapy in China using best practice principles and incorporating longer term disease risks. Menopausal hormone therapy is potentially cost saving in the context of China.
Introduction
Menopausal Hormone Therapy (MHT) is commonly used worldwide to treat menopause and International, American and European Medical Society guidelines advocate an individualized treatment approach with a focus on MHT as the most effective interventionCitation1–7. The Chinese Menopause Society Clinical Guideline for Menopause Management also recommends an individualized approach to the prescription of MHTCitation8,Citation9.
Despite these recommendations, uptake of MHT is comparatively low in China. This has consequences for the estimated 168 million women aged between 45 and 59 years who are potentially experiencing menopausal symptoms and eligible for MHT. The reasons for low uptake are likely to be patient, provider and payer-relatedCitation10–14. For example, patient’s awareness of MHT was only 3.5% in a cross-sectional sample of 3,619 women in ShanghaiCitation11. High discontinuation rates were reported in a sample of previous and current users South ChinaCitation12. Interestingly, one third of women discontinued MHT because symptoms decreased while on treatment, which potentially speaks to the effectiveness of MHTCitation12. From the prescriber perspective, a multicentre cross-sectional survey of 194 gynaecologists from 100 gynaecology departments of major hospitals in 45 cities in China reported that although more than 90% were familiar with MHT guidelines, only 70% reported following them frequentlyCitation13. A survey conducted in Jiaxing, showed that among 2,158 medical care personnel the main reason for not prescribing MHT was fear of cancer (43.8%)Citation14.
In 2020, China published an updated version of the Guidelines for Pharmacoeconomic Evaluations (2019), signalling the increasingly important role that Pharmacoeconomics (PE) plays in decision making in ChinaCitation15–17. Since 2017 pharmacoeconomic evaluation and budget impact analyses have formed part of the supporting evidence for price negotiations in ChinaCitation15. Despite the importance of MHT and PE to date there are no studies evaluating the cost effectiveness of MHT in China. Literature suggests that barriers to using Pharmacoeconomics in China include limited expertise to develop cost utility analysis (CUA), clinician’s suspicion of CUA, and patient’s reluctance to participate in CUACitation15,Citation16. More broadly, cost effectiveness studies done in other countries have been criticized for not including longer term disease risks related to MHT and for the limited availability of utility dataCitation18.
In view of the global importance of MHT, the increasing role of PE and the lack of cost utility analysis for MHT in China, the objective of our research was to evaluate the cost utility of MHT for the treatment of women with menopause in China.
Methods
The decision question was to determine the cost effectiveness of MHT for symptomatic perimenopausal women in China. A bespoke cost utility model was developed and programmed in Microsoft Excel. We applied a systematic approach to selecting the appropriate model structure as set out by Barton and BrennanCitation19,Citation20. A Markov model was most suited to our decision question because we needed to model the long-term outcomes of costs and effects over a lifetime model horizon. We identified nine health states that needed to be modelled, with the cohort transitioning to and from health states without the model becoming overly complex. We decided against a decision tree model because of the model states and recursive pathways of patients would render it overly complex. A discrete event simulation was not warranted as we did not need to consider interaction or transmission for perimenopausal women. The same model structure was used in four comparable models from other countriesCitation21–24. The population modelled is a cohort of perimenopausal females over the age of 45 years, with intact uterus, experiencing irregular periods and menopausal symptoms and with no risk factors for MHT. The treatment context is the outpatient primary provider as prescriber, where chronic conditions are treated in the inpatient setting (hospitalized). Healthcare payer and societal perspectives were evaluated. The intervention was the oral combination estradiol plus dydgrogesterone MHT (both continuous sequential and continuous combined formulations). The comparator was no treatment which aligns with the Chinese menopause guidelineCitation8,Citation9. Five-year treatment duration was modelled, in keeping with the definition of short-term MHT by the National Institute for Health and Clinical Excellence which recommends treating women “for the shortest possible time and with the lowest possible doseCitation15,Citation25. Long term (10-year) treatment was also modelled. The cycle length was 12 months and discount rates of 5% were applied to costs and quality adjusted life years (QALYs) in line with the China Pharmacoeconomic GuidelinesCitation17. The measure of benefit was the quality of life associated with an improvement in menopausal symptoms. The model structure consisted of nine health states, seven of which represent the long-term disease risks associated with MHTCitation26.
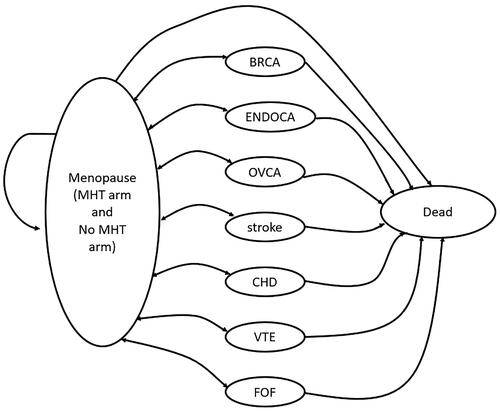
A schematic of the model structure is shown in .
The cohort enters the model in the perimenopausal, symptomatic state. During the first five or ten years (depending on treatment duration) patients move to health states according to disease risk data that shows that patients on MHT have an adjusted risk for breast cancer (BRCA), endometrial cancer (ENDOCA), ovarian cancer (OVCA), stroke, coronary heart disease (CHD), venous thromboembolic events (VTE) and fracture of femur (FOF) Citation26. From these health states a disease-specific mortality rate was applied to transition to death. Age-dependent all-cause mortality for China was applied to patients in the menopausal stateCitation27. The face validity of the model was verified by the clinical co-author (TS). In this paper, we describe the base case and results, alternate scenarios are described in detail in the supplement.
Input data for parameters
Focused literature searches were conducted according to the methods described by Paisley et al. and Edlin et al. to identify utility, disease risk and mortalityCitation28,Citation29. For every focused search, two stage literature selection was performed independently by two reviewers (TR, MD). The quality and relevance of all studies meeting inclusion criteria were then weighed against each other according to the principles described by NuijtenCitation30. The PRISMA information is presented in the supplement Table 1.
Utility data
There was no published utility for perimenopausal women in China (only pre- and post- menopause) therefore we conducted primary research to measure the utility of menopausal aged women in China and report these findings separatelyCitation32. The utility of perimenopausal women with intact uterus with symptoms was 0.863 and not experiencing symptoms was 0.956Citation32. For the base case, our clinical co-authors ranked each health state in order of severity and we applied a disutility to the health states according to the ranking, anchored to the utility of symptomatic menopausal women (0.863). We applied a disutility of 0.03 for least severe (VTE), 0.06 for moderately severe (BRCA, ENDOCA, FOF) and 0.09 for most severe (OVCA, STROKE, CHD) health states as shown in .
Table 1. Base case model parameters and references.
Disease risk data
Focused searches were conducted to identify disease risk data for the respective health states, ranked according to quality, relevance, and recency. The disease risks for BRCA came from a nested case control study of 98,611 cases between 1998 and 2018 matched to 457,498 controlsCitation33. A total of 33,703 (34%) women with breast cancer and 134,391 (31%) controls used MHT one year prior to the index dateCitation33. In patients who used oestradiol plus dydrogesterone the combined adjusted odds ratio was 0.88 (95% CI 0.76 to 1.01) for treatment duration 3–4 years and 1.11 (0.94–to 1.31) for 5–9 yearsCitation33. The disease risks for CHD, VTE and stroke came from a systematic review and meta-analysis of MHT on cardiovascular diseaseCitation34. For CHD the summary estimate was 1.02 (95%CI 0.94–1.10) based on five randomized trials; for stroke the relative risk was 1.14 (1.04–1.25) based on 13 randomized trials and for VTE 1.70 (1.33–2.16) based on 15 randomized trialsCitation34. The disease risks for FOF came from a systematic review and meta-analysis by the United States Preventive Services Task Force on the impact of MHT on chronic conditionsCitation35. For combination MHT, the relative risk of fractures was 0.80 (0.68–0.94)Citation35. The disease risks for OVCA and ENDOCA came from the UK Medicines and Healthcare products Regulatory AgencyCitation26.
Mortality data
Focused searches were conducted to identify mortality data for the respective health states, ranked according to quality, relevance, and recency. We identified two meta-analysis that found a correlation between MHT and decreased mortality, however the samples did not match the modelled population therefore a conservative approach was adopted applying all-cause mortality (treatment related) to both arms in the modelCitation27,Citation34,Citation41. For BRCA the data came from the GLOBOCAN 2018 study and reported age standardized abridged incidence and mortality rates for BRCA from ChinaCitation36. For OVCA the estimates came from ten geographically diverse areas (5 urban 5 rural) across China and reported age standardized females, urban areasCitation42. For ENDOCA the age standardized, abridged evidence came from a systematic subnational analysis for the Global Burden of Disease Study 2013 during 1990–2013 in ChinaCitation37. For CHD age standardized, abridged, data from 2016 came from China cardiovascular diseases report 2018: an updated summaryCitation43. The evidence from stroke came from the same study and was for first-ever stroke in adults aged 40–74 yearsCitation43. For VTE the evidence came from a report to evaluate trends in Hospitalization and In-Hospital Mortality between 2007 and 2016. Age standardized, abridged in hospital mortality in China was providedCitation39. For FOF the data came from a systematic review and meta-analysis to determine 1-year mortality rate after hip fracture based on data from mainland China between the years 2000 and 2018Citation40. Age-specific, abridged data were reported for femoral intertrochanteric fracture for the age group <60 years (55–59)Citation40.
Cost data
The base case used the China Diagnosis Related Group (C-DRG) data. The cost of no MHT was 749$ (4,990¥) based on the sum of two C-DRGs for menopause (genitourinary syndrome of menopause 136$ (907¥) added to depression of menopause 612$ (4,082¥))Citation31. The following values were used for the C-DRG for BRCA (C50.900) ¥11,649; ENDOCA and OVCA (C54.100) ¥17,421; STROKE (I63.900) ¥59,748; CHD (I25.103) ¥5,346; VTE (I82.900 × 004) ¥49,492 and FOF (S72.900) ¥68,441Citation31.The C-DRG for ENDOCA was not available and OVCA was used as a conservative proxyCitation31. The health state costs were the same for MHT and non-MHT arms in the model and that the drug costs of sequential and combination oestradiol plus dydrogesterone were included in the model. Base case parameters are summarized in .
The model underwent rigorous validation by an independent senior third-party modeller. Validation was performed by replicating the Excel model in TreeAge Pro Healthcare (Version 2020 – TreeAge Software, Inc.). The results were found to be well within the acceptable range of a “successful” model replicationCitation44.
Results
Results are presented in Yuan (¥) and United States Dollars ($), converted using the exchange rate from 01 March 2023 (0.15). For the base case, the total cost for MHT was 22,516$ (150,106¥) and the total quality adjusted life years was 12.32 versus no MHT which was 30,824$ (205,495¥) and the total quality adjusted life years which was 11.16 resulting in a dominant incremental cost effectiveness ratio (ICER) of −7,184$ (−47,898¥) per QALY.
Sensitivity analysis
Univariate deterministic sensitivity analysis
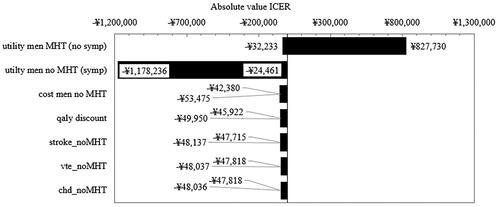
All parameters were explored in univariate sensitivity analysis. There were three parameters that resulted in variation of the ICER more than 5%. Two of these were the utility of women on MHT and the utility of women not on MHT. Therefore, we performed threshold analysis to determine at what utility threshold the ICER moved from being cost saving/cost effective to not cost effective. Keeping all other parameters constant, when the utility of women on MHT with no symptoms was equal to the utility of not on MHT with symptoms, MHT was no longer cost effective. MHT remained cost saving in all instances except when the utility of women on MHT (no symptoms) decreased from 0.956 (base case) to 0.860 (arbitrary ten percent reduction) in which case the ICER increased to 124,160$ (827,730¥) (Supplementary Table 10). This finding confirmed that if MHT does not improve symptoms, then MHT will not be cost-effective. The third parameter that showed variation >5% was the cost of women not on MHT (see discussion).
Results for parameters influencing the results >1% are shown in .
Scenario analysis
A range of plausible scenarios were explored altering treatment type (combination continuous, sequential continuous); treatment duration (5 years, 10 years), start age (45, 50, 55, 60), disease risk data (single or multiple source); cost data (literature search, China DRGs); utility data (literature search, constant disutility, variable ranked disutility); perspective (healthcare payer, societal) and type of analysis (deterministic, probabilistic). Changing treatment duration from 5 years to 10 years resulted in an ICER of −6,785$ (-45,230¥); changing start age from 45 to 50, 55 and 60 resulted in an ICER of −7,165$ (−47,767¥), 7,108$ (−47,385¥) and 6,994$ (−46,627¥), respectively; changing the source of cost data from China DRG to literature search resulted in an ICER of 1,007$ (6,711¥) (the cost data in the literature was higher than the China DRGs, however this version assumes no cost for no MHT). Changing the utility data to constant disutility and literature search utilities resulted in ICERs of −7,183$ (−47,884¥) and −7,189$ (−47,928¥) respectively. Changing from societal to healthcare perspective resulted in an ICER of 7,502$ (50,017¥). The scenarios and results are shown in .
Table 2. Incremental cost utility results for deterministic range of scenarios.
Probabilistic sensitivity analysis
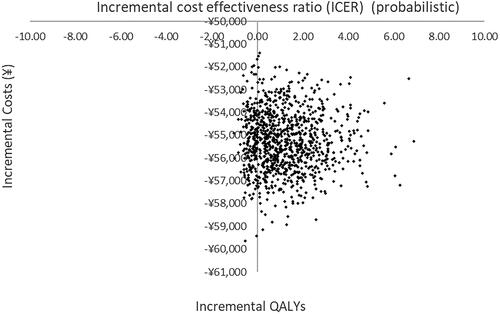
To evaluate the uncertainty in the parameter estimates, probabilistic sensitivity analysis was performed by fitting distributions to the deterministic estimates and running 1000 iterations of a Monte Carlo simulation. We used gamma distributions for cost parameters and beta distributions for utility and disease risk parameters. According to the probabilistic sensitivity analysis, 90% of the estimates fall in the south east quadrant demonstrating lower cost and higher QALY for MHT versus no MHT and 10% lie in the south-west quadrant indicating lower cost and lower QALYs. The probabilistic sensitivity analysis plot is shown in .
Figure 3. Scatterplot of probabilistic sensitivity analysis (PSA) for 1,000 iterations of Monte Carlo simulation for the base case analysis (Yuan).
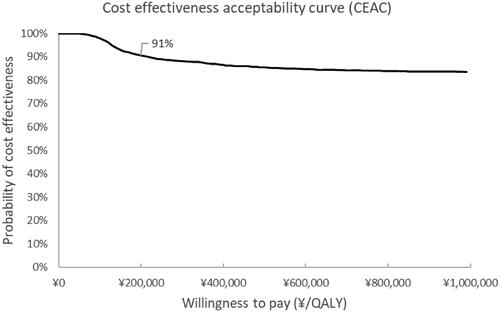
The cost effectiveness acceptability curve showed a 91% probability of being cost saving at a willingness to pay three times the GDP per capita in China (29,821$, 200,000¥) and is shown in .
Discussion
This is the first cost effectiveness model evaluating MHT for menopause in China. The base case model demonstrated that MHT is potentially cost-saving for the base case and a range of scenarios resulted in a dominant incremental cost effectiveness ratio (ICER) of −7,184$ −47,898¥) per QALY. Although there are no comparable studies in China, our results align with other published work evaluating MHT in other countries. A systematic review identified five cost effectiveness studies demonstrating cost effectiveness of MHT in the United States, United Kingdom, Sweden and FinlandCitation18,Citation21–24,Citation45.
Strengths
Our model was developed according to best practice guidelines, reported according to international reporting standards, and aligned with both the China Pharmacoeconomics Guidelines and Chinese Menopausal GuidelinesCitation8,Citation15,Citation46,Citation47. Furthermore, our model benefited from a meta-analysis of the utility of breast cancer in women in China (supplement scenario) and primary data from a study measuring EQ5D5L data in a cohort of menopausal-aged women in China (base case scenario)Citation32,Citation38. In line with best practice, where assumptions were required, a conservative approach was adopted to bias in favour of the comparator. To the best of our knowledge this is also the first cost effectiveness model in China and the rest of world, that transparently includes the long-term disease risks identified by the United Kingdom’s Medicines and Healthcare Products Regulatory Agency relating to MHTCitation26. The model developed by Zethraeus for Sweden and later adapted to USA and UK used similar health states except it did not include endometrial cancer or ovarian cancer, presumably disease risk data were not available at that timeCitation21,Citation22,Citation45. The implication being that all evidence-based disease risks known at this time have been incorporated into the model transparently, overcoming the limitations of previous models reviewed by Velentzis et al.Citation18,Citation26.
We performed comprehensive literature searches to identify China-specific utility, disease risk and mortality data and extensively explored all parameters in univariate sensitivity analysis, probabilistic sensitivity analysis and extensive scenario analysis. Sensitivity and scenario analysis demonstrated that the model results were consistent across cost, utility and disease risk data sources explored in the base case and supplementary scenarios.
The model underwent rigorous independent validation by a senior third-party modeller. The validation was performed by replicating the Excel model in TreeAge Pro Healthcare (Version 2020 – TreeAge Software, Inc.) The results are well within a range that is accepted as “successful” model replicationCitation44.
Limitations
The strengths of our study should be considered along with the limitations common to model-based economic evaluations. Sensitivity analysis showed that the model was most sensitive to three main parameters, the first of which were the (i) utility of symptomatic women not on MHT and the (ii) utility of asymptomatic women on MHT. To derive this parameter in the model our literature searches identified only one study measuring the utility of menopausal women in ChinaCitation48. The study by Liu (2014) was performed on data from 2010 that measured the utility of women aged 40–59 who with natural menopause in premenopausal and post-menopausal womenCitation48. However, Liu et al. report values only for pre- and post-menopausal groups rather than the perimenopausal group. Due to this lack of published evidence we conducted primary research to measure the utility in a cohort of women in China using the EQ5D5LCitation32. In 649 perimenopausal women with symptoms the mean utility measured by EQ5D5L was 0.864, and for 66 perimenopausal women with no symptoms the mean utility was 0.956Citation32. Threshold analysis confirmed that as long as the utility of women on MHT is higher than those not on MHT then MHT will remain cost effective and potentially cost saving (Supplementary Table 10).
The third parameter that the model was sensitive to was the (iii) cost of no MHT. In the model the cost of having menopause and not being on MHT is based on two DRGs for menopause (). We believe that this is conservative estimate of the cost of symptomatic women not on MHT. Women not on MHT are likely to use over-the-counter drugs, adjunct therapies such as acupuncture or Chinese remedies to alleviate symptoms which are unlikely to be borne by payers but are rather out of pocket expenses. Therefore, we believe that the base case represents a conservative estimate of the cost of not being on MHT.
The reader should be aware of the uncertainty around the utility parameters attached to the health states (BRCA, ENDOCA, OVCA, CHD, VTE, stroke, FOF). For the base case, we applied a ranked variable disutility decrement according to the severity ranking from our co-authors and since these rankings are based on clinical expert opinion, they are subjective. Nevertheless, the sensitivity analysis showed that when these utilities were varied (±10%), there was minimal impact on the results (change in ICER ranged from −0.035 to 0.003%; −2¥ to +50¥). We originally performed focused literature searches to identify utility values for the health states, however we found that the utility values in the literature were arguably high (supplement page 8). Even so, when we ran the model using the literature search values and an alternate constant disutility approach (both described in the supplement) there was negligible impact on the ICER (base case variable ranked utility −47,898¥; literature review −47,928¥; constant disutility −47,884¥).
A further consideration is that we applied the reduced utility uniformly across the patient’s remaining life. We considered that each of the health states in the model represents a complex clinical diagnosis representing a spectrum of severity, for example mild to severe stroke. We were reassured that although the constant utility approach has limitations, the health state utility values differentially affect both MHT and no MHT cohorts in the same direction and to the same magnitude. The reality is that patients with FOF and VTE may have an increase in utility over time whereas patients with BRCA, ENDOCA and OVCA are likely to have a decrease in utility over time. Patients with CHD and stroke may arguably have an increase or decrease in utility over time, depending on the severity of their condition and most recent event. The reader should consider that by assuming a constant disutility, we have underestimated the QALY gain (smaller area under the curve) in patients who might otherwise increase their utility over time and overestimated the QALY gain (larger area under the curve) in patients who might otherwise decrease their utility over time. Notably the model did not show sensitivity to these parameters.
In the model, the comparator was no MHT which aligns with other models and recommendations on the management of menopauseCitation6,Citation21–24,Citation45. The Chinese Menopausal Society Clinical Guideline states that “non-MHT therapy” is mainly used in patients with MHT contraindicationsCitation8. The guideline lists the following non-MHT therapies: Selective serotonin reuptake inhibitors, selective dual serotonin and norepinephrine reuptake inhibitors, and clonidine; Chinese patent medicines (Xiangshao Granules, Kuntai Capsules, black cohosh); Bio-identical hormones, Phytoestrogens, Mindfulness-based stress reduction therapy, stellate ganglion block, acupuncture and hypnosis may contribute to the therapeutic effects). For selective serotonin reuptake inhibitors, selective dual serotonin and norepinephrine reuptake inhibitors the guidelines states that they cannot be used as a substitute for MHTCitation8. For Chinese patent medicines the guideline stated that further research data was required to support the long-term safetyCitation8,Citation49. Soy isoflavones (Phytoestrogens) and bio-identical hormones are not recommendedCitation8. The challenges of Pharmacoeconomics of traditional Chinese medicine are widely reportedCitation50. During model conceptualisation we performed literature searches to identify efficacy data for non-MHT therapies to include as potential comparators, however we found no evidence to inform the model. For example, a study protocol was published describing a study to evaluate use of acupuncture for menopause, however despite study completion in 2015, no published results could be identifiedCitation51. Note that oestrogen only therapy for hysterectomised females was not a relevant comparatorCitation6,Citation8.
Taken as a whole, clinical guidelines advocate for an individualized approach to treatment, which includes treatment duration. In practice, our clinical co-authors agreed that in China a five-year treatment duration would represent the average patient. Five-year treatment duration was also in keeping with the definition of short-term MHT by the National Institute for Health and Clinical Excellence which recommends treating women “for the shortest possible time and with the lowest possible dose”Citation15,Citation25.
The simplest model structure believed to represent the clinical situation was used, in keeping with the best practice recommendation that “overly complex models should be avoided if a simpler one will accurately reflect all aspects of the decision problem”Citation46. To incorporate the evidence, it was necessary to assume that the health states were mutually exclusive (that is, that women on MHT would get BRCA or OVCA or ENDOCA etc). The clinical reality is that health states may co-exist, however from a model structure perspective a structure accounting for that would have required combined health states and in the absence of data to inform competing risks, strong assumptions would have had to be made to parameterise such a structure. Similarly, there was no utility data available for combined health states. It was judged that the added structural complexity of such a model outweighed the added value, especially considering that co-conditions would affect both intervention and comparator arms equally. It was also considered reasonable to assume that the 45-year-old female cohort modelled represented the average patient with no predisposing risks to MHT, the majority of whom are unlikely to develop co-conditions. A similar thought process ruled out the inclusion of subsequent events (see supplement).
We included two disease modifiers in our model (treatment type and duration) however there is evidence that other disease modifiers may also play a role in risk factors, for example body mass index may influence risk breast cancerCitation18. From a modelling perspective including BMI as risk modifier for BRCA would require strong assumptions about the effect of BMI on disease risks for the other health states, because BMI specific disease risks are not available in the literature. The alternative was to include them, which would necessitate assumptions on disease risk and quality of life, but we judged that the additional assumptions required would not add sufficient value to the results to warrant the additional modelling complexity. Alternately the assumption could be made that BMI is normally distributed in China and that the modelled cohort consists of patients with average (mean) BMI and that excluding BMI is therefore a satisfactory approach. Furthermore, other potential disease modifiers include urinary incontinence; gallbladder disease; and dementiaCitation18. Again, it seems reasonable to assume that the modelled cohort of the “average patient” does not suffer any of the above.
The results of our study will be useful for informing decision making in the context of symptomatic perimenopausal women in China, however the reader should exercise caution in applying findings to other populations or healthcare settings.
Future model-based evaluations would benefit from China-specific utility values for patients with endometrial and ovarian cancer, venous thromboembolic events, and fractured femur. A longitudinal study measuring the utility of patients on MHT and not on MHT would be useful, including measurement of hormone levels and symptoms.
Conclusion
Contingent on the structure and assumptions of the model, combination estradiol plus dydrogesterone MHT is potentially cost saving in symptomatic women over the age of 45 years in China.
Transparency
Author contributions
TR: evidence synthesis, model conceptualisation, programming, validation, reporting. manuscript writing, review, editing; MD: supervision, writing, review, editing; TS: clinical contribution, face validity, writing, review, editing; YQ: clinical contribution, face validity, writing, review, editing; MR: clinical contribution, face validity, writing, review, editing; YW: clinical contribution, face validity, writing, review, editing; MCG: clinical contribution, face validity, writing, review, editing; KK: supervision, writing, review, editing.
Acknowledgements
Thank you to Ute Zerwes who performed the literature searches and Bjoern Schwander from the Agency for Health Economic Assessment and Dissemination (AHEAD) who performed independent model validation.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Previous presentations
None.
Supplemental Material
Download MS Word (1.6 MB)Declaration of funding
This research was funded by Abbott Products Operations AG. The research team and authors had the final decision on the methods used for modelling, the parameters used and the content of the manuscript.
Declaration of financial/other interests
TR, MD have received research funding from Abbott. TS, YQ, MR, YW have received honorarium from Abbott. KK and MGC are employed by Abbott.
References
- Gambacciani M, Biglia N, Cagnacci A, et al. Menopause and hormone replacement therapy: the 2017 recommendations of the Italian Menopause Society. Minerva Ginecol. 2018;70(1):27–34.
- Baber RJ, Panay N, Fenton A, The IMS Writing Group. IMS recommendations on women’s midlife health and menopause hormone therapy. Climacteric. 2016;19(2):109–150. doi: 10.3109/13697137.2015.1129166.
- The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24(7):728–753. doi: 10.1097/GME.0000000000000921.
- Stuenkel CA, Davis SR, Gompel A, et al. Treatment of symptoms of the menopause: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2015;100(11):3975–4011. doi: 10.1210/jc.2015-2236.
- Cobin RH, Goodman NF, AACE Reproductive Endocrinology Scientific Committee. Goodman NF on behalf of the AACE reproductive endocrinology scientific committee. Endocr Pract. 2017;23(7):869–880. doi: 10.4158/EP171828.PS.
- National Institute for Health and Care Excellence (NICE). Clinical guideline. Methods, evidence and recommendations. Version 1.2; 2015 [cited 2021 Feb 21]. Available from: https://www.nice.org.uk/guidance/ng23/documents/menopause-full-guideline2.
- Hamoda H, Panay N, Pedder H, et al. The British Menopause Society & Women’s Health concern 2020 recommendations on hormone replacement therapy in menopausal women. Post Reprod Health. 2020;26(4):181–209. doi: 10.1177/2053369120957514.
- Menopause Group of the Chinese Medical Association Obstetrics and Gynecology Branch. Menopause management and menopausal hormone therapy in China. Chinese J Obstet Gynecol. 2018;53(11):729–739. doi: 10.3760/cma.j.issn.0529-567x.2018.11.001.
- ] Menopause Subgroup of the Chinese Society of Obstetrics and Gynecology, Chinese Medical Association. The 2023 Chinese menopause symptom management and menopausal hormone therapy guidelines. Zhonghua Fu Chan Ke Za Zhi. 2023;58(1):4–21. doi: 10.3760/cma.j.cn112141-20221118-00706.
- Yu Q. Population education of menopausal knowledge in China. Climacteric. 2019;22(4):323–323. doi: 10.1080/13697137.2019.1609767.
- Jin F, Tao MF, Teng YC, et al. Knowledge and attitude towards menopause and hormone replacement therapy in chinese women. Gynecol Obstet Invest. 2015;79(1):40–45. doi: 10.1159/000365172.
- Chu K, Song Y, Chatooah ND, et al. The use and discontinuation of hormone replacement therapy in women in South China. Climacteric. 2018;21(1):47–52. doi: 10.1080/13697137.2017.1397622.
- Chen R, Yu Q. Current status of physicians’ awareness and clinical practice of menopause hormone replacement therapy in China: a multicenter, cross-sectional survey. Maturitas. 2015;82(3):316–317. doi: 10.1016/j.maturitas.2015.06.012.
- Gao L, Wu X, Liu X, et al. Awareness of hormone replacement therapy in medical care personnel in Jiaxing, China: a questionnaire survey. Gynecol Endocrinol. 2018;34(4):332–335. doi: 10.1080/09513590.2017.1405929.
- Zhen X, Sun X, Dong H. Health technology assessment and its use in drug policies in China. Value Health Reg Issues. 2018;15:138–148. doi: 10.1016/j.vhri.2018.01.010.
- Bao Peng L, Tan CQ, Wan XM, et al. Cost-utility analysis in China: differences and difficulties compared with developed countries. Pharmacoeconomics. 2007;25(7):619. doi: 10.2165/00019053-200725070-00007.
- Liu GG, Shanlian H, Jiuhong W, et al. China guidelines for pharmacoeconomic evaluations. 2020 Edition. Beijing: China Society for Pharmacoeconomics and Outcomes Research; 2020.
- Velentzis LS, Salagame U, Canfell K. Menopausal hormone therapy: a systematic review of cost-effectiveness evaluations. BMC Health Serv Res. 2017;17(1):326. doi: 10.1186/s12913-017-2227-y.
- Barton P, Bryan S, Robinson S. Modelling in the economic evaluation of health care: selecting the appropriate approach. J Health Serv Res Policy. 2004;9(2):110–118. doi: 10.1258/135581904322987535.
- Brennan A, Chick SE, Davies R. A taxonomy of model structures for economic evaluation of health technologies. Health Econ. 2006;15(12):1295–1310. doi: 10.1002/hec.1148.
- Lekander I, Borgström F, Ström O, et al. Cost-effectiveness of hormone therapy in the United States. J Womens Health. 2009;18(10):1669–1677. doi: 10.1089/jwh.2008.1246.
- Lekander I, Borgström F, Ström O, et al. Cost effectiveness of hormone therapy in women at high risks of fracture in Sweden, the US and the UK-results based on the women’s health initiative randomised controlled trial. Bone. 2008;42(2):294–306. doi: 10.1016/j.bone.2007.09.059.
- Zethraeus N, Borgström F, Jönsson B, et al. Reassessment of the cost-effectiveness of hormone replacement therapy in Sweden: results based on the women’s health initiative randomized controlled trial. Int J Technol Assess Health Care. 2005;21(4):433–441. doi: 10.1017/S0266462305050609.
- Ylikangas S, Bäckström T, Heikkinen J. Cost-effectiveness of continuous combined hormone replacement therapy in long-term use: economic evaluation based on a 9-year study in Finland. Curr Med Res Opin. 2007;23(1):57–64. doi: 10.1185/030079907X159542.
- Lumsden MA, Davies M, Sarri G, Guideline Development Group for Menopause: diagnosis and Management (NICE Clinical Guideline No. 23). Sarri G for the guideline development group for menopause: diagnosis and management. Diagnosis and management of menopause the national institute of health and care excellence guideline. JAMA Intern Med. 2016;176(8):1205–1206. doi: 10.1001/jamainternmed.2016.2761.
- Medicines and Healthcare Products Regulatory Agency. Drug safety update volume 13, issue 2: September 2019. First published online 30 August; 2019 [cited 2021 Mar 5]. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/832681/Sept-2019-PDF.pdf.
- World Health Organisation. China Life Table, females, age specific, all-cause mortality; 2020 [cited 2020 Jun 24]. Available from: https://apps.who.int/gho/data/view.main.60340.
- Paisley S. Classification of evidence in decision-analytic models of cost-effectiveness: a content analysis of published reports. Int J Technol Assess Health Care. 2010;26(4):458–462. doi: 10.1017/S026646231000098X.
- Edlin RM, Hulme C, Hall P, et al. Cost effectiveness modelling for health technology assessment, a practical course. Switzerland: Springer; 2015.
- Nuijten MJ. The selection of data sources for use in modelling studies. Pharmacoeconomics. 1998;13(3):305–316. doi: 10.2165/00019053-199813030-00005.
- China diagnosis related group codes and data provided by Abbott Affiliate. 2020.
- Rautenberg TA, Ng SKA, Downes M. A cross-sectional study of symptoms and health-related quality of life in menopausal-aged women in China. BMC Women’s Health. 2023;23(1):563. doi: 10.1186/s12905-023-02728-y.
- Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of breast cancer: nested case-control studies using the QResearch and CPRD databases. BMJ. 2020;371:m3873. doi: 10.1136/bmj.m3873.
- Kim J-E, Chang J-H, Jeong M-J, et al. A systematic review and meta-analysis of effects of menopausal hormone therapy on cardiovascular diseases. Sci Rep. 2020;10(1):20631. doi: 10.1038/s41598-020-77534-9.
- Gartlehner G, Patel SV, Feltner C, et al. Hormone therapy for the primary prevention of chronic conditions in postmenopausal women: evidence report and systematic review for the US preventive services task force. JAMA. 2017;318(22):2234–2249. doi: 10.1001/jama.2017.16952.
- Feng R-M, Zong Y-N, Cao S-M, et al. Current cancer situation in China: good or bad news from the 2018 global cancer statistics? Cancer Commun. 2019;39(1):22. doi: 10.1186/s40880-019-0368-6.
- Zhou M, Wang H, Zhu J, et al. Cause-specific mortality for 240 causes in China during 1990–2013: a systematic subnational analysis for the global burden of disease study 2013. Lancet. 2016;387(10015):251–272. doi: 10.1016/S0140-6736(15)00551-6.
- Rautenberg T, Hodgkinson B, Zerwes U, et al. Meta-analysis of health state utility values measured by EuroQol 5-dimensions (EQ5D) questionnaire in Chinese women with breast cancer. BMC Cancer. 2022;22:52. doi: 10.1186/s12885-021-09140-5.
- Zhang Z, Lei J, Shao X, et al. Trends in hospitalization and in-Hospital mortality from VTE 2007 to 2016, in China. Chest. 2019;155(2):342–353.
- Cui Z, Feng H, Meng X, et al. Age-specific 1-year mortality rates after hip fracture based on the populations in mainland China between the years 2000 and 2018: a systematic analysis. Arch Osteoporos. 2019;14(1):55. doi: 10.1007/s11657-019-0604-3.
- Boardman HMP, Hartley L, Eisinga A, et al. Hormone therapy for preventing cardiovascular disease in post-menopausal women. Cochrane Database Syst Rev. 2015;2015(3):Cd002229. doi: 10.1002/14651858.CD002229.pub4.
- Pan R, Zhu M, Yu C, et al. Cancer incidence and mortality: a cohort study in China, 2008–2013. Int J Cancer. 2017;141(7):1315–1323. doi: 10.1002/ijc.30825.
- Ma LY, et al. China cardiovascular diseases report 2018: an updated summary. J Geriatric Cardiology. 2020;17(1):1–8.
- Schwander B, Hiligsmann M, Nuijten M, et al. Replication of published health economic obesity models: assessment of facilitators, hurdles and reproduction success. Pharmacoeconomics. 2021;39(4):433–446. doi: 10.1007/s40273-021-01008-7.
- Salpeter SR, Buckley NS, Liu H, et al. The cost-effectiveness of hormone therapy in younger and older postmenopausal women. Am J Med. 2009;122(1):42–52.e2. doi: 10.1016/j.amjmed.2008.07.026.
- Caro JJ, Briggs AH, Siebert U; on behalf of the ISPOR-SMDM Modeling Good Research Practices Task Force, et al. Modeling good research practices–overview: a report of the ISPOR-SMDM modeling good research practices task force-1. Value Health. 2012;15(6):796–803. doi: 10.1016/j.jval.2012.06.012.
- Husereau D, Drummond M, Petrou S, et al. Consolidated health economic evaluation reporting standards (CHEERS) statement. Value Health. 2013;16(2):e1-5–e5. doi: 10.1016/j.jval.2013.02.010.
- Liu K, He L, Tang X, et al. Relationship between menopause and health-related quality of life in middle-aged Chinese women: a cross-sectional study. BMC Womens Health. 2014;14:7.
- Palacios S, Stevenson JC, Schaudig K, et al. Hormone therapy for first-line management of menopausal symptoms: practical recommendations. Women Health. 2019;15:1–8.
- Yang Y, Tian K, Bai G, et al. Health technology assessment in traditional Chinese medicine in China: current status, opportunities, and challenges. Global Health J. 2019;3(4):89–93. doi: 10.1016/j.glohj.2019.11.002.
- Li Y, Zheng H, Zheng Q, et al. Use acupuncture to relieve perimenopausal syndrome: study protocol of a randomized controlled trial. Trials. 2014;15(1):198. doi: 10.1186/1745-6215-15-198.