Abstract
Aims
Intraurethral catheter balloon inflation is a substantial contributor to significant catheter-related urethral injury. A novel safety valve has been designed to prevent these balloon-inflation injuries. The purpose of this evaluation was to assess the cost-effectiveness of urethral catheterisation with the safety valve added to a Foley catheter versus the current standard of care (Foley catheter alone).
Materials and methods
The analysis was conducted from the UK public payer perspective on a hypothetical cohort of adults requiring transurethral catheterization. A decision tree was used to capture outcomes in the first 30 days following transurethral catheterization, followed by a Markov model to estimate outcomes over a person’s remaining lifetime. Clinical outcomes included catheter balloon injuries [CBIs], associated short-term complications, urethral stricture disease, life years and QALYs. Health-economic outcomes included total costs, incremental cost-effectiveness ratio, net monetary benefit (NMB) and net health benefit.
Results
Over a person’s lifetime, the safety valve was predicted to reduce CBIs by 0.04 per person and CBI-related short-term complications by 0.03 per person, and nearly halve total costs. The safety valve was dominant, resulting in 0.02 QALYs gained and relative cost savings of £93.19 per person. Probabilistic sensitivity analysis indicated that the safety valve would be cost-saving in 97% of simulations run versus standard of care.
Conclusions
The addition of a novel safety valve aiming to prevent CBIs during transurethral catheterization to current standard of care was estimated to bring both clinical benefits and cost savings.
Introduction
Urethral catheterization is a common medical procedure. Recent studies conducted in the United Kingdom reported catheter prevalence of 7.0–10.8% in the community setting and 18.6% in the general hospital setting, rising to as much as 76.6% in critical care patientsCitation1,Citation2.
Given the blind nature of urethral catheterization, associated iatrogenic injuries may, unfortunately, occur. In a 2016 prospective study conducted over 6 months in two Irish tertiary care hospitals, 37 urethral catheterization injuries occurred, representing an overall incidence of 6.7/1,000 catheterizations (1.3% of male catheterizations)Citation3. None of these patients were known or subsequently found to have to have a stricture in the acute phase. In 26/37 cases balloon inflation was confirmed and in the remainder it was uncertain if it had occurred. The incidence of catheterization injuries was very similar in a subsequent expanded study spanning three UK and four Irish hospital Trusts/Groups, comprising a total of 3,836 beds, which identified 66 cases of catheterization injury over a mean follow-up of 3.4 months, resulting in an injury incidence of 6.16/1,000 catheterizations (1.23% of male catheterizations)Citation4. This equated to an average of 3 injuries occurring per every 500 hospital beds each monthCitation4.
Catheterization injuries have marked implications in terms of both patient wellbeing and health economics. An estimated acute care cost of €9,000 per injury has been calculated in a previous study; this figure does not include follow-up, management of long-term complications, or medico-legal costingCitation3. Of the 37 patients who suffered a catheterization injury in the former study, 12 patients developed sepsis and 3 patients developed acute kidney injuryCitation3. As a result of these acute complications, three patients required admission to an intensive care unit and one patient diedCitation3. In a 3-year follow-up study of the same patients, 29 (78%) had developed a urethral stricture including 10 who required a permanent suprapubic or urethral catheterCitation5. These longer-term consequences impact both secondary care and community resources, such as the regular replacement of long-term catheters and management of a urethral stricture. This highlights the fact that the long-term consequences frequently arise following acute catheterization injuries, with a potentially profound impact on an individual. Urethral stricture disease (USD) has a well-recognised deleterious impact on health-related quality of lifeCitation6–8. Furthermore, major cost implications in addition to the aforementioned acute care expenditure become apparent as the provision of ongoing care for all-cause recurring USD imparts a financial burden on healthcare servicesCitation9.
Intraurethral catheter balloon inflation is one cause of significant catheter-related urethral injuriesCitation10. A series of porcine and human ex vivo experiments were conducted, resulting in the establishment of a threshold pressure for urethral injury of 150 kPaCitation11. This pressure level suggests the inflating balloon is incorrectly positioned within the urethra. Based on this knowledge, a suitable safety valve, the transurethral catheter safety valve (CE-approved TUC Safety Valve, Class Medical, Limerick, Ireland) was designedCitation12 to vent at this predetermined pressure level. Venting of the safety valve causes the inflation fluid to leak externally, preventing further inflation of the balloon and thus protecting the urethra, as well as alerting the user of the need to cease the inflation attempt and to reposition the catheter (Supplementary Figure 1).
In two studies conducted to date, no cases of catheter balloon injuries (CBI) were observed with the use of the safety valve,Citation13,Citation14. In a pre-post study conducted over 6 months in two Irish hospitals (and including 2 community care groups), 13 CBIs were recorded over 3 months prior to safety valve useCitation14. During the 3-monthly period following safety valve introduction, 12 episodes of the safety valve venting were recorded over 699 catheterizations, with each episode of the valve venting indicating a CBI preventedCitation14.
In light of the favourable clinical evidence, the purpose of this evaluation was to assess the cost-effectiveness, from a UK public payer perspective, of urethral catheterization with the safety valve added to a standard Foley catheter when compared with the current standard of care, the use of a standard Foley catheter alone.
Methods
No ethics approval was required, as the study only involved the modelling of a hypothetical patient cohort based on published data.
The reporting in this manuscript follows the CHEERS checklist for health economic evaluation reportingCitation15.
Description of the economic model
The model assessed the addition of a novel safety valve to standard of care (SoC), i.e. transurethral catheterization with a Foley catheter and the safety valve versus a Foley catheter alone in UK adults requiring transurethral catheterization. The perspective was that of the UK public payer (the National Health Service [NHS]) and outcomes were assessed over a lifetime horizon. Clinical outcomes of interest included the total number of adverse events (catheter balloon injuries [CBI], its short-term complications, and development of urethral stricture disease [USD]), total life years (LYs), and quality-adjusted life-years (QALYs). Health-economic outcomes included total costs, incremental cost-effectiveness ratio (ICER, representing the cost per QALY gained), net monetary benefit (NMB, representing the value of an intervention in monetary terms at a defined willingness-to-pay threshold, with positive incremental NMB indicating that the intervention is cost-effective relative to the comparator) and net health benefit (NHB, representing the impact of the intervention on population health, with positive NHB indicating an improvement in overall population health with the intervention). Both costs and QALYs were discounted at 3.5% per annum and a cost-effectiveness threshold of £20,000–£30,000 per QALY gained was adopted, in line with the recommendations from the National Institute of Health and Care Excellence (NICE)Citation16. The model was developed using Microsoft Excel.
The model adopted a hybrid structure using a decision tree to capture short-term outcomes in the first 30 days following transurethral catheterization (), followed by a Markov model to estimate long-term outcomes over the remaining lifetime of the person (). The choice of model structure was informed by a pragmatic literature review. The model structure was validated with clinical experts (consultant urologists) in order to capture all the key relevant outcomes.
Figure 1. Structure of the model. A: Short-term decision tree component, B: long-term Markov model. Abbreviations. CBI: Catheter balloon injury; USD: urethral stricture disease

The decision tree separated short-term catheter users, who may only require the catheter for a few days while an unrelated procedure is being conducted in the hospital, and people who require long-term catheterization. Both groups were considered at risk of CBI, following which they could develop short-term complications. People who suffered short-term complications were at risk of mortality. All surviving individuals who had developed a CBI, regardless of short-term complication status, could suffer longer-term from USD. The cohort mix at the final stages of the decision tree components determined the initial allocation of people across the Markov health states.
In the Markov model component, the “Alive” health state captured individuals who had had a one-time catheterization and did not suffer USD; therefore, they can be considered “equivalent” to the general population. The “Recurring USD treatment” health state captured one-time catheter users who had had a CBI and developed USD, incurring associated health decrements and costs. During each model cycle, individuals in this health state could have successful treatment and move to the “Asymptomatic USD” health state or have unsuccessful treatment and remain in the “Recurring USD treatment” state. The “Asymptomatic USD” state was associated with the costs of follow-up by a urologist every 6-months, but no health decrement. When in the “Asymptomatic USD” health state, people whose symptoms recurred could move back to the “Recurring USD treatment” state or require a more advanced treatment option (a long-term indwelling transurethral or suprapubic catheter) captured by the “End of line USD treatment” health state. This latter state captured the health decrement and costs associated with long-term catheter use, including the risk of CBI and the associated management and short-term complications. People who required repeat catheterization that had not developed USD entered the Markov component of the model in the “No stricture” health state. This health state captured the costs of catheter changes and the risk of CBI and subsequent complications with each change. People in the “No stricture” health state who developed USD moved to the “End of line USD treatment” health state. Transitions to the absorbing “Dead” state, representing mortality, were possible from all model states. The Markov cycle length was 1 month with half-cycle correction applied. The half-cycle correction allows the model to better account for the fact that events driving state changes could occur at any point during the monthly cycle.
This structure of the model was associated with two important assumptions. Firstly, it assumed long-term use of an indwelling catheter continued over the person’s lifetime and therefore did not account for people who discontinue its use. Nonetheless, it is believed those who have been on an indwelling catheter for the longer-term already are very unlikely to transition away from this, resulting in life-time catheter use. Secondly, no one-time catheter users who developed USD were assumed to immediately receive an end of line treatment (i.e. a long-term indwelling catheter). Instead, it was assumed those people would first receive treatment aiming to alleviate their symptoms (e.g. urethrotomy), represented by the “Recurring USD treatment” state.
Key model inputs
The key source of clinical inputs related to the novel safety valve was a real-world pre-post study describing the incidence of CBI in two Irish hospitals over 3-month periods before and after the safety valve introductionCitation14. A prospective real-world study by Davis et al. conducted in two teaching hospitals in Ireland was a major source of clinical data for the SoC armCitation3,Citation5. The initial report from a 6-month study periodCitation3 provided information on CBI incidence and short-term complications, and the long-term follow-up study (mean 37 months, range 31–42 months) of people who experienced CBICitation5 largely informed transition probabilities in the Markov model component. More detail on the derivation of Markov transition probabilities is provided in Supplementary Table 1.
The data from the two aforementioned studies was supplemented with a variety of literature sources to inform long-term clinical outcomes and disutilities associated with CBI management, short-term CBI complications, and long-term Markov health states. Utilities are measures of health-related quality of life (HRQoL) defined on a scale of 0 (representing death) to 1 (representing perfect health). Age- and gender-specific UK general population utilitiesCitation17, representing the HRQoL of the UK general population served as the basis for calculating the utilities of the modelled cohort. Disutilities are values subtracted from the health state-associated utility to reflect the worsening of HRQoL associated with a particular event, e.g. disease worsening, treatment, procedure, or an adverse event. Disutilities associated with CBI management and short-term complications were applied only in the model cycle in which the causative events occurred, while disutilities associated with a particular Markov health state were applied at each monthly cycle during which the person remained in that state.
Resource use associated with acute CBI management, management of short-term complications, recurring USD treatment, and long-term indwelling catheter use (end of line treatment) was primarily sourced from Davis et al.Citation3,Citation5. Details on the proportion of people requiring each type of healthcare resource are provided in Supplementary Table 2. Costs were derived from standard UK sources such as NHS reference costs and the Personal Social Services Research Unit (PSSRU)Citation18,Citation19. The cost of the safety valve was £15. Key clinical and HRQoL inputs are summarised in and unit costs are summarised in .
Table 1. Key clinical and HRQoL inputs.
Table 2. Unit costs applied in the model.
Sensitivity analyses
Deterministic and probabilistic sensitivity analyses were performed to assess uncertainty around the model estimates. One-way deterministic sensitivity analyses (DSA) estimated the effect of varying input parameter values, one at a time, on model results. Parameters were varied by ±20% from the base case input value unless otherwise specified. Probabilistic sensitivity analyses were performed to estimate multivariate and stochastic uncertainty in the model. All inputs were simultaneously sampled from plausible distributions for 2,000 iterations, and the incremental costs and QALYs generated from each iteration were examined collectively by computing the mean and 95% credible intervals (CrIs). In addition, the spread of the results from the iterations was visually presented on a cost-effectiveness plane.
Results
Base case results
In the base case analysis, the addition of the safety valve during catheterization was estimated to result in both QALY gains (0.02 QALYs per person) and cost savings (£93.19 per person) over a lifetime horizon when compared with the SoC of catheterization with a Foley catheter alone. The safety valve was therefore the dominant technology. A summary of the base case results is presented in . At a willingness-to-pay threshold of £20,000 per QALY (the lower range used in the UK), the NMB associated with introducing the safety valve was £536.57 per person, and at the willingness to pay threshold of £30,000 per QALY (upper range used in the UK) the NMB was £758.27. The NHB associated with introducing the safety valve was estimated at 0.03 QALYs per person at both willingness-to-pay thresholds.
Table 3. Results of the base case and scenario analyses, per patient.
The base case results indicated important clinical benefits associated with introducing the safety valve. The estimated reduction in the number of CBIs relative to the use of a Foley catheter alone was 0.04 per catheterized person over a lifetime horizon, including in those who require repeat, life-time catheterization. Important reductions were also predicted in the number of CBI-related short-term complications, which fell by 0.03 per person. Both of these events were reduced by 100% by introducing the safety valve.
Overall, the introduction of the safety valve was predicted to nearly halve the total costs from £186.75 to £93.56 (a 49.8% reduction) over a persons lifetime. The detailed breakdown of costs predicted by the model is presented in Supplementary Table 3.
Sensitivity and scenario analyses
Deterministic sensitivity analyses revealed that the cost-effectiveness estimates derived from the model were most sensitive to the starting age of the cohort, the proportion of people experiencing a CBI per catheterization when using the Foley catheter alone (SoC arm of the model), the proportion of people who develop USD after a CBI, the efficacy of the safety valve, and the disutility associated with having an indwelling catheter (Supplementary Figure 2, top panel). The case was similar when only costs were considered, with additional hospital stay associated with a CBI becoming a more important factor, and disutilities no longer having an impact (Supplementary Figure 2, bottom panel) However, no individual parameter variation changed the direction of the results. For example, even using a lower bound for the frequency of CBIs, the safety valve remained cost-effective.
In probabilistic sensitivity analyses at a £20,000 per QALY gained willingness-to-pay threshold, the safety valve was cost-effective in all simulations and was cost-saving in 97.0% of the simulations (Supplementary Figure 3), increasing the confidence in the model predictions. The 95% CrIs around the base case incremental cost (mean −£105.44; 95% CrI: −£301.74 to £2.16) and the incremental QALY gain (mean 0.02; 95% CrI: 0.01–0.05) were tight.
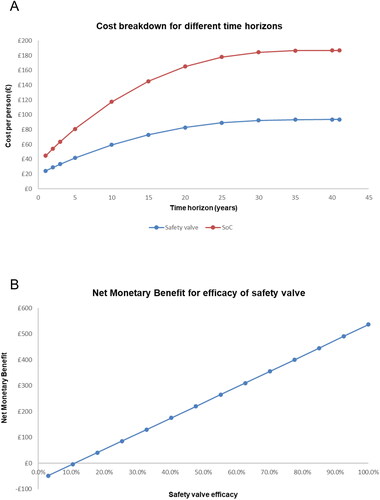
Finally, two series of scenario analyses were run: (1) truncating the model’s time horizon and assessing the costs generated by the model and (2) varying the efficacy of the safety valve and assessing the cost-effectiveness (expressed as NMB). Detailed results for 1-, 5-, and 10-year time horizons are presented in and the per-person costs accrued over all studied time horizons are presented in (top panel) for both the safety valve and the SoC arm. As a large proportion of the costs, particularly in long-term catheter users, are expected to be generated over the person’s lifetime, these scenario analyses specifically assessed the shorter-term impact of introducing the safety valve. Across all horizons assessed, the introduction of the safety valve was consistently associated with substantially lower costs than SoC, suggesting that the cost savings resulting from introducing the safety valve begin early after its introduction and continue as long-term complications are spared. In the other series of scenario analyses, the efficacy of the safety valve was varied from 5% to the base case value of 100% and the NMB was assessed at the £20,000 per QALY willingness-to-pay threshold (, bottom panel). The NMB was positive, indicating the safety valve was cost-effective relative to SoC, at all efficacy levels higher than 10%, suggesting that any uncertainty around the real-world efficacy of the safety valve is very unlikely to change the direction of model results in such a way that the use of a Foley catheter alone would be the more cost-effective technology.
Discussion
Deterministic and probabilistic analyses were conducted over a lifetime horizon from the UK NHS perspective to assess the cost-effectiveness of the safety valve combined with a standard Foley catheter versus the standard catheter alone for urethral catheterization. The addition of the safety valve was estimated to be the dominant strategy, i.e. associated with both improved clinical outcomes and cost savings. Furthermore, since the valve was estimated to prevent various short- and long-term complications such as urosepsis, kidney injury, flexible/rigid cystoscopy, and others, its introduction can be expected to have a positive health benefit to society, with a gain of 0.02 QALYs per catheterized person over their lifetimes. We acknowledge that CBI is an under-researched area and that further studies are desirable to investigate the incidence and long term sequelae of CBI on a global scale. Importantly, however, no individual parameter variation changed the direction of the results. Hence, even at higher starting ages or lower CBI incidence, the safety valve remained cost-effective.
To the authors’ knowledge, no cost-effectiveness assessments of a similar safety device have been published. Therefore, this analysis presented is important to highlight the impact that preventing CBI can have on the UK health care system. Acute management and short-term complications, which occur in many cases due to preventable errors by the clinician performing the catheterization can be particularly costly for the NHS. If people who are not necessarily long-term catheter users already end up having an injury in the community, the development of urethral stricture and longer-term consequences could have a large impact on community resource use through the need for repeating catheterisation or treatment and recovery for alleviating the stricture symptoms. Furthermore, given the greater introduction of step-down care from hospital, it is difficult to identify how much extra resource use would be required in the community for short-term complications of a catheter-related injury.
The analysis conducted suggests that the acute management and short-term complications prevented by the safety valve more than outweighs the cost of implementing the valve, without including other prevented costs such as USD. Importantly, as the safety valve is compatible with all types of commercially available indwelling catheters, it can be expected to improve the clinical outcomes and cost-effectiveness of urethral catheterization regardless of the preferred strategy or catheter type used by a given practitioner or centre. Given the number of catheters a clinician is likely to place, particularly in community settings, the expected training cost per person catheterised in terms of clinician time is likely to be minimal and would be captured in the bounds of sensitivity analysis on the cost of using the valve (given that no charge is provided for this training). Whilst the catheterization procedure takes a few seconds longer if the safety valve is used, the additional time required to use the safety valve is negligible for any cost impact.
While the present analysis aimed to assess the cost-effectiveness of the safety valve relative to standard care, the budget impact of introducing the valve is also of interest in the setting of limited resources within the NHS. Although a full budget impact assessment is out of scope of this analysis, it can be approximated based on the published number of catheter users within NHS England and the cost saving associated with the introduction of the safety valve in the current model. In the NHS, the annual number of people using urinary catheters has previously been estimated to be 997,814 per yearCitation20. Some of these will be long-term users, however, the majority of cases are expected to be one-time catheter users. Assuming, for simplification, that all catheter users are one-time users, over a one-year time horizon, the model estimated that introducing the safety valve will bring savings of £17.27 per person. Across the published number of catheter users in the NHS, this would result in a saving of £17,232,248 per year. Although likely an underestimate due to the exclusion of long-term catheter users, clinical opportunity costs and medico-legal claims, this simplified analysis highlights the large potential impact the safety valve may have on NHS finances.
Catheter injuries have been reported to occur more frequently when catheterization was performed by junior doctors at early stages of training, particularly when they were unsupervisedCitation10. Since the current analysis did not factor in the experience and training level of the personnel performing catheterization or the setting (hospital, community, or hospice), it is possible that in particular settings where junior personnel training takes place (i.e. there is a higher abundance of junior doctors), such as training hospitals, the health and cost benefits of safety valve introduction would be even more pronounced, given the complete prevention of CBI observed in clinical studies conducted to dateCitation13,Citation14 and the results of the present analysis. The results of the sensitivity analysis highlight how one of the key drivers of the model is the frequency at which catheter injuries occur, where a higher base line incidence of these injuries will result in the safety valve being more cost-effective.
A considerable strength of the current evaluation was the robustness of the results when sensitivity and scenario analyses were performed. None of the individual model parameters were able to change the direction of model results (i.e. result in catheterization with a Foley catheter alone being more cost effective than with the safety valve added to the Foley catheter). In probabilistic sensitivity analyses, catheterization using the safety valve was cost-effective in all iterations and cost-saving in 97.0% of the iterations.
Scenario analyses truncating the time horizon over which the costs and benefits were calculated resulted in lower incremental costs saved; however, the relative proportional cost savings remained the same between treatment arms, with the safety valve saving around half the costs when compared to using a Foley catheter alone. When the effectiveness of the safety valve was varied in another set of scenario analyses, the results indicated that effectiveness below 10% would be required to change the direction of model results. Given the 100% effectiveness of the safety valve observed to dateCitation13,Citation14, the results of this scenario analysis indicate that as more evidence on real-world use of the safety valve emerges, the valve is very likely to remain cost-effective. Although it may be an overestimate that the safety valve is 100% effective, this scenario analysis highlights how the efficacy of the safety valve would have to be substantially different to the current evidence in order to change the direction of results.
In terms of study limitations, the analysis was limited to transurethral catheterization only, although there is no reason the safety valve could not be used for suprapubic catheterization, in which instance it would prevent urethral balloon inflation in the instance of inadvertent antegrade urethral passage of the catheter. Therefore, the full population that could potentially benefit from the introduction of the safety valve was not captured.
Another limitation, pertaining to the model structure itself, was that the model did not account for people who no longer required a long-term indwelling catheter; this was due to the paucity of available data to inform such an option in the model. The assumption that a long-term indwelling catheter was used for the person’s lifetime was validated through engaging with clinical experts.
Whilst there are a small number of cases where this assumption does not hold, such as for those catheterized for urinary retention who go on to have prostate surgery, it was expected that the majority of the assessed population would be catheterized for life. Inputs were collated from various sources to assess the cost-effectiveness of the safety valve based upon available data. It would be beneficial to conduct future data collection to validate and improve upon the robustness of the results. However, a randomized controlled trial would not be appropriate for this type of intervention. Firstly, for this type of intervention there is an inability to randomise, given both the clinician and individual are aware of what treatments are being used, and sham catheterization is not possible. Secondly, events are rare, meaning that a very large sample size would be required for a trial. Thirdly, resource use when there is an event may not reflect real-world resource use or patient management in a clinical trial.
The model starting age was based upon the mean age of catheterization from a very large study of catheterization across the UK. Given the previous study on the TUC valve reflected all people catheterised over a 6-month period in an Irish hospital, the average age used in the model should reflect the overall population, not those most likely to incur the event. Although it is noted this is more likely to occur in older people, if an older mean age was used, then a higher incidence of catheter injuries should also be used.
It is worth noting that, whilst the majority of the saving and benefit is likely to be in secondary care, a proportion of these costs will be incurred in the community setting in many cases, due to catheter users being more commonplace in the community. However, the model is not able to distinguish the exact costs for either due to step-down care of events, while there may be variation of where procedures are conducted (between the community or secondary care) by region or ICB.
Regarding model inputs, no published utility values for many of the complications or health impacts associated with CBI could be identified, necessitating the use of clinical expert opinion or applying utilities for similar complications as proxies. While this produces some uncertainty about the health impact of the safety valve, such concerns can be largely alleviated by the PSA results that strongly indicated the safety valve remained cost-effective when all model inputs were repeatedly varied by random sampling from appropriate distributions.
Finally, the total QALY gains and cost savings associated with the introduction of the safety valve may appear to be small. This is because the majority of people who undergo transurethral catheterization would not be inflicted with any urethral trauma, and the valve would have a large positive impact for only the small subset of people who suffer a CBI. Given the burden of these injuries and their consequences to the person, as well as the costs associated with treating acute and long-term CBI complicationsCitation3,Citation5,Citation9, prevention of CBI with an effective and cost-effective device is likely to be beneficial to people requiring catheterization, the health system, and society as a whole, despite the small number of affected people.
Conclusion
CBIs are recurring, preventable iatrogenic injuries that may have profound health consequences to the person undergoing catheterization and incur unnecessary costs to health systems. The results of this analysis strongly indicate that the addition of a novel safety valve aiming to prevent CBIs during transurethral catheterization is both clinically beneficial and cost-saving compared with the use of a standard Foley catheter alone, suggesting the device has the potential to improve the outcomes of individuals, improve overall population health, and bring savings to the already stretched healthcare budgets.
Transparency
Author contributions
All authors were involved in the conceptualization of the model design and economic analysis. RM, SM and BA conducted the economic analysis. All authors were involved in the interpretation of the results. RM, SM and BA were responsible for the initial draft of the manuscript, with medical writing assistance. SC, HF, JW, GL, ND, and MW, provided critical revision of the manuscript at multiple stages.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplemental Material
Download MS Word (1.3 MB)Acknowledgements
Medical writing and editorial assistance with the preparation of this manuscript was provided by Karolina Badora.
Declaration of funding
As part of this analysis, the York Health Economics Consortium were funded by Class Medical to construct the health economic model and co-author the manuscript.
Declaration of financial/other relationships
None stated.
References
- Prieto J, Wilson J, Bak A, et al. A prevalence survey of patients with indwelling urinary catheters on district nursing caseloads in the United Kingdom: the community urinary catheter management (CCaMa) study. J Infect Prev. 2020;21(4):129–135. doi: 10.1177/1757177420901550.
- Shackley DC, Whytock C, Parry G, et al. Variation in the prevalence of urinary catheters: a profile of national health service patients in England. BMJ Open. 2017;7(6):e013842. doi: 10.1136/bmjopen-2016-013842.
- Davis NF, Quinlan MR, Bhatt NR, et al. Incidence, cost, complications and clinical outcomes of iatrogenic urethral catheterization injuries: a prospective multi-institutional study. J Urol. 2016;196(5):1473–1477. doi: 10.1016/j.juro.2016.05.114.
- Croghan SM, Riogh AN, Madden A, et al. Abstract 30 - incidence of iatrogenic urethral catheterisation injuries: a prospective multicentre audit. Eur Urol Open Sci. 2021;31:S13. doi: 10.1016/S2666-1683(21)00196-8.
- Davis NF, Bhatt NR, MacCraith E, et al. Long-term outcomes of urethral catheterisation injuries: a prospective multi-institutional study. World J Urol. 2020;38(2):473–480. doi: 10.1007/s00345-019-02775-x.
- Bertrand LA, Warren GJ, Voelzke BB, et al. Lower urinary tract pain and anterior urethral stricture disease: prevalence and effects of urethral reconstruction. J Urol. 2015;193(1):184–189. doi: 10.1016/j.juro.2014.07.007.
- Lubahn JD, Zhao LC, Scott JF, et al. Poor quality of life in patients with urethral stricture treated with intermittent self-dilation. J Urol. 2014;191(1):143–147. doi: 10.1016/j.juro.2013.06.054.
- Breyer BN, Edwards TC, Patrick DL, et al. Comprehensive qualitative assessment of urethral stricture disease: toward the development of a patient centered outcome measure. J Urol. 2017;198(5):1113–1118. doi: 10.1016/j.juro.2017.05.077.
- Osterberg EC, Murphy G, Harris CR, et al. Cost-effective strategies for the management and treatment of urethral stricture disease. Urol Clin North Am. 2017;44(1):11–17. Feb doi: 10.1016/j.ucl.2016.08.002.
- Thomas AZ, Giri SK, Meagher D, et al. Avoidable iatrogenic complications of urethral catheterization and inadequate intern training in a tertiary-care teaching hospital. BJU Int. 2009;104(8):1109–1112. doi: 10.1111/j.1464-410X.2009.08494.x.
- Davis NF, Cunnane EM, Mooney RO, et al. Characterisation of human urethral rupture thresholds for urinary catheter inflation related injuries. J Mech Behav Biomed Mater. 2018;83:102–107. doi: 10.1016/j.jmbbm.2018.04.015.
- Davis NF, Mooney RO, Cunnane CV, et al. Preventing urethral trauma from inadvertent inflation of catheter balloon in the urethra during catheterization: evaluation of a novel safety syringe after correlating trauma with urethral distension and catheter balloon pressure. J Urol. 2015;194(4):1138–1145. doi: 10.1016/j.juro.2015.02.083.
- Davis NF, Cunnane EM, Mooney ROC, et al. Clinical evaluation of a safety-device to prevent urinary catheter inflation related injuries. Urology. 2018;115:179–183. doi: 10.1016/j.urology.2018.02.026.
- O'Connor E, Croghan S, Baird O, et al. Pd31-01 transurethral catheterisation safety valve (tucsv©) for the prevention of catheter balloon inflation injury of the urethra – A prospective multi-institutional study. J Urol. 2022;207(Suppl 5):e541.
- Husereau D, Drummond M, Petrou S, et al. Consolidated health economic evaluation reporting standards (CHEERS) statement. BMJ. 2013;346(1):f1049. doi: 10.1136/bmj.f1049.
- National Institute of Health and Care Excellence. NICE health technology evaluations: the manual. 2022 [cited 2022 June]. https://www.nice.org.uk/process/pmg36/resources/nice-health-technology-evaluations-the-manual-pdf-72286779244741.
- Kind P, Hardman G, Macran S. UK population norms for EQ-5D. 1999 [cited 2022 June]. https://www.york.ac.uk/che/pdf/DP172.pdf.
- National Health Service. NHS reference costs. 2019/20. 2021 [cited 2022 June]. https://www.england.nhs.uk/publication/2019-20-national-cost-collection-data-publication/.
- Beecham J, Curtis L. Unit costs of health and social care personal social services research unit. 2021 [cited 2022 June]. https://www.pssru.ac.uk/project-pages/unit-costs/.
- Smith DRM, Pouwels KB, Hopkins S, et al. Epidemiology and health-economic burden of urinary-catheter-associated infection in English NHS hospitals: a probabilistic modelling study. J Hosp Infect. 2019;103(1):44–54. doi: 10.1016/j.jhin.2019.04.010.
- Gage H, Avery M, Flannery C, et al. Community prevalence of long‐term urinary catheters use in England. Neurourol Urodyn. 2017;36(2):293–296. doi: 10.1002/nau.22961.
- Bayne DB, Gaither TW, Awad MA, et al. Guidelines of guidelines: a review of urethral stricture evaluation, management, and follow-up. Transl Androl Urol. 2017;6(2):288–294. Apr doi: 10.21037/tau.2017.03.55.
- Pickard R, Goulao B, Carnell S, et al. Open urethroplasty versus endoscopic urethrotomy for recurrent urethral stricture in men: the OPEN RCT. Health Technol Assess. 2020;24(61):1–110. Nov doi: 10.3310/hta24610.
- John G, Bardini C, Combescure C, et al. Urinary incontinence as a predictor of death: a systematic review and meta-analysis. PLoS One. 2016;11(7):e0158992. doi: 10.1371/journal.pone.0158992.
- Office for National Statistics. England and wales national life tables 2018–2020 [cited 2022 June]. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/datasets/mortalityratesqxbysingleyearofage
- National Institute of Health and Care Excellence. NHS Assessment Report. Implantable cardioverter defibrillators for the treatment of arrhythmias and cardiac resynchronisation therapy for the treatment of heart failure (review of TA95 and TA120). 2013 [cited 2022 July]. https://www.nice.org.uk/guidance/ta314/documents/arrythmias-icds-heart-failure-cardiac-resynchronisation-assessment-report2.
- Bermingham SL, Hodgkinson S, Wright S, et al. Intermittent self catheterisation with hydrophilic, gel reservoir, and non-coated catheters: a systematic review and cost effectiveness analysis. BMJ. 2013;346(15):e8639. doi: 10.1136/bmj.e8639.
- Constanti M, Floyd CN, Glover M, et al. Cost-Effectiveness of initiating pharmacological treatment in stage one hypertension based on 10-year cardiovascular disease risk: a Markov modeling study. Hypertension. 2021;77(2):682–691. doi: 10.1161/HYPERTENSIONAHA.120.14913.
- National Institute of Health and Care Excellence. NICE guideline [NG148]. Acute kidney injury: prevention, detection and management. 2019 [cited 2022 June]. https://www.nice.org.uk/guidance/ng148.
- Sullivan PW, Slejko JF, Sculpher MJ, et al. Catalogue of EQ-5D scores for the United Kingdom. Med Decis Making. 2011;31(6):800–804. doi: 10.1177/0272989X11401031.
- Liu C, Attar K, Gall A, et al. The relationship between bladder management and health-related quality of life in patients with spinal cord injury in the UK. Spinal Cord. 2010;48(4):319–324. doi: 10.1038/sc.2009.132.
- Ara R, Brazier J. Deriving an algorithm to convert the eight mean SF‐36 dimension scores into a mean EQ‐5D preference‐based score from published studies (where patient level data are not available). Value Health. 2008;11(7):1131–1143. doi: 10.1111/j.1524-4733.2008.00352.x.
- National Institute of Health and Care Excellence. Medtech innovation briefing [MIB116]. Urethrotech UCD for difficult or failed catheterisation. 2017 [cited 2022 June]. https://www.nice.org.uk/advice/mib116/chapter/The-technology.
- Cox E, Saramago P, Kelly J, et al. Effects of bladder cancer on UK healthcare costs and patient health-related quality of life: evidence from the BOXIT trial. Clin Genitourin Cancer. 2020;18(4):e418–e442. doi: 10.1016/j.clgc.2019.12.004.
- Guest JF, Keating T, Gould D, et al. Modelling the annual NHS costs and outcomes attributable to healthcare-associated infections in England. BMJ Open. 2020;10(1):e033367. doi: 10.1136/bmjopen-2019-033367.
- NHS Prescription Services. Drug Tariff, Part IXA (Appliances). 2021 [cited 2022 June]. https://www.nhsbsa.nhs.uk/pharmacies-gp-practices-and-appliance-contractors/drug-tariff.
- National Institute of Health and Care Excellence. Medtech innovation briefing [MIB241]. Optilume for anterior urethral strictures. 2021 [cited 2022 June]. https://www.nice.org.uk/advice/mib241.
- National Institute of Health and Care Excellence. Medtech innovation briefing [MIB210]. TUC safety valve to prevent balloon inflation in the urethra during transurethral catheterisation. 2020 [cited 2022 June]. https://www.nice.org.uk/advice/mib210.
- NHS Greater Glasgow and Clyde. NHS greater glasgow urology formulary. 2019 [cited 2022 July]. https://ggcmedicines.org.uk/media/uploads/other_formularies/ggc_adtc_urology_formulary_26_november__2019_final.pdf.