Abstract
Aim
To assess, from a United States (US) payer’s perspective, the cost-effectiveness of gels designed to separate the endometrial surfaces (intrauterine spacers) placed following intrauterine surgery.
Materials and methods
A decision tree model was developed to estimate the cost-effectiveness of intrauterine spacers used to facilitate endometrial repair and prevent the formation (primary prevention) and reformation (secondary prevention) of intrauterine adhesions (IUAs) and associated pregnancy- and birth-related adverse outcomes. Event rates and costs were extrapolated from data available in the existing literature. Sensitivity analyses were conducted to corroborate the base case results.
Results
In this model, using intrauterine spacers for adhesion prevention led to net cost savings for US payers of $2,905 per patient over a 3.5-year time horizon. These savings were driven by the direct benefit of preventing procedures associated with IUA formation ($2,162 net savings) and the indirect benefit of preventing pregnancy-related complications often associated with IUA formation ($3,002). These factors offset the incremental cost of intrauterine spacer use of $1,539 based on an assumed price of $1,800 and the related increase in normal deliveries of $931. Model outcomes were sensitive to the probability of preterm and normal deliveries. Budget impact analyses show overall cost savings of $19.96 per initial member within a US healthcare plan, translating to $20 million over a 5-year time horizon for a one-million-member plan.
Limitations
There are no available data on the effects of intrauterine spacers or IUAs on patients’ quality of life. Resultingly, the model could not evaluate patients’ utility related to treatment with or without intrauterine spacers and instead focused on costs and events avoided.
Conclusion
This analysis robustly demonstrated that intrauterine spacers would be cost-saving to healthcare payers, including both per-patient and per-plan member, through a reduction in IUAs and improvements to patients’ pregnancy-related outcomes.
PLAIN LANGUAGE SUMMARY
Every year, women in the United States (US) undergo surgery to treat intrauterine abnormalities to maintain or improve the uterus’ ability to support fetal development and result in a term delivery. Despite the benefits of these procedures, damage caused to the endometrium (uterine lining) is associated with a risk of adherence of the endometrial cavity surfaces with scar tissue known as intrauterine adhesions (IUAs).
Damage to the endometrium and the resulting IUAs may be associated with infertility, light or absent menstruation, pregnancy loss, and other pregnancy-related complications. Treating these conditions within the US healthcare system consumes resources and adds costs for healthcare payers (public and private insurance providers).
To facilitate endometrial repair and to reduce or prevent IUAs, researchers have developed materials to place within the endometrial cavity following surgery to separate the endometrial surfaces during the early healing period. These intrauterine “spacers” are intended to improve patients’ subsequent clinical outcomes and save money for healthcare payers. It is unknown whether these improved clinical outcomes offset the cost of the routine use of spacers following “at-risk” procedures that involve the endometrial cavity.
We developed a model designed to determine the cost-effectiveness of an intrauterine spacer by quantifying improvements in clinical outcomes and the resultant cost savings for patients undergoing uterine surgeries with or without spacers. Our model predicted that routinely using such spacers following at-risk procedures would improve patient outcomes and reduce costs to US payers.
Introduction
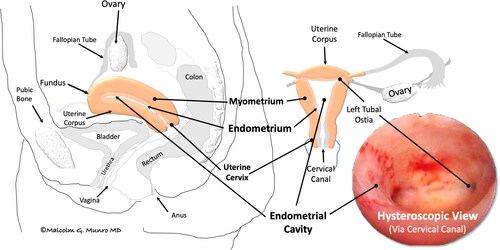
Surgical procedures involving the endometrial cavity can damage the basal layers of the endothelial linings, leading to scar formation, including the development of endocervical and/or intrauterine adhesions (IUAs). These adhesions joining the opposing epithelial surfaces, the endometrium in the endometrial cavity, and the columnar layer in the cervix contribute to developing a spectrum of symptoms and findings collectively known as Asherman SyndromeCitation1–3. The terms used to describe the uterine anatomy, and its potential disorders and treatments are defined in Supplementary Table 1 and the uterine anatomy is shown in .
Figure 1. Simplified uterine anatomy and physiology. Depicted is the normally pear-shaped uterus situated anterior to (in front of) the colon, posterior to (behind) the bladder, and attached to the vagina. The hollow organ includes a corpus, primarily comprising specialized muscle (myometrium), lined by a layer of tissue called the endometrium, and a cervix, connecting the endometrial cavity to the vagina via the cervical canal. After conception, the embryo is transferred via the fallopian tube to the endometrial cavity, where it attaches to and is then enveloped by the endometrium, where, as a fetus, it develops until reaching maturity. At that point, in the process of labor, it is expelled from the endometrial cavity by dilation of the cervical canal and contractions of the muscular uterine corpus. If a pregnancy does not occur, the superficial portion of the endometrium, the functionalis, is discharged during menstruation.
Different IUA phenotypes are associated with various conditions, the most common of which are amenorrhea, light or irregular menstruation, infertility, recurrent miscarriages, and pregnancy-related complicationsCitation2,Citation4,Citation5. Among infertile patients, the prevalence of IUAs is estimated to be approximately 4.6%Citation6. Hooker et al. reported an IUA incidence of 21.2% of those who had undergone surgical termination of pregnancy in the first trimester, while the rate following second-trimester termination was 16.1%Citation7.
When adhesions form in the endometrial cavity following surgical procedures, they are known as primary IUAs. The standard treatment for IUAs is hysteroscopic lysis of adhesions (LOA), a surgical procedure conducted within the endometrial cavity and occasionally the cervical canal involving transection of the adhesions under direct visualization with the goal of restoring a normal configurationCitation4. When adhesions reform in the endometrial cavity after LOA, they are known as secondary IUAs. The rate of secondary IUAs has been reported to range from 3% to 62.5%Citation2,Citation4,Citation8,Citation9.
Studies have demonstrated a considerable economic burden associated with LOA. Sikirica et al. reported 351,777 hospitalizations in the United States (US) in 2005 for LOA, accounting for 967,332 hospital daysCitation10. The estimated number of inpatient days attributed to LOA as a secondary procedure was 270,245Citation10. The burden related to IUAs likely extends significantly beyond this estimate, which covers LOA alone, without considering pregnancy-related costs.
There are no currently approved products validated for safe and effective prevention of IUAs in the US. To reduce the risk or severity of IUA formation after at-risk procedures, researchers have investigated systemic pharmacologic agents and agents for placement within the endometrial cavity following surgery, including medical devices, gels, and biologic agents. Medical devices used to prevent adhesion formation include intrauterine contraceptive devices (IUDs) and modified Foley catheter balloons, each of which have all been used off-label. Gel-based intrauterine spacers are often composed of physically cross-linked hyaluronic acid. Intrauterine biologics described include amnion grafts, stem cells, and platelet-rich plasma. These approaches aim to facilitate endometrial repair and prevention of adhesions by separating the damaged endometrial surfaces or to facilitate the generation of endometrial cells during the healing processCitation11–14. Pharmacologic agents, primarily estrogens, often combined with progestins but also including antibiotics, are also intended to prevent or reduce the severity of adhesions without physically separating the endometrial cavity surfacesCitation2,Citation12,Citation14.
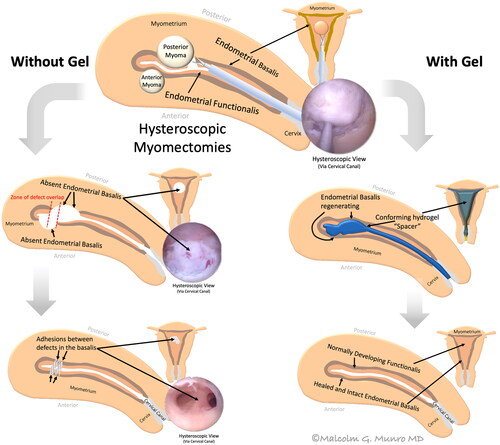
Modified Foley catheter balloons have been placed within the endometrial cavity to act as spacers during the early post-operative healing stage. These spherical balloons have limitations as they will not conform to the roughly triangular shape of the endometrial cavity and must be removed in a subsequent procedure. Compared with Foley balloons, gel-based intrauterine spacers conform to the endometrial cavity to act as a “conforming spacer” to prevent the formation of IUAs in areas that are not separated using balloons (e.g. ostial and fundal regions as well as the lower segmentCitation15). displays a hypothetical mechanism of adhesion formation and the postulated mechanism of adhesion prevention through the introduction of a gel-based intrauterine spacer.
Figure 2. Conceptualized genesis and prevention of intrauterine adhesions following hysteroscopic multiple myomectomy. The hysteroscopic myomectomy is depicted in the top center image with dissection into the pseudocapsule between the leiomyoma and the myometrium. (Left) The two leiomyomas have been removed with overlapping defects that facilitate the formation of intrauterine adhesions (IUAs) while preventing healing of the endometrial basalis. (Right) A hydrogel instilled into the endometrial cavity acts as a conforming “spacer,” separating the defects in a way and for a duration of time that facilitates healing of the endometrium’s basal layer, thereby preventing the formation of IUAs.
The intrauterine instillation of hyaluronic acid-derived auto-cross-linked polysaccharide (ACP) gel as a physically cross-linked conforming spacer following LOA demonstrated a significant reduction of secondary IUAs compared with those who received no intrauterine spacer (14% vs. 32%, p < .05)Citation16. Additionally, a meta-analysis by Fei et al. demonstrated that immediate post-procedure instillation of hyaluronic acid gel after the evacuation of retained products of conception reduced the incidence of postoperative IUAs (risk ratio [RR]: 0.44, p = .0001)Citation17. This study also corroborated the effectiveness of a conforming hyaluronic acid gel spacer in significantly increasing subsequent pregnancy rates (RR: 1.94, p = .00001)Citation17. Similarly, Mao et al. found that 26% of women experienced spontaneous pregnancy after receiving ACP gel post-adhesiolysis, compared with 15% of women who underwent LOA without an intrauterine spacerCitation18. Following dilation and curettage (D&C), the rates of miscarriage were 17% lower with the use of ACPs of hyaluronic acid compared to those without gel (21% vs. 38%, p = .081)Citation19.
Despite the evidence demonstrating the efficacy of adjuvant treatments like intrauterine spacers, to date, none have been approved by the US Food and Drug Administration (FDA) for preventing IUA formation following procedures involving the endometrial cavityCitation20,Citation21. The real-world use and cost-effectiveness of these adjuvants in the US health system is undocumented.
Ideally, an intrauterine spacer should conform to the various sizes and irregular shapes of the patient’s endometrial cavity, and it should remain in place long enough to facilitate independent post-surgical healing of the endometrial surfaces, the latter which can be referred to as “residence time”. The use of Foley balloons is reported for a planned residence time of 7–10 days before removal. The residence time of physically cross-linked gels following an intrauterine procedure is unknown due to the nature of physically cross-linked gels to not solidify and can be rapidly expelled from the endometrial cavity.
Recent novel covalently cross-linked hydrogel spacers have been developed that combine the conforming properties of gels and a known residence time of 2–3 weeks, which is specifically aligned with known tissue healing processesCitation21. Clinical trials are underway to evaluate the efficacy of such a covalently cross-linked conforming hydrogel intrauterine spacer intended for the prevention/minimization of IUAs, which may ultimately support the FDA’s approvalCitation21. In the absence of available data regarding this novel hydrogel spacer, in the design of this model, we opted to focus on in-situ forming physically cross-linked conforming gel spacers, regardless of their unknown residence time, as the data for these conforming intrauterine spacers represent an effective strategy for preventing the formation of IUAs following surgeryCitation16,Citation17.
The primary objective of this analysis was to estimate the cost-effectiveness of conforming spacers if used within the US healthcare system. The clinical outcome estimation was based on the available literature regarding the impact of conforming spacers on the incidence and recurrence of IUAs and fertility and pregnancy-related outcomes. Consequently, such an analysis compares predicted patient expenditures with and without routine use of endometrial cavity spacers in at-risk procedures. The outcomes of interest included the predicted number of events experienced and projected costs associated with subsequent LOA due to IUA formation and the potential impact of intrauterine trauma as reflected by IUA on fertility and pregnancy outcomes. Whereas a cost-minimization approach would assume equal efficacy of treatment with or without intrauterine spacers, this analysis used data from the available literature to inform different efficacies for each treatment approach. Therefore, this cost-effectiveness analysis (CEA) is designed to aid healthcare payers’ and providers’ decisions regarding the reimbursement of conforming intrauterine spacers should they come to market. Additionally, the model evaluates the potential clinical benefit of using conforming intrauterine spacers with extended residence time that can be used to establish these as a breakthrough intervention in the IUA disease paradigm.
Methods
Model overview
The economic model was designed to assess the cost-effectiveness of conforming intrauterine spacers among patients at risk of developing IUAs following primary intrauterine procedures (e.g. without prior adhesions). We utilized a decision tree model framework, an algorithm that analyzes different clinical pathways for patients undergoing primary procedures. A decision tree is defined by three different types of nodes: chance node (risk of developing IUA), decision node (choice of treatment), and end node (clinical outcomes). This framework allowed a direct comparison between the intervention and the comparator arms. Further, our model estimated the clinical benefits of the intervention arm.
The analysis was conducted from a US payer perspective over a simulated three-and-a-half-year timeframe. This time horizon was considered sufficient to capture the majority of costs and events related to IUAs, including those pertaining to follow-up treatments, multiple pregnancy attempts, and risks of miscarriage and pregnancy-related complicationsCitation19. No discounting was applied to the costs nor health outcomes, as the short time horizon of this analysis was thought to render this adjustment unmeaningful. This analysis considered direct healthcare costs associated with IUA treatment, including gel acquisition, procedure, diagnostic, and pregnancy-related costs. All cost inputs were determined using the existing literature and online databases. Costs were inflated to 2022 United States Dollars (USD), the latest year for which complete data were available, using the medical care component of the consumer price indexCitation22. The model was run using a specific set of inputs and settings in the base case, and alternate settings were explored via scenario and sensitivity analysis. As described in the Scenario and Sensitivity Analysis section, these settings included an alternate time horizon, treatment population, and other factors affecting costs.
Model structure
To capture the relevant clinical pathways and health outcomes for patients undergoing primary and secondary procedures, we utilized a decision tree model framework that evaluates patients who receive one of the following two treatments: procedures that affect the endometrial cavity with a conforming intrauterine spacer intervention (intervention arm), or procedures that affect the endometrial cavity without a conforming spacer intervention (comparator arm). All assumptions related to patient treatment pathways were validated using a survey of multiple physician subject matter experts. This survey was designed to be geographically representative of the US and therefore selected eight physicians with direct experience in diagnosing and treating patients with fertility issues and IUAs.
As shown in , patients modeled not to develop clinically significant IUAs after a primary procedure within the endometrial cavity pursue pregnancy and non-pregnancy-related clinical outcomes. Conversely, patients modeled with IUA-related infertility after a primary procedure were postulated to undergo one or more (up to 3) rounds of secondary LOA procedures to restore the baseline configuration of the endometrial cavity and functionality of the endometrium.
Figure 3. Decision tree model framework. Abbreviations. IUA, intrauterine adhesion; LOA, lysis of adhesions
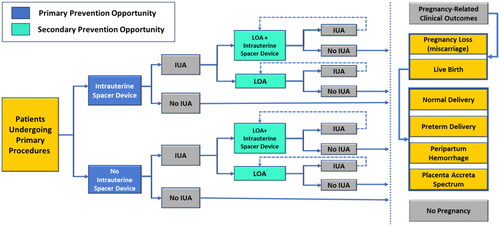
The model assumes equal randomization at each decision node. This assumption allows patients who would or would not receive conforming intrauterine spacers following the primary procedure to have a chance of receiving them following the secondary procedure. As presented in , the decision tree culminates in two broad clinical outcomes: pregnancy and no pregnancy. The modeled pregnant patients could experience miscarriage, a successful term live birth with a normal peri-delivery experience, a live birth from preterm delivery, and/or other pregnancy-related complications such as placenta accreta spectrum (PAS) and peripartum hemorrhage, as seen in . Consequently, results in our analysis are measured in terms of total incremental costs and the number of events experienced by patients in each treatment arm.
Study population
The model study population included premenopausal women (aged 18–44) undergoing a primary procedure. This group, the primary prevention population, represents those at risk of developing IUAs without any prior incidence of IUA. Additionally, we report outcomes for a modeled subgroup of patients previously diagnosed with IUA who would undergo LOA with or without conforming intrauterine spacers. This subgroup of patients is referred to as the secondary prevention population as they would be at risk of reoccurrence of IUA.
Intervention and comparator
Model outcomes were calculated for the intervention and the comparator arms. The intervention arm included patients undergoing transcervical procedures receiving adjuvant treatment with an intrauterine spacer following surgery. In contrast, patients within the comparator arm were modeled to undergo transcervical procedures without adjuvant therapy.
Patient outcomes
Considering the study population of premenopausal women, the model evaluated pregnancy-related outcomes, such as the likelihood of a viable pregnancy and live birth. The model time horizon of three-and-a-half-years allowed each modeled patient to experience multiple pregnancy-related outcomes. Therefore, the outcomes were non-mutually exclusive and measured in terms of events per patient. Live birth as an outcome was further stratified into normal delivery, defined as full-term birth without complications, and preterm birth, defined as delivery before 37 weeks of gestation. The analysis also included the risk of a miscarriage, wherein a patient could undergo multiple attempts before achieving a successful pregnancy and delivery.
The model also included other critical clinical events that could occur during and around parturition that are potentially reduced with the routine use of intrauterine spacers. These events include preterm births (defined as occurring before), peripartum hemorrhage (defined as blood loss of greater than 500 ml for a vaginal delivery and greater than 1000 ml for cesarean deliveryCitation23) and PASCitation24. PAS is an umbrella term for various degrees of abnormal placental attachment to the myometrium related to trauma-related defects in the integrity of the endometrium, a circumstance highly correlated to peripartum hemorrhageCitation24. Patients with PAS were considered to include those with placenta previa, a condition in which the placenta implants over or near the cervix, impeding normal delivery and posing a risk of peripartum hemorrhage.
Efficacy-related inputs
The efficacy inputs utilized in our analysis were derived through a meta-analysis of randomized control trials (RCTs), identified using a systematic literature review (SLR). The search component of this SLR was conducted using terms for the patient population, intervention, and outcomes of interest. This led to the identification of 2,214 unique articles reporting on outcomes of patients undergoing intrauterine surgeries. Two independent reviewers subsequently screened these articles according to guidelines laid out by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) organizationCitation25. A subset of the SLR-identified articles included following screening (8 studies) was deemed suitable to inform efficacy inputs of this study, which assessed the incidence and recurrence of IUAs following transcervical surgeries and LOA, respectively, and compared patients treated with conforming intrauterine spacers to those treated without adjuvants, using an RCT design.
We then applied meta-analyses to derive the efficacy of these spacers compared to no treatment for the primary and secondary prevention of IUAs. The available literature on conforming intrauterine spacers comprised six studies of hyaluronic acid gels, which were included in the primary prevention analysisCitation26–31. Two studies were included for the secondary prevention analysis that compared the efficacy of hyaluronic acid gel with no adjuvant therapyCitation16,Citation18. General details regarding the included studies are presented in Supplementary Table 2.
After selecting sufficiently homogenous RCTs in terms of study design, patient population, and outcomes evaluated, meta-analyses were applied. First, a proportional meta-analysis was conducted to derive a pooled incidence and recurrence rate of IUAs for the comparator arm (without an intrauterine spacer). In contrast, the efficacy of conforming intrauterine spacers was estimated using the relative risk ratio obtained via a comparative meta-analysis of the included studies. The analysis was conducted in R Studio version 4.2.2. The probability inputs used in the model are presented in below.
Table 1. Probability inputs.
Pregnancy-related efficacy inputs
Pregnancy-related efficacy inputs were sought to inform the number of events per patient for each event described in the Patient Outcomes section. Pregnancy-related parameters are presented in and were informed by a long-term follow-up study of patients who underwent D&C post-miscarriage conducted by Hooker et al.Citation19. That study was identified through the SLR above and was selected for use in this model due to the investigators’ long-term follow-up and reporting of pregnancy. These outcomes interest US payers since they reflect the use or non-use of a conforming intrauterine spacer in a relevant population of women aged 18–44Citation19.
Patients in the Hooker et al. study underwent a follow-up hysteroscopy 8–12 weeks after their primary procedure, D&C, to assess whether IUAs were present. Patients diagnosed with IUAs then underwent LOA procedures to improve the chance of conception and normal pregnancy. Over the following three and a half years, pregnancy outcomes (such as miscarriages, premature births, normal delivery, and peripartum hemorrhage) were evaluated. Due to the similarity between this real-world clinical practice and the model described herein, the results published by Hooker et al. were used directly as inputs for the current analysisCitation19.
Cost inputs
In alignment with the US payer perspective, the model considered direct healthcare costs to US payers that could be influenced by intrauterine spacers, patients’ subsequent probability of developing IUAs, and costs associated with pregnancy-related outcomes. This analysis assumed that each patient’s payer would cover the cost of each spacer. Resource use estimates and assumptions related to patient treatment pathways were validated by multiple physician subject matter experts with direct experience in diagnosing and treating patients with IUAs.
Patients modeled to develop clinically significant IUAs following a primary transcervical procedure were assumed to experience infertility and would undergo several diagnostic tests before discovering IUAs. Multiple physician subject matter experts informed these to include a visit to a reproductive endocrinology and infertility specialist for all patients with IUAs, as well as other tests that may be conducted in some cases, with the proportion of patients undergoing each of these tests informed by the survey mentioned above of subject matter experts. These additional tests include hysterosalpingogram, sonohysterography, hysteroscopy, and a large variety of laboratory tests. The complete list of these tests and their unit costs are included in .
Table 2. Unit costs and resource use inputs.
Any identified IUAs would then be treated with LOA, after which the patients undergo hysteroscopy to assess whether IUAs were stably eliminated or had recurred. If IUAs were modeled to recur, repeat LOA could be performed to a maximum of three LOA procedures (if IUAs recur repetitively), after which all IUAs were considered to be fully transected with a normal appearing endometrial cavity.
Patients within the conforming spacer group were modeled to incur the cost of using a spacer, as did any patients who received a spacer in subsequent LOA procedures. Finally, the model included pregnancy-related costs associated with each of the pregnancy-related events included in the model. A key cost input, the cost of preterm delivery, was calculated as the sum of a normal delivery, published by Rae et al.Citation33, and the additional costs of newborns within their first six months of life, based on a claims data analysis by Beam et al.Citation34. The cost of PAS was calculated according to the costs reported by Han et al. for PAS with and without placenta previaCitation35, based on the proportion of patients with placenta previa (3/23) reported by Tavcar et al.Citation32. The cost of peripartum hemorrhage was calculated to be $28,438 per occurrence. It included the cost for the required procedure (extravasation)Citation36, anesthesia (for one surgery)Citation37, blood transfusion (4 units)Citation38, and an ICU stay of 3 daysCitation39, each inflated to 2022 values. It was assumed that patients without pregnancy did not incur any pregnancy-related costs. The inputs related to unit costs and resource use are presented in .
Sensitivity and scenario analysis
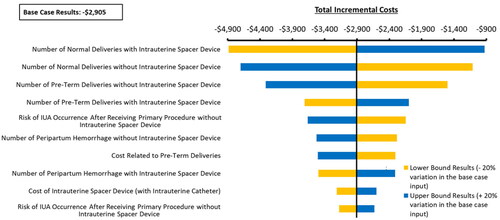
The model was also used to conduct additional analyses to corroborate the findings from the base case results, including one-way sensitivity analysis (OWSA) and several scenario analyses. For the OWSA, each model parameter was varied by ± 20% of the corresponding base case value to estimate the lower and upper bound values of the model inputs. The model was then run using these values to obtain the lower and upper bounds of the model results, respectively. The top 10 model parameters that yielded the greatest variation from the base case results are presented using a tornado diagram in the Model outcomes section.
Additionally, through scenario analyses, alternate model inputs and settings were used to estimate the cost-effectiveness of conforming intrauterine spacers for US payers in different scenarios applicable in the real world. Several scenarios were implemented in the model and are presented in . Finally, a pricing analysis was conducted to derive the base case and scenario results while varying the unit cost per spacer within the model.
Table 3. Scenario analysis description.
Budget impact analysis
A budget impact analysis (BIA) was conducted to present the monetary impact of conforming intrauterine spacers on the overall cost of treating IUAs. This BIA extrapolated cost outcomes from the CEA to a hypothetical national health plan of one million covered persons. The analysis was run over a time horizon of 5 years. As shown in Supplementary Figure 1, the model assumed a plan population of 1,000,000 patients, and the population was set to grow at 0.51% per year to approximate the growth rate of the US populationCitation65. The study population of premenopausal women undergoing transcervical procedures mirrored that of the CEA, as did the treatment options: transcervical procedures conducted with and without post-procedure placement of intrauterine spacers.
Relevant costs of the spacer, procedure, diagnostics, and pregnancy-related costs were included and were derived from the per-patient CEA results. These modeled costs were then calculated over five years and measured per one-million initial member plan and initially enrolled plan member, assuming that the plan would continue to treat the same proportion of its members each year and that plan growth would mirror that of the general US population.
Costs were calculated separately for the scenarios in which patients did or did not use a conforming intrauterine spacer, and the difference was reported as the budget impact. The former scenario assumed the routine use of intrauterine spacers following at-risk intrauterine procedures. In contrast, the latter assumed no primary usage of intrauterine spacers, although patients in both scenarios were assumed to receive the spacers in 50% of LOA procedures after their initial transcervical procedure. As the total costs in each scenario were calculated based on these 100% or 0% market shares, the results herein represent the maximum possible budget impact given the parameter values used. Epidemiological and cost inputs used for the BIA are presented in Supplementary Table 3 and 4, respectively.
Model outcomes
Base case analysis
The base case analysis demonstrates that post-procedure use of a conforming intrauterine spacer improved various patient outcomes. This could be expected from greater efficacy values for spacers compared with no adjuvant treatments (). Following intrauterine procedures, patients modeled to have an intrauterine spacer positioned had substantially fewer miscarriages (166 fewer per 1000 patients) and certain pregnancy-related complications (5 and 33 fewer for peripartum hemorrhage and preterm delivery, respectively). Conversely, patients treated with post-procedure spacers experienced more normal deliveries and live births than those without such spacers (73 and 40 more, respectively).
Table 4. Base case results – primary population.
Intrauterine spacers in the primary prevention population resulted in an overall cost savings of $2,905 per patient (). These cost savings were primarily driven by the direct benefit of preventing IUAs ($2,162 net savings) and the indirect benefit of preventing preterm births ($3,002). These factors offset the incremental cost associated with more normal deliveries in the intrauterine spacer arm ($931) and the incremental cost of the spacers ($1,539). As patients in each arm could use intrauterine spacers in LOA procedures beyond their primary transcervical procedure ($1,800 per use), those on the spacer arm accumulated greater than $1,800 in intrauterine spacer costs on average, and those on the comparator arm also accumulated some cost of intrauterine spacer use for LOA.
Incremental costs relating to pregnancy losses, and deliveries were generally lower for patients treated with spacers (cost per occurrence is described in the Cost Inputs section). Peripartum hemorrhage had minimal effect on total incremental costs when comparing patients treated with and without conforming intrauterine spacers. Incremental differences in the costs of pregnancy losses were minimal due to a relatively low cost per miscarriage, as most miscarriages do not require procedural treatment. Although the costs of PAS and peripartum hemorrhage are relatively high ($10,342 and $28,438, respectively), incremental differences in the frequency of these complications were minimal (difference of 4 and −5 events per 1,000 patients, respectively, comparing patients treated with and without conforming intrauterine spacers). Consequently, the incremental costs of PAS and peripartum hemorrhage were relatively low ($45 and −$139, respectively).
One-way sensitivity analysis
We found that the total incremental cost savings from the routine use of conforming intrauterine spacers ranged from $4,886 to $925, within the range of sensitivity analyses conducted. This is presented as a tornado diagram in . The most influential parameters impacting the model results include the number of normal deliveries with and without intrauterine spacers, the number of preterm deliveries, and the risk of IUA occurrence post-transcervical procedures without the use of spacers.
Scenario analysis
presents the total incremental cost results when comparing patients modeled to receive or not receive an intrauterine spacer following primary procedures under the various scenarios. As presented, the unit cost of an intrauterine spacer was varied to validate the model outcomes against potential unit cost variability. Across the scenarios and tested unit costs, the results tend to favor conforming spacers and range from −$3,510 to $1,154. In scenarios with positive incremental cost results, these are lower than the costs of the intrauterine spacer, providing a partial offset of the spacer cost. Using the base case cost of $1,800, the scenario that assumes a high proportion of patients receiving miscarriage surgery (see ) has the most favorable results for patients treated with spacers (cost savings of $2,997). In contrast, the scenario evaluating the secondary population while assuming equal efficacy (in terms of pregnancy-related outcomes) across the treatment arms yielded the least favorable results for spacer-treated patients (incremental cost of $590).
Table 5. Scenario analyses.
Budget impact analysis outcomes
The calculation of the number of patients undergoing primary procedures eligible for treatment with a conforming intrauterine spacer in 2024 is presented in Supplementary Figure 1, which estimated 1,360 patients within a hypothetical one-million-member plan to undergo primary procedures in the first year, with the treated population growing slightly in each subsequent year. Based on the number of treated patients within a plan and a cost savings of $2,905 per patient derived from the CEA, the BIA results predict that the routine use of an intrauterine spacer following primary procedures would lead to a five-year overall cost savings of $19.96 per initial plan member (Supplementary Table 5). This translates to a cost savings of approximately $20 million over a 5-year time horizon for a plan that begins in 2024 (the first year of the BIA) with one million members, based on the assumptions described in the corresponding Methods section. The relatively low budget impact associated with the per-plan member measurement is expected as most plan members do not undergo intrauterine procedures.
As these results extrapolate the cost-effectiveness results, cost savings originate from the same factors identified within the CEA. Namely, treatment with intrauterine spacers was associated with a predicted reduced cost of LOA and diagnostics (by $1,295 and $867 per treated patient in the first year, respectively), as well as a reduced cost associated with lower incidence of preterm births ($3,002 per treated patient in the first year) when compared to primary intrauterine surgery without intrauterine spacers. These savings to US payers are predicted to grow slightly each year in alignment with the predicted US population growth, to an expected savings of $2,905 per current patient in the fifth year, compared with a first-year savings of $2,965 per current patient.
Discussion
IUAs are a major contributor to recurrent pregnancy loss, infertility, and pregnancy-related complicationsCitation4. They are commonly caused by intrauterine surgical trauma, which may lead to partial or complete closure of the endometrial cavity. Patients undergoing procedures involving the endometrial cavity, such as myomectomy, septum transection, surgical removal of retained products of conception, and D&C, among others, are at a high risk of developing IUAs, which can have substantial effects on women’s reproductive health. Although post-surgical adhesions are commonly treated with LOA, recurrence of these adhesions remains a significant problem. A patient may require repeat LOA before the endometrial cavity is adhesion-freeCitation9. The average recurrence rate post-LOA is 28.7%Citation9 which in severe IUAs can be as high as 62.5%Citation66.
The prevention of adhesions appears to be an ideal treatment strategy. As described earlier, various adjuvant therapies have been used following surgical procedures to prevent the occurrence and recurrence of IUAs. Several studies combining the findings from RCTs have demonstrated the efficacy of adhesion spacers in preventing IUAs (RR: 0.68, 95% CI: 0.53–0.86 p = .03) and improving pregnancy-related outcomes in patients (RR: 0.99, 95% CI: 0.46–2.13, p = .98)Citation67–69. However, the evidence from these studies were derived from RCTs conducted with relatively small sample sizes and therefore additional evidence is necessary to validate these conclusions. This analysis assesses the effectiveness of intrauterine spacers from an economic perspective, considering both the cost and improvements to health-related outcomes associated with intrauterine spacers compared with no treatment, thereby evaluating their cost-effectiveness in treating IUAs from a payer’s perspective. As this model-based analysis predicted that the use of conforming spacers facilitates the reduction of costs and detrimental clinical outcomes, these events were reported separately only, rather than in the conventional mode, as a ratio of cost per event averted.
The base case analysis revealed that the use of conforming intrauterine spacers resulted in an estimated cost-savings of $2,905 per patient, which is primarily attributable to a reduced cost of preterm deliveries, with additional savings from reduced costs of miscarriage and peripartum hemorrhage. The model also demonstrated improved pregnancy outcomes for patients in the intrauterine spacer arm with more live births and normal deliveries (40 and 73 per 1000 patients treated with and without intrauterine spacers, respectively). Patients undergoing transcervical surgeries with spacers also tend to avoid pregnancy-related complicationsCitation18,Citation19, which could significantly reduce the overall economic burden of IUAs on the healthcare system, as evidenced by the results of the present analysis.
Sensitivity analyses revealed that the model results are most sensitive to the number of preterm and normal deliveries in each treatment arm, the incidence of IUAs following transcervical procedures, and the cost of preterm deliveries. This outcome can be expected, as the cost of preterm deliveries is far higher than that of any other outcome evaluated in the model ($90,016 per preterm delivery, compared with $28,438 per peripartum hemorrhage, the next most costly outcome).
Additional scenarios were implemented in the model to evaluate the outcomes using alternate model settings, which help to represent alternative real-world scenarios or methods of determining cost-effectiveness for payers. Using a short-term, six-month time horizon, the analysis determined that the cost savings associated with intrauterine spacers in primary procedures would be $705 per patient. This is primarily due to the short follow-up time, which did not include the costs incurred for birth-related outcomes such as preterm delivery, which are favorable for patients treated with intrauterine spacers compared to those treated without spacers.
The scenario evaluating the secondary prevention population had a cost savings of $1,691 per patient, supporting the clinical viability of intrauterine spacers in the secondary prevention of IUAs. However, a limitation of this scenario was that pregnancy-related outcomes were informed by the same study used to inform these outcomes following primary procedures wherein an intrauterine spacer (or no spacer) was used. This was necessary, as no study was found comparing patients who underwent LOA with or without intrauterine spacers and also reported on pregnancy-related outcomes. To assess the impact of this assumption, an alternative version of the secondary prevention scenario was implemented, wherein patients had identical pregnancy outcomes on each arm (intrauterine spacer vs. no spacer). In this case, cost differences were reduced in comparison to the base case or the prior secondary prevention scenario, and the scenario resulted in an incremental cost of $590.
The model outcomes were consistent when considering variability in unit cost per intrauterine spacer. Even at the highest unit cost tested, the model yielded overall cost savings. Based on the BIA, the addition of intrauterine spacers in the treatment mix of IUAs is estimated to yield cost savings of $14,674 over five years per currently enrolled insurance plan patient undergoing a primary procedure, assuming growth proportional to the US population. As observed in the CEA, the greater costs of spacers are offset by the cost savings associated with improved pregnancy-related outcomes and lower recurrence rates of IUAs, leading to savings at the US payer level.
This analysis faced several limitations, including a lack of data within the literature about the impact of IUAs on the patient’s quality of life (QoL). IUAs are not life-threatening but may significantly impact a patient’s QoL from pain, pregnancy loss, and menstrual abnormalities. However, multiple factors (such as in vitro fertilization treatment, partner fertility, etc.) may impact clinical outcomes, which cannot be controlled for in a clinical study; hence, there is a shortage of studies evaluating adhesions in this domain. Further research is needed to quantify the effects of adjuvant therapies, IUAs, and the clinical outcomes captured by this model on patients’ QoL.
Given the lack of approved products in the US, the effectiveness of the spacers was derived from a meta-analysis of RCTs conducted in other geographical settings (primarily in Europe and Asia). The model does not compare intrauterine spacers with other adjuvant therapies, such as nonconforming spacers (balloons), IUDs, hormone therapy, cell therapy, and amniotic tissues. Future analyses could be undertaken to evaluate the effectiveness of intrauterine spacers relative to alternative therapies, which may help guide clinicians and payers as to which adjuvant therapies should be applied. This study was conducted only from a payer’s perspective and therefore included only direct healthcare costs borne by the payer. Including indirect costs, such as those related to productivity losses, would help to determine the overall economic burden of IUAs on society.
Strengths of this analysis include the holistic model design, which includes all relevant costs and clinical outcomes related to IUAs and the use of intrauterine spacers to accurately capture the disease burden from a payer’s perspective. The model structure effectively includes several subsequent events and consequences to ensure the disease was well captured, and that patient treatment pathways within the model apply to the real world. The efficacy parameters related to the risk of adhesions and recurrence were derived using meta-analyses by combining the findings from multiple RCTs, thus improving the robustness of our estimation of efficacy parameter values and reducing the potential for bias in the model outcomes stemming from these efficacies.
Sensitivity analyses were conducted to analyze the consistency of model outcomes by varying the model inputs at their respective lower- and upper-bound values. To incorporate alternate settings applicable in the real world and further corroborate the model findings, scenario analyses were implemented, most of which resulted in little change to model results, demonstrating their robustness across different circumstances. Finally, the assumptions related to determining model inputs were informed using a thorough survey of practicing surgeons to ensure these were aligned with the clinical practice.
This model is the first published analysis of the potential cost-effectiveness of conforming intrauterine spacers. Overall, the findings herein support the cost-effectiveness of such spacers for primary and secondary prevention of IUAs and related complications in the US from a payer perspective. However, further research is needed to verify the applicability of the results to other countries. This can be achieved by using location-specific model inputs and settings. Given the disease burden and lack of approved therapies with established efficacy, there is a clear unmet need for effective treatments that reduce the risk of IUAs following surgeries conducted within the endometrial cavity, including LOA for existing IUAs.
In the US, there is a single ongoing, randomized controlled study designed to evaluate the effectiveness of a novel gel adjuvant in reducing the incidence/recurrence and severity of IUACitation21. The application of this model to the trial above and other future trials will rely upon similarity in efficacy to hyaluronic acid gels evaluated herein. Assumptions are also required to connect trial results reporting reduction in IUA formation to improvement in pregnancy- and birth-related outcomes. As the strongest driver of cost savings in the model described herein, a reduction in the number of preterm births is a key factor in the ability of a spacer to achieve cost-effectiveness. Long-term clinical trials or real-world evidence studies will be necessary to demonstrate this connection and verify the cost-effectiveness predicted by this model. If approved, this will be the first adjuvant therapy for IUA prevention available in the US. Assuming similar or improved efficacy to previously published compared data on gels containing hyaluronic acid (the subject of the current analysis), the model and resulting predictions described herein suggest that approving and adopting such adjuvant therapies may lead to substantial cost savings and a beneficial budget impact for US payers.
Conclusion
A cost-effectiveness model was developed to evaluate conforming intrauterine spacers for preventing IUAs from a US payer perspective over a 3.5-year time horizon. The model results presented herein suggest that the routine use of conforming intrauterine spacers following at-risk intrauterine procedures would be associated with an overall cost savings of $2,905 per patient, primarily due to reduced occurrence of IUAs and preterm births. The analysis also demonstrated improved patient outcomes for those treated with spacers, reflected in more pregnancies and full-term live births without complications. These findings were robust to variations applied through OWSA and several scenario analyses. Overall, conforming intrauterine spacers are likely to be a cost-effective option for the prevention of IUAs and preparation for conception and ongoing healthy pregnancies.
Transparency
Author contributions
All authors participated in the design of this study. The analysis was performed by DS, CM, AM, AKJ, LS, and JK. The writing for this manuscript was reviewed and edited by all authors. All authors approve this as the final version of the manuscript to be published and agree to be accountable for all aspects of the work.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Geolocation information
This work was conducted by employees of Axtria Inc., Berkeley Heights, New Jersey, USA, with funding from Rejoni Inc., Bedford, Massachusetts, USA, and is intended for global audiences, focusing on those within the USA.
Supplemental Material
Download MS Word (26.3 KB)Supplemental Material
Download MS Word (21.1 KB)Supplemental Material
Download MS Word (23.8 KB)Supplemental Material
Download MS Word (31.4 KB)Supplemental Material
Download MS Word (26.4 KB)Supplemental Material
Download MS Word (37.4 KB)Acknowledgements
No assistance in the preparation of this article is to be declared. This article and the results herein have not been presented at any previous presentations.
Declaration of funding
This study was funded by Rejoni Inc., Bedford, Massachusetts, USA, who commissioned Axtria Inc. to work with MGM and other physician subject matter experts to review the literature, design, and develop the model described herein.
Declaration of financial/other relationships
DS, CM, AM, AKJ, LS, WCL, and JK are paid employees of Axtria Inc. MGM and IF are consultants for Rejoni Inc.
References
- Dreisler E, Kjer JJ. Asherman’s syndrome: current perspectives on diagnosis and management. Int J Womens Health. 2019;11:191–198. doi: 10.2147/IJWH.S165474.
- AAGL Elevating Gynecologic Surgery. AAGL practice report: practice guidelines on intrauterine adhesions developed in collaboration with the European Society of Gynaecological Endoscopy (ESGE). J Minim Invasive Gynecol. 2017;24(5):695–705. doi: 10.1016/j.jmig.2016.11.008.
- Hooker AB, Lemmers M, Thurkow AL, et al. Systematic review and meta-analysis of intrauterine adhesions after miscarriage: prevalence, risk factors and long-term reproductive outcome. Hum Reprod Update. 2014;20(2):262–278. doi: 10.1093/humupd/dmt045.
- Yu D, Wong YM, Cheong Y, et al. Asherman syndrome–one century later. Fertil Steril. 2008;89(4):759–779. doi: 10.1016/j.fertnstert.2008.02.096.
- Hooker AB, Mansvelder FJ, Elbers RG, et al. Reproductive outcomes in women with mild intrauterine adhesions; a systematic review and meta-analysis. J Matern Fetal Neonatal Med. 2022;35(25):6933–6941. doi: 10.1080/14767058.2021.1931103.
- Baradwan S, Baradwan A, Al-Jaroudi D. The association between menstrual cycle pattern and hysteroscopic march classification with endometrial thickness among infertile women with Asherman syndrome. Medicine. 2018;97(27):e11314. doi: 10.1097/MD.0000000000011314.
- Hooker A, Fraenk D, Brölmann H, et al. Prevalence of intrauterine adhesions after termination of pregnancy: a systematic review. Eur J Contracept Reprod Health Care. 2016;21(4):329–335. doi: 10.1080/13625187.2016.1199795.
- Trinh TT, Nguyen KD, Pham HV, et al. Effectiveness of hyaluronic acid gel and intrauterine devices in prevention of intrauterine adhesions after hysteroscopic adhesiolysis in infertile women. J Minim Invasive Gynecol. 2022;29(2):284–290. doi: 10.1016/j.jmig.2021.08.010.
- Hanstede MM, van der Meij E, Goedemans L, et al. Results of centralized asherman surgery, 2003-2013. Fertil Steril. 2015;104(6):1561.e1–1568.e1. doi: 10.1016/j.fertnstert.2015.08.039.
- Sikirica V, Bapat B, Candrilli SD, et al. The inpatient burden of abdominal and gynecological adhesiolysis in the US. BMC Surg. 2011;11(1):13. doi: 10.1186/1471-2482-11-13.
- Bosteels J, Weyers S, Mol BW, et al. Anti-adhesion barrier gels following operative hysteroscopy for treating female infertility: a systematic review and meta-analysis. Gynecol Surg. 2014;11(2):113–127. doi: 10.1007/s10397-014-0832-x.
- Konci R, Caminsky N, Tulandi T, et al. Supplements to conventional treatment after hysteroscopic lysis of intrauterine adhesions: a systematic review. J Obstet Gynaecol Can. 2020;42(8):984–1000. doi: 10.1016/j.jogc.2019.09.008.
- Deffieux X, Gauthier T, Menager N, et al. Hysteroscopy: guidelines for clinical practice from the French College of Gynaecologists and Obstetricians. Eur J Obstet Gynecol Reprod Biol. 2014;178:114–122. doi: 10.1016/j.ejogrb.2014.04.026.
- Orhue AA, Aziken ME, Igbefoh JO. A comparison of two adjunctive treatments for intrauterine adhesions following lysis. Int J Gynaecol Obstet. 2003;82(1):49–56. doi: 10.1016/s0020-7292(03)00030-4.
- Kou L, Jiang X, Xiao S, et al. Therapeutic options and drug delivery strategies for the prevention of intrauterine adhesions. J Control Release. 2020;318:25–37. doi: 10.1016/j.jconrel.2019.12.007.
- Acunzo G, Guida M, Pellicano M, et al. Effectiveness of auto-cross-linked hyaluronic acid gel in the prevention of intrauterine adhesions after hysteroscopic adhesiolysis: a prospective, randomized, controlled study. Hum Reprod. 2003;18(9):1918–1921. doi: 10.1093/humrep/deg368.
- Fei Z, Xin X, Fei H, et al. Meta-analysis of the use of hyaluronic acid gel to prevent intrauterine adhesions after miscarriage. Eur J Obstet Gynecol Reprod Biol. 2020;244:1–4. doi: 10.1016/j.ejogrb.2019.10.018.
- Mao X, Tao Y, Cai R, et al. Cross-linked hyaluronan gel to improve pregnancy rate of women patients with moderate to severe intrauterine adhesion treated with IVF: a randomized controlled trial. Arch Gynecol Obstet. 2020;301(1):199–205. doi: 10.1007/s00404-019-05368-6.
- Hooker AB, de Leeuw RA, Twisk JWR, et al. Pregnancy and neonatal outcomes 42 months after application of hyaluronic acid gel following dilation and curettage for miscarriage in women who have experienced at least one previous curettage: follow-up of a randomized controlled trial. Fertil Steril. 2020;114(3):601–609. doi: 10.1016/j.fertnstert.2020.04.021.
- Sutton C. Adhesions and their prevention. The Obstetric & Gynaecologis. 2005;7(3):168–176. doi: 10.1576/toag.7.3.168.27096.
- C. g. I. NCT05394662. Safety and effectiveness of Juveena™ hydrogel system following TCGP at high-risk for intrauterine adhesions. https://clinicaltrials.gov/ct2/show/record/NCT05394662?cond=Intrauterine+Adhesion&map_cntry=US&draw=2&rank=3.
- FRED. Consumer price index for all Urban consumers: Medical care in U.S. city average [CPIMEDSL]. https://fred.stlouisfed.org/series/CPIMEDSL.
- Smith JR. Postpartum hemorrhage. https://emedicine.medscape.com/article/275038-overview#a5.
- ACOG. Placenta accreta spectrum. https://www.acog.org/clinical/clinical-guidance/obstetric-care-consensus/articles/2018/12/placenta-accreta-spectrum.
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339(1):b2700–b2700. doi: 10.1136/bmj.b2700.
- Di Spiezio Sardo A, Spinelli M, Bramante S, et al. Efficacy of a polyethylene oxide-sodium carboxymethylcellulose gel in prevention of intrauterine adhesions after hysteroscopic surgery. J Minim Invasive Gynecol. 2011;18(4):462–469. doi: 10.1016/j.jmig.2011.04.007.
- Guida M, Acunzo G, Di Spiezio Sardo A, et al. Effectiveness of auto-crosslinked hyaluronic acid gel in the prevention of intrauterine adhesions after hysteroscopic surgery: a prospective, randomized, controlled study. Hum Reprod. 2004;19(6):1461–1464. doi: 10.1093/humrep/deh238.
- Tafti SZG, Javaheri A, Firoozabadi RD, et al. Role of hyaluronic acid intrauterine injection in the prevention of Asherman’s syndrome in women undergoing uterine septum resection: an RCT. Int J Reprod Biomed. 2021;19(4):339–346. doi: 10.18502/ijrm.v19i4.9060.
- Huang C-Y, Chang W-H, Cheng M, et al. Crosslinked hyaluronic acid gels for the prevention of intrauterine adhesions after a hysteroscopic myomectomy in women with submucosal myomas: a prospective, randomized, controlled trial. Life. 10(5):2020. doi: 10.3390/life10050067.
- Hooker AB, de Leeuw R, van de Ven PM, et al. Prevalence of intrauterine adhesions after the application of hyaluronic acid gel after dilatation and curettage in women with at least one previous curettage: short-term outcomes of a multicenter, prospective randomized controlled trial. Fertil Steril. 2017;107(5):1223.e3–1231.e3. doi: 10.1016/j.fertnstert.2017.02.113.
- Vatanatara J, Tingthanatikul Y, Lertvikool S, et al. Alginate carboxymethylcellulose hyaluronic acid for preventing intrauterine adhesion after vacuum aspiration for first-trimester abortion: a prospective, randomized controlled trial. J Gynecol Surg. 2021;37(5):402–407. doi: 10.1089/gyn.2020.0196.
- Tavcar J, Movilla P, Carusi DA, et al. Incidence and clinical implications of placenta accreta spectrum after treatment for Asherman syndrome. J Minim Invasive Gynecol. 2023;30(3):192–198. doi: 10.1016/j.jmig.2022.11.013.
- Rae M, Twitter CC, Dingel H. Health costs associated with pregnancy, childbirth, and postpartum care. https://www.healthsystemtracker.org/brief/health-costs-associated-with-pregnancy-childbirth-and-postpartum-care/.
- Beam AL, Fried I, Palmer N, et al. Estimates of healthcare spending for preterm and low-birthweight infants in a commercially insured population: 2008–2016. J Perinatol. 2020;40(7):1091–1099. doi: 10.1038/s41372-020-0635-z.
- Han X, Guo Z, Yang X, et al. Association of placenta previa with severe maternal morbidity among patients with placenta accreta spectrum disorder. JAMA Netw Open. 2022;5(8):e2228002. doi: 10.1001/jamanetworkopen.2022.28002.
- 2021 Embolization Coding and Reimbursement Guide. https://www.bostonscientific.com/content/dam/bostonscientific/Reimbursement/peripheral-intervention/pdf/Embolization_Coding_and_Reimbursement_Guide.pdf.
- Mulkey M. How much does anesthesia cost? https://dpianes.com/how-much-does-anesthesia-cost/.
- Forbes JM, Anderson MD, Anderson GF, et al. Blood transfusion costs: a multicenter study. Transfusion. 1991;31(4):318–323. doi: 10.1046/j.1537-2995.1991.31491213295.x.
- Dasta JF, McLaughlin TP, Mody SH, et al. Daily cost of an intensive care unit day: the contribution of mechanical ventilation. Crit Care Med. 2005;33(6):1266–1271. doi: 10.1097/01.ccm.0000164543.14619.00.
- Medicare. Hysteroscopy, surgical; with lysis of intrauterine adhesions (any method). https://www.medicare.gov/procedure-price-lookup/cost/58559/.
- Medicare. Treatment of incomplete abortion, any trimester, completed surgically. https://www.medicare.gov/procedure-price-lookup/cost/59812/.
- Nanda K, Lopez LM, Grimes DA, et al. Expectant care versus surgical treatment for miscarriage. Cochrane Database Syst Rev. 2012;2012(3):CD003518. doi: 10.1002/14651858.CD003518.pub3.
- Hysteroscopy, diagnostic (separate procedure). https://www.medicare.gov/procedure-price-lookup/cost/58555/.
- Saline infusion sonohysterography (sis), including color flow doppler, when performed. https://www.medicare.gov/procedure-price-lookup/cost/76831/.
- Gynecology procedures 2020 coding and reimbursement guide. https://conference.globalcastmd.com/files/resources/2020%20Hysteroscopy%20CPT%20Code%20Reimbursement%20Guide.pdf.
- Medicare. Ultrasound, transvaginal. https://www.medicare.gov/procedure-price-lookup/cost/76830.
- Larsen RG, Bowdino CS, Mathes MA, et al. Minimal access to male fertility prices online: an analysis of the society for assisted reproductive technology (SART) clinics. Transl Androl Urol. 2020;9(5):2107–2112. doi: 10.21037/tau-20-944.
- FindACode.com. 86901 – CPT® Code in category: blood typing, serologic. https://www.findacode.com/code.php?set=CPT&c=86901.
- Machlin SR, Mitchell EM. Expenses for office-based physician visits by specialty and insurance type, 2016. In: Statistical brief (medical expenditure panel survey (US)). Rockville (MD): Agency for Healthcare Research and Quality; 2001.
- FindACode.com. 82670 – CPT® code in category: estradiol. https://www.findacode.com/code.php?set=CPT&c=82670.
- FindACode.com. 84144 – CPT® code in category: chemistry procedures. https://www.findacode.com/code.php?set=CPT&c=84144.
- FindACode.com. 84443 – CPT® code in category: chemistry procedures. https://www.findacode.com/code.php?set=CPT&c=84443.
- FindACode.com. 84146 – CPT® code in category: chemistry procedures. https://www.findacode.com/code.php?set=CPT&c=84146.
- FindACode.com. 84403 – CPT® code in category: testosterone. https://www.findacode.com/code.php?set=CPT&c=84403.
- FindACode.com. 83001 – CPT® Code in category: gonadotropin. https://www.findacode.com/code.php?set=CPT&c=83001.
- FindACode.com. 83002 – CPT® Code in category: gonadotropin. https://www.findacode.com/code.php?set=CPT&c=83002.
- Labcorp. Anti-Müllerian Hormone (AMH) (Endocrine Sciences). https://www.labcorp.com/tests/500183/anti-m-llerian-hormone-amh-endocrine-sciences.
- MDsave.com. Chlamydia test. https://www.mdsave.com/procedures/chlamydia-test/df84f5.
- MDsave.com. Gonorrhea test. https://www.mdsave.com/procedures/gonorrhea-test/df85fc.
- MDsave.com. Syphilis test. https://www.mdsave.com/procedures/syphilis-test/d786fbc5.
- Labcorp. Human Immunodeficiency Virus 1 & 2 (HIV-1/HIV-2), Qualitative, RNA. https://www.labcorp.com/tests/139825/human-immunodeficiency-virus-1-amp-2-hiv-1-hiv-2-qualitative-rna.
- MDsave.com. Hepatitis B surface antigen screening. https://www.mdsave.com/procedures/hepatitis-b-surface-antigen-screening/d483ffc9.
- STDcheck.com. Hepatitis C test. https://www.stdcheck.com/hepatitis-c-test.php?cjdata=MXxOfDB8WXww&coupon=10OffOrder&source=CJ&utm_source=affiliate&utm_medium=website&utm_campaign=9126524&cjevent=01a38bbd60e811ee80b301620a18b8f8.
- Waitzman NJ, Jalali A, Grosse SD. Preterm birth lifetime costs in the United States in 2016: an update. Semin Perinatol. 2021;45(3):151390. doi: 10.1016/j.semperi.2021.151390.
- U.S. population growth rate 1950–2023. https://www.macrotrends.net/countries/USA/united-states/population-growth-rate.
- Pan LZ, Wang Y, Chen X. A randomized controlled study on an integrated approach to prevent and treat re-adhesion after transcervical resection of moderate-to-severe intrauterine adhesions. Clinics. 2021;76:e1987. doi: 10.6061/clinics/2021/e1987.
- Bosteels J, Weyers S, D'Hooghe TM, et al. Anti-adhesion therapy following operative hysteroscopy for treatment of female subfertility. Cochrane Database Syst Rev. 2017;11(11):CD011110. doi: 10.1002/14651858.CD011110.pub3.
- Liu H, Xu Y, Yi N, et al. Efficacy and safety of hyaluronic acid gel for the prevention of intrauterine adhesion: a meta-analysis of randomized clinical trials. Gynecol Obstet Invest. 2018;83(3):227–233. doi: 10.1159/000486674.
- Yan Y, Xu D. The effect of adjuvant treatment to prevent and treat intrauterine adhesions: a network meta-analysis of randomized controlled trials. J Minim Invasive Gynecol. 2018;25(4):589–599. doi: 10.1016/j.jmig.2017.09.006.