Abstract
Aims
To describe healthcare costs of patients with metastatic castration-resistant prostate cancer (mCRPC) initiating first-line (1 L) therapies from a US payer perspective.
Methods
Patients initiating a Flatiron oncologist-defined 1 L mCRPC therapy (index date) on or after mCRPC diagnosis were identified from linked electronic medical records/claims data from the Flatiron Metastatic Prostate Cancer (PC) Core Registry and Komodo’s Healthcare Map. Patients were excluded if they initiated a clinical trial drug in 1 L, had <12 months of insurance eligibility prior to index, or no claims in Komodo’s Healthcare Map for the Flatiron oncologist-defined index therapy. All-cause and PC-related total costs per-patient-per-month (PPPM), including costs for services and procedures from medical claims (i.e. medical costs) and costs from pharmacy claims (i.e. pharmacy costs), were described in the 12-month baseline period before 1 L therapy initiation (including the baseline pre- and post- mCRPC progression periods) and during 1 L therapy (follow-up).
Results
Among 459 patients with mCRPC (mean age 70 years, 57% White, 16% Black, 45% commercially-insured, 43% Medicare Advantage-insured, and 12% Medicaid-insured), average baseline all-cause total costs (PPPM) were $4,576 ($4,166 pre-mCRPC progression, $8,278 post-mCRPC progression). Average baseline PC-related total costs were $2,935 ($2,537 pre-mCRPC progression, $6,661 post-mCRPC progression). During an average 1 L duration of 8.5 months, mean total costs were $13,746 (all-cause) and $12,061 (PC-related) PPPM. The cost increase following 1 L therapy initiation was driven by higher PC-related outpatient and pharmacy costs. PC-related medical costs PPPM increased from $1,504 during baseline to $5,585 following 1 L mCRPC therapy initiation.
Limitations
All analyses were descriptive; statistical testing was not performed.
Conclusion
Incremental costs of progression to mCRPC are significant, with the majority of costs driven by higher PC-related costs. Using contemporary data, this study highlights the importance of utilizing effective therapies that slow progression and reduce healthcare resource demands despite the initial investment in treatment costs.
PLAIN LANGUAGE SUMMARY
Prostate cancer is one of the most common causes of cancer death in men. While outcomes are good if treated early, some patients may develop advanced disease that is more difficult and costly to treat. The most advanced form is late-stage hormone-resistant prostate cancer. Previous studies found that healthcare costs and use of medical services increase when prostate cancer advances to this stage. New medications are available, but their costs are not well known. Our study reviewed clinical information and health insurance data to estimate the healthcare costs and medical services used by 459 men who received drug treatment for late-stage hormone-resistant prostate cancer between 2017 and 2021 in the United States. In the year before a diagnosis and the start of drug treatment for late-stage hormone-resistant prostate cancer, total healthcare costs per month were approximately $4,000. After diagnosis of but before drug treatment for late-stage hormone-resistant prostate cancer, monthly healthcare costs nearly doubled, to approximately $8,000. Most of the additional $4,000 were specifically related to prostate cancer. After the start of the first drug treatment, total healthcare costs increased to approximately $13,500 every month. Most of the costs were related to medical office/clinic visits and medications. As their disease progressed, men received additional therapies, and office/clinic visits and use of chemotherapy also increased. These results emphasize the high healthcare costs and large number of medical services used by men with late-stage hormone-resistant prostate cancer. Therapies that slow progression to advanced prostate cancer may help to reduce high healthcare costs.
Introduction
Prostate cancer (PC) is the second most common cause of cancer-death among men, with over 288,300 new cases and 34,700 deaths expected in the United States (US) in 2023Citation1. When PC is detected early, there is a favorable prognosis; however, advanced metastatic disease is associated with substantial declines in prognosis, with a 5-year relative survival rate of 32.3% and deterioration of quality of lifeCitation2,Citation3. As such, preventing and delaying metastasis is an important goal of clinical disease management.
Androgen deprivation via bilateral orchiectomy or medical castration with luteinizing hormone–releasing hormone (LHRH) analogues has been a backbone of therapy for PCCitation4. Despite treatment with androgen deprivation therapy (ADT), patients typically progress to castration-resistant prostate cancer (CRPC) within 2-3 years on averageCitation5, while approximately 60% of men with non-metastatic CRPC (nmCRPC) eventually develop metastatic CRPC (mCRPC) within 3 years of CRPC onsetCitation6.
Treatment guidelines recommend androgen-receptor signaling inhibitors (ARSIs; e.g. abiraterone acetate, enzalutamide) in combination with ADT, or chemotherapy, immunotherapy, and/or radiopharmaceuticals for treatment of mCRPC - the most aggressive form of PCCitation7,Citation8. In addition, Phase 3 clinical trials of poly (ADP-ribose) polymerase (PARP) inhibitors have demonstrated treatment benefit when combined with ARSIs in the first-line (1 L) treatment of patients with mCRPC who also have alterations in genes encoding homologous recombination repair enzymesCitation9,Citation10.
In addition to the increased complexity of clinical management when patients progress to mCRPC, real-world studies have shown a significant increase in economic burdenCitation11,Citation12. For example, in a large claims-based study of patients with nmCRPC who progressed to mCRPC, healthcare resource utilization (HRU; including hospitalizations, emergency room visits, office visits, outpatient encounters, and prescription fills) was 1.5 to 2.5 times greater after a diagnosis of mCRPC, depending on the HRU category, while total healthcare costs increased by over 5-foldCitation11. Another claims-based study also observed significant increases in HRU and costs after disease progression from nmCRPC to mCRPCCitation12. However, a limitation of these studies is reliance on algorithms for the identification of mCRPC and absence of clinical data confirming disease stage (i.e. metastasis status and/or castration status).
Since the approval of ARSIs and PARP inhibitors to treat mCRPC (abiraterone approved as 1 L therapy in 2012, enzalutamide approved as 1 L therapy in 2014, and for patients with mutations in homologous recombination repair genes, such as BRCA, olaparib was approved in 2020 and niraparib was approved in 2023)Citation13–16, few real-world studies have combined clinical and healthcare claims data to characterize the economic burden across stages of advanced PC disease, as patients progress to mCRPC and initiate 1 L mCRPC therapy. Using contemporary data to reflect the current mCRPC treatment guidelines and landscape, this study utilized clinical data to identify patients with mCRPC combined with claims-based data to describe HRU and costs before and after progression to mCRPC and by line of mCRPC therapy (LOT) from a US payer perspective. Results from this study are intended to provide payers and clinicians with a description of treatment utilization, HRU, and costs among patients with mCRPC as their disease progresses in order to inform clinical decision making.
Methods
Data sources
This study relied on linkage of the Flatiron Metastatic PC Core Registry, containing clinical data from 280 US cancer clinics (∼800 sites of care) between 01/01/2013-12/01/2021, and Komodo’s Healthcare Map (claims data) from 01/01/2014-12/01/2021. Both databases contain de-identified and Health Insurance Portability and Accountability Act (HIPAA)-compliant patient records per Title 45 of Code of Federal Regulations, Part 46.101(b)(4). The Flatiron Metastatic PC Core Registry is a longitudinal database, comprising de-identified, patient-level, structured and unstructured data, curated via technology-enabled abstractionCitation17,Citation18. The data source contains detailed clinical data extracted from structured electronic medical records (EMRs), unstructured data abstracted from physicians’ notes using natural language processing and other documents, including demographics, diagnoses, visits, laboratory tests and vitals, medication administration, medication prescriptions and orders, performance status, and insurance data. Other oncology-specific data elements include PC-related characteristics that may not be available in other databases (e.g. dates of initial/metastatic PC/CRPC diagnoses, stage at initial diagnosis, LOTs, prostate-specific antigen (PSA) levels, mortality).
The study comprised de-identified patients from the Flatiron Metastatic PC Core Registry included in the registry based on a diagnosis of PC (International Classification of Diseases, Ninth/Tenth Revision, Clinical Modification [ICD-9-CM: 185.x or ICD-10-CM: C61.x]) and documentation of both metastatic disease and CRPC in EMR data, as well as ≥2 documented clinical visits in the Flatiron network, on different days, occurring on or after 01/01/2013. The identification of metastatic disease and CRPC status was abstracted by Flatiron based on information available in patients’ medical records. Explicit physician documentation was required to confirm metastatic disease while the identification of CRPC status required that patients met one of the following three criteria: (1) explicit physician documentation of either castration resistance or that the patient is no longer responding to ADT alone; (2) a rising PSA value of ≥2ng/mL on their initial hormonal therapy and ≥1 higher subsequent PSA value within 3 months of their first PSA value; (3) physician documentation of a rising PSA or PSA progression on the 1 L of hormonal therapy with a change in treatment (if PSA values were unavailable).
To provide information on HRU and costs, payer claims data for patients with complete medical and prescription benefit information, including insurance eligibility, were obtained from Komodo’s Healthcare Map healthcare encounters data. The Komodo Health database contains over 320 million US patients across Medicaid, Commercial and Medicare Advantage insurers. Payer claims analyzed in this study from Medicare Advantage and Medicaid insurers comprised of claims from Medicare Advantage and Medicaid managed care plans.
The linkage of de-identified, tokenized, patient-level records from the Flatiron and Komodo Health databases was performed by DatavantCitation19. Komodo used an algorithm to impute medical costs from a payer’s perspective, which was derived based on several factors, including type of claim, payer channel (e.g. Medicare Advantage, commercial insurance), type of service, and setting of careCitation20. When costs from a payer’s perspective for pharmacy claims were unavailable, they were imputed with the median cost from a payer’s perspective for the medication by national drug code and payer channel, or in reference to National Average Drug Acquisition Cost and Medicare Advantage Average Sales Price.
The analysis of this study is exempt from institutional review for the following reasons: (a) it is a retrospective analysis of existing data with no patient intervention or interaction, and (b) no patient identifiable information is included in the EMR and claims datasetsCitation21. Flatiron Health, Inc., Komodo Health Solutions, and Datavant did not participate in data analyses.
Study design
This study used a descriptive, retrospective longitudinal cohort study design. The index window spanned from 01/01/2017 until 12/01/2021 to observe outcomes among patients initiated on more recently approved regimens for the treatment of mCRPC. The date of mCRPC was defined as the later date of confirmation of metastasis or castration resistance. The index date was defined as the date of initiation of a Flatiron oncologist-defined 1 L mCRPC regimen, that occurred on or after evidence of mCRPC, and represented the earlier of: the first observed claim in Komodo Health for an mCRPC therapy or the Flatiron oncologist-defined LOT start date (). LOTs for mCRPC consisted of treatment with an ARSI, chemotherapy, an estrogen, immunotherapy, a PARP inhibitor, or a radiopharmaceutical. Flatiron oncologist-defined LOTs were numbered such that the 1 L therapy was defined as the LOT initiated on or after evidence of mCRPC.
Figure 1. Study design scheme.
1L: first-line; HRU: Healthcare resource utilization; LOT: line of therapy; mCRPC: metastatic castration-resistant prostate cancer.
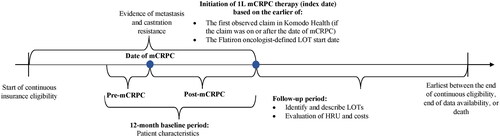
The baseline period was defined as the 12 months preceding the index date and was stratified into two periods: pre-mCRPC progression and post-mCRPC progression. The pre-mCRPC progression period was the portion of the 12-month baseline period during which the patient had localized PC, nmCRPC, or metastatic castration-sensitive PC (mCSPC), and ended before the latest of a diagnosis of metastasis or castration resistance. The post-mCRPC progression period was the portion of the 12-month baseline period that occurred on and after the latest of a diagnosis of metastasis or castration resistance and before the initiation of 1 L mCRPC therapy. Each patient could contribute to one or both periods depending on the timing of the metastasis diagnosis and castration resistance. The follow-up period was defined as the period spanning from the index date to the earliest of the end of continuous insurance eligibility, end of data availability (i.e. 12/01/2021), or death.
HRU and costs were assessed using Komodo Health closed claims data and were evaluated during the 12-month baseline period (stratified by the pre- and post-mCRPC progression periods) and during the follow-up period. In the follow-up period, HRU and costs were reported overall, and stratified by 1 L, 2 L, and third-line (3 L) therapy periods. HRU and costs in the 1 L, 2 L, and 3 L therapy periods were reported for the duration of the LOT (i.e. from therapy initiation until the day before the initiation of a subsequent LOT, if observed) among patients with ≥1 claim in Komodo Health for an agent included as part of 1 L, 2 L, and 3 L regimens, respectively.
Lines of therapy
An algorithm created by Flatiron identified LOTs in a stepwise fashion based on review and summary of the medication orders and administrations recorded in the EMRs. In this algorithm, drug episodes of the same medication occurring close in time were grouped into longitudinal components that were then summarized into LOTs based on the start and end dates of the components. Medications that were initiated within a 28-day window of the start of the first drug episode were considered as part of a single LOT. For each LOT, the associated regimen included all the drugs that were given as part of that LOT. A LOT was designated as starting with the initiation of a new regimen after a diagnosis of mCRPC and ending when the patient switched to a subsequent treatment regimen or end of clinical activity (i.e. the day before the last record in the database observed across all component tables or the end of data availability [i.e. 12/01/2021]). Treatment re-initiation after a gap of more than 90 days from the last day of supply was considered a new LOT. Therefore, based on the definition of an LOT, there may have been a period where patients were untreated before they initiated their next LOT. Of note, ADT is not included in the Flatiron algorithm to identify LOTs; as such, patients treated with ADT monotherapy do not contribute to any LOT and were not included in this study. However, the use of ADT in combination with Flatiron oncologist-defined LOTs was evaluated (i.e. LOTs during which patients had ≥1 claim for an ADT agent in Komodo Health).
Study sample
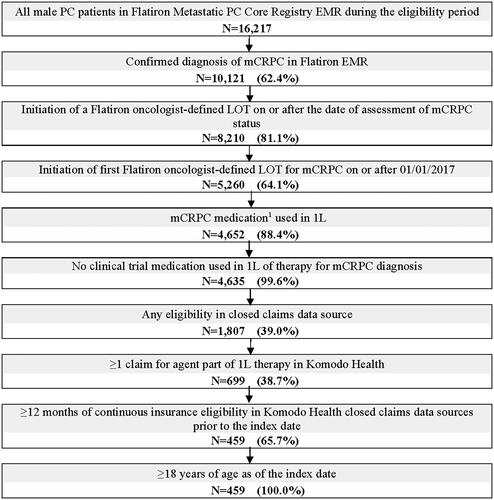
Patients meeting the following criteria were included in the study sample (): (1) A chart-confirmed diagnosis for metastatic PC and confirmed CRPC (from Flatiron data, based on information available in patients’ EMR, incorporating physician-reported CRPC in medical charts, observed rise in PSA levels while on hormone therapy, or physician-documented rise in PSA levels on hormone therapy plus a change in treatment); (2) ≥1 Flatiron oncologist-defined LOT for mCRPC on or after mCRPC diagnosis and 01/01/2017; (3) ≥1 claim in Komodo Health records for patients’ Flatiron oncologist-defined 1 L mCRPC therapy; (4) ≥18 years of age; and, (5) ≥12 months of continuous insurance eligibility in Komodo Health records prior to the index date. Patients were excluded from the study if they used a clinical trial medication as part of 1 L therapy for mCRPC.
Figure 2. Patient identification flowchart.
1L: first-line; ARSIs: androgen receptor signaling inhibitors; EMR: electronic medical records; LOT: line of therapy; mCRPC: metastatic castration-resistant prostate cancer; PARP: poly ADP-ribose polymerase; PC: prostate cancer.
Note:
1. Medications considered for 1L mCRPC therapy were: ARSIs (i.e. apalutamide, darolutamide, enzalutamide, abiraterone acetate), chemotherapy (i.e. cabazitaxel, carboplatin, cisplatin, docetaxel, etoposide, mitoxantrone), PARP inhibitors (i.e. niraparib, olaparib, rucaparib, talazoparib), immunotherapy (i.e. sipuleucel-T, pembrolizumab), estrogens (i.e. estramustine phosphate, diethystillbestrol, polyestradiol phosphate), radiopharmaceuticals (i.e. radium-223, lutetium-177-PSMA-617).
Outcomes
Demographic and clinical characteristics were evaluated in the 12-month baseline period that preceded the index date.
LOTs were evaluated using Flatiron EMR data and regimens used as Flatiron oncologist-defined 1 L, 2 L, and 3 L therapy for mCRPC were reported. The use of ADT in combination with Flatiron oncologist-defined LOTs (i.e. LOTs during which patients had ≥1 claim in Komodo Health for an ADT agent) was evaluated. All-cause and PC-related HRU were identified based on medical claims and included inpatient admissions (number of admissions, number of days with admissions, and mean length of admission), number of days with emergency room visits, number of days with outpatient visits, and number of days with other services were reported. The number of days with all-cause or PC-related pharmacy claims were identified based on dates with a dispensed medication (i.e. a filled pharmacy claim). In addition, all-cause and PC-related healthcare costs, including medical costs (i.e. sum of inpatient, emergency room, outpatient, other costs), pharmacy costs and total healthcare costs (sum of medical and pharmacy costs) were reported overall and by payer channel (i.e. commercial and Medicare Advantage; Medicaid coverage was not included as it represented a small proportion of the study sample). HRU and costs (including the costs associated with imaging and testing) were assessed using closed claims data from Komodo Health and were reported for the baseline period (overall and stratified by pre- and post-mCRPC progression), and follow-up period (overall and stratified by 1 L, 2 L, and 3 L therapy periods). PC-related HRU and costs were defined based on claims for malignant neoplasm of prostate (identified with the ICD-10-CM code C61) or claims with procedure codes for LHRH or other therapies for mCRPC (i.e. ARSIs, chemotherapy, PARP inhibitors, immunotherapy, estrogens, and radiopharmaceuticals). All HRU and costs were reported per-patient-per-month (PPPM), expressed in 2022 US dollars (USD), and measured from a payer’s perspective.
Further, to evaluate whether the COVID-19 pandemic was a strong driver of all-cause HRU observed in this study, COVID-related HRU (Table S1) were described in the baseline and follow-up periods.
Statistical analyses
Baseline characteristics, medications used for 1 L/2L/3L therapy, and HRU and costs were described using means, medians, and standard deviations (SDs) for continuous variables, and frequencies and proportions for categorical variables. All analyses were conducted using SAS Enterprise Guide software Version 7.15.
Results
Baseline characteristics
A total of 459 patients with mCRPC and represented in both databases were included. Mean age was 70.0 years, 57.1% were White, and 15.5% were Black (). Most patients were treated in a community oncology setting (92.8%) and had either commercial (45.1%) or Medicare Advantage (42.9%) insurance; fewer patients had Medicaid (11.8%). The most recent ECOG performance score was available for 76.0% of patients, the majority of whom had a score of 0 (45.6%) or 1 (43.3%). The mean Quan-Charlson Comorbidity Index score was 9.1 (median: 9.0). Most patients (79.3%) transitioned directly from mCSPC to mCRPC, while fewer patients transitioned from nmCRPC (19.8%) or localized PC (<1%) to mCRPC as their next diagnosis recorded. On average, patients initiated 1 L mCRPC therapy 3.8 months (median: 1.2) after mCRPC diagnosis. In the 12-month baseline period, the majority of patients used anti-androgens (72.1%; ARSI: 56.0%) while few patients (8.9%) had undergone chemotherapy. Most patients had evidence of ADT use before 1 L mCRPC therapy (93.5%), among whom 39.8% used ADT in monotherapy (i.e. without use of ARSIs, chemotherapy, PARP inhibitors, immunotherapy, estrogens, or radiopharmaceuticals). While not included in this study, 42 patients were treated with ADT monotherapy following a diagnosis of mCRPC (i.e. did not initiate a Flatiron oncologist-defined LOT for mCRPC).
Table 1. Baseline characteristics.
HRU during baseline
On average, the baseline pre-mCRPC progression period was 10.0 months (median: 10.9) and 3.3 months (median: 3.3) post-mCRPC progression. Most patients contributed time to both periods (90.6% pre-mCRPC progression, 87.1% post-mCRPC progression; ). A trend towards higher HRU in the post-mCRPC progression period relative to the pre-mCRPC progression period was observed for all HRU categories. For example, a mean PPPM of 1.18 days with all-cause inpatient admissions was observed in the pre-mCRPC progression period, and 1.57 days PPPM in the post-mCRPC progression period. Mean PPPM days with PC-related inpatient admissions was 0.96 in the pre-mCRPC progression period and 1.49 in the post-mCRPC progression period. On average, 2.56 days PPPM all-cause outpatient visits were observed in the pre-mCRPC progression period and 4.94 in the post-mCRPC progression period. PC-related outpatient visits were observed for a mean of 1.34 days PPPM in the pre-mCRPC progression period and 3.67 days in the post-mCRPC progression period.
Table 2. Baseline and follow-up HRU1 PPPM.
HRU following 1 L mCRPC therapy initiation
Patients were treated with a 1 L agent for a longer average treatment duration than observed in other LOTs. Overall, patients were followed for an average of 15.2 months (median: 12.3), with an average duration of 1 L therapy of 8.5 months (median: 5.6; ). There were 166 patients (36.2%) who had a claim in Komodo Health for a Flatiron oncologist-defined 2 L therapy and were evaluated for an average of 6.5 months (median 5.1) during the 2 L therapy period. In 3 L, 86 patients (18.7%) had a claim in Komodo Health for the Flatiron oncologist-defined 3 L therapy and were evaluated during the 3 L therapy period over an average 3 L therapy period of 5.2 months (median: 3.2).
There were no clear differences in HRU for 1 L, 2 L and 3 L treatments during the follow-up period (). Most patients had ≥1 all-cause or PC-related inpatient admission during the entire follow-up period (all cause: 61.2%; PC-related: 56.4%), ranging from 34.9% to 38.0% of patients across LOTs for all-cause admissions and from 31.4% to 37.2% for PC-related admissions. Of note, 3 L therapy tended to have higher all-cause (5.33 days PPPM) and PC-related (4.49 days PPPM) outpatient visits than 1 L (all-cause: 3.75 days PPPM; PC-related: 2.62 days PPPM) and 2 L (all-cause: 3.94 days PPPM; PC-related: 2.91 days PPPM) therapy periods. Similarly, the mean number of all-cause pharmacy claims PPPM was noticeably higher in the 3 L period (5.33 claims PPPM) than the 1 L (4.44 claims PPPM) and 2 L (4.65 claims PPPM) periods. COVID-related HRU did not appear to be an important driver of all-cause HRU in the baseline or follow-up periods (Table S2).
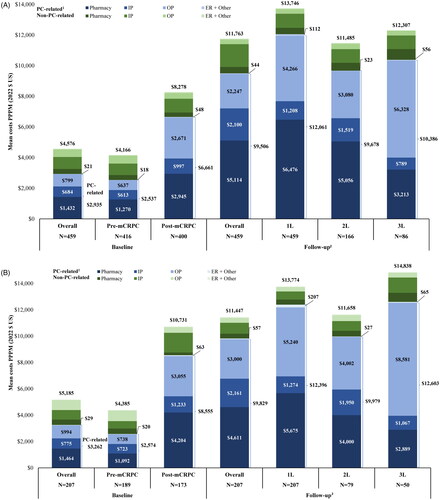
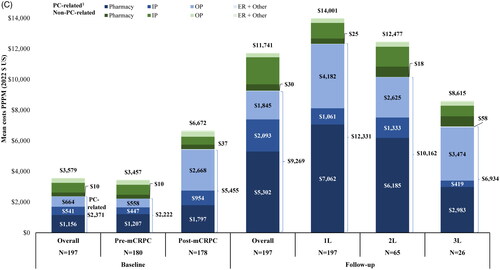
Costs during baseline
During the 12-month baseline period before 1 L mCRPC therapy initiation, the mean total all-cause costs PPPM were $4,576; medical costs accounted for most of this total ($2,823) while pharmacy costs contributed $1,753 ( and Table S3A). The mean total PC-related costs PPPM were $2,935. When evaluating the pre- and post-mCRPC progression components of the baseline period, total all-cause costs were observed to nearly double in the post-mCRPC progression period ($8,278 PPPM) prior to 1 L treatment initiation as compared to the pre-mCRPC progression period ($4,166 PPPM). PC-related costs increased from $2,537 PPPM pre-mCRPC progression to $6,661 PPPM post-mCRPC progression. Specifically, PC-related pharmacy costs more than doubled; PC-related outpatient costs more than quadrupled, and conventional imaging costs more than tripled (Tables S3A, S4). During the pre-mCRPC progression period, PC-related total costs represented 60.9% of total all-cause costs, while during the post-mCRPC progression period 80.5% of total costs were PC-related.
Figure 3. (A) Baseline and follow-up costs PPPM. (B) Baseline and follow-up costs PPPM among patients with Commercial insurance. (C) Baseline and follow-up costs PPPM among patients with Medicare Advantage insurance
1L: first-line; 2L: second-line; 3L: third-line; ARSI: androgen receptor signaling inhibitor; ER: emergency room; ICD-10-CM: International Classification of Diseases 10th Revision Clinical Modification; IP: inpatient; LHRH: luteinizing hormone-releasing hormone; OP: outpatient; mCRPC: metastatic castration-resistant prostate cancer; PARP: poly ADP-ribose polymerase; PPPM: per-patient-per-month; US: United States.
Notes:
1. PC-related HRU and costs were identified with the ICD-10-CM code C61 and procedure codes for LHRH or of the following guideline-recommended therapies for mCRPC: ARSIs, chemotherapy, PARP inhibitors, immunotherapy, estrogens, and radiopharmaceuticals.
2. Outpatient costs during 3L were driven by an outlier who had costs of $153,660 PPPM during 3L; excluding this patient would have resulted in outpatient costs of $4,642 PPPM.
When analyses were stratified by payer, total all-cause costs during the overall baseline period were higher among patients with commercial insurance than those with Medicare Advantage, but the proportion of total costs that were PC-related were similar in both groups (62.9% among patients with commercial insurance and 66.2% among patients with Medicare Advantage). In addition, the increase in costs from the pre-mCRPC progression period to the post-mCRPC progression period was greater among patients with commercial insurance than those with Medicare Advantage insurance ( and Tables S3B,C).
Costs following 1 L mCRPC therapy initiation
Once 1 L mCRPC therapy was initiated, all-cause costs PPPM increased by 66.1% (1 L: $13,746 PPPM; medical costs: $6,856; pharmacy costs: $6,890; ; Table S3A). This cost increase was predominantly related to the initiation of PC-related therapies ($6,476 PPPM) and higher PC-related outpatient costs ($4,266 PPPM). All-cause costs associated with 2 L were $11,485 PPPM (medical costs: $5,920; pharmacy costs: $5,565) of which $9,678 was PC-related (medical costs $4,622; pharmacy costs: $5,056). During 3 L, all-cause costs were $12,307 PPPM (medical costs: $8,391; pharmacy costs: $3,916) with $10,386 PPPM attributable to PC (medical costs: $7,173; pharmacy costs: $3,213). Compared to 1 L therapy, all-cause costs were 16.4% lower in 2 L and 10.5% lower in 3 L. PC-related total costs represented 80.8% of total all-cause costs during the overall follow-up period, 87.7% in 1 L, 84.3% in 2 L, and 84.4% in 3 L.
When analyses were stratified by payer channel (i.e. Commercial or Medicare Advantage), the distribution of costs by LOTs differed. Among patients with Commercial insurance, all-cause 3 L costs were the highest ($14,838 PPPM; medical costs: $11,261; pharmacy costs: $3,577) followed by 1 L ($13,774 PPPM; medical costs: $7,715; pharmacy costs: $6,059) and 2 L ($11,658 PPPM; medical costs: $7,229; pharmacy costs: $4,429), while all-cause costs decreased with successive LOTs for patients with Medicare Advantage insurance (1 L: $14,001 PPPM; medical costs: $6,596; pharmacy costs: $7,405; 2 L: $12,477 PPPM; medical costs: $5,614; pharmacy costs: $6,863; 3 L: $8,615 PPPM; medical costs: $4,966; pharmacy costs: $3,649; and Tables S3B,C).
Regimens used in Flatiron oncologist-defined lines of therapy
ARSI use with or without ADT (+/- ADT) was the most common type of Flatiron oncologist-defined 1 L therapy; 35.7% of patients were treated with abiraterone acetate, 26.8% were treated with enzalutamide, and 2.8% were treated with apalutamide, for a total of 65.4% of patients overall (). Of those initiating ARSIs as 1 L therapy, 69.7% of patients had a claim for ADT during 1 L, among whom 88.9% used leuprolide. Chemotherapy was the second most common type of 1 L therapy, with 16.3% of patients overall undergoing chemotherapy (the majority of whom were treated with docetaxel). Radium-223, combination therapy and other types of monotherapy were relatively uncommon as 1 L therapy agents.
Figure 4. Regimens used as Flatiron oncologist-defined 1 L, 2 L, and 3 L mCRPC therapyCitation1,Citation2.
1L: first-line; 2L: second-line; 3L: third-line; ARSI: androgen receptor signaling inhibitor; Chemo: chemotherapy; mCRPC: metastatic castration-resistant prostate cancer.
Notes:
1. All individual agents reported were used as monotherapy in Flatiron oncologist-defined 1L, 2L, or 3L mCRPC therapy.
2. Medications considered for other monotherapy were: ARSIs (i.e. darolutamide), chemotherapy (i.e. cisplatin, mitoxantrone), PARP inhibitors (i.e. olaparib), and immunotherapy (i.e. pembrolizumab).
3. Among 164 patients treated with abiraterone acetate monotherapy as 1L, 54 (32.9%) had a claim for generic abiraterone acetate while 110 (67.1%) had a claim for branded abiraterone acetate (i.e. Zytiga [108] or Yonsa [2]) as their first claim on or after the date of mCRPC.
![Figure 4. Regimens used as Flatiron oncologist-defined 1 L, 2 L, and 3 L mCRPC therapyCitation1,Citation2.1L: first-line; 2L: second-line; 3L: third-line; ARSI: androgen receptor signaling inhibitor; Chemo: chemotherapy; mCRPC: metastatic castration-resistant prostate cancer.Notes:1. All individual agents reported were used as monotherapy in Flatiron oncologist-defined 1L, 2L, or 3L mCRPC therapy.2. Medications considered for other monotherapy were: ARSIs (i.e. darolutamide), chemotherapy (i.e. cisplatin, mitoxantrone), PARP inhibitors (i.e. olaparib), and immunotherapy (i.e. pembrolizumab).3. Among 164 patients treated with abiraterone acetate monotherapy as 1L, 54 (32.9%) had a claim for generic abiraterone acetate while 110 (67.1%) had a claim for branded abiraterone acetate (i.e. Zytiga [108] or Yonsa [2]) as their first claim on or after the date of mCRPC.](/cms/asset/dfd9058b-f7ca-4e52-94f1-e17a49376d31/ijme_a_2303890_f0004_c.jpg)
Relative to 1 L therapy, the proportion of patients treated with ARSI (+/- ADT) as 2 L therapy decreased. ARSIs were used in 41.0% of patients with 2 L therapy. Approximately 17.5% of patients were treated with abiraterone acetate, 22.9% with enzalutamide, and 0.6% with apalutamide. As compared to 1 L, the proportion of patients using chemotherapy increased substantially in 2 L to 28.9%; combination therapy was also much more common (22.9%).
Chemotherapy was the most common type of 3 L therapy, used by over 50% of patients (26.7% for both docetaxel and cabazitaxel). The proportion of patients using ARSI (+/- ADT) as a 3 L therapy (20.9%) was lower than 1 L or 2 L therapy.
Among patients who did not have a subsequent Flatiron oncologist-defined LOT, the proportion who died within each LOT was 28.7% for 1 L, 41.6% for 2 L, and 36.5% for 3 L (Table S5).
Discussion
This study used novel methodology to describe the real-world HRU and economic burden of mCRPC in the US by linking EMR and claims data to adequately identify mCRPC, with a study recency that increases the generalizability of results by reflecting the current treatment guidelines and landscape. Prior to 1 L therapy initiation, PC-related total costs increased as patients developed mCRPC but before initiating a Flatiron oncologist-defined 1 L treatment. PC-related total costs increased further upon starting 1 L therapy. Across Flatiron oncologist-defined LOTs, ARSIs were prioritized in early lines, and chemotherapy and combination therapy were utilized more often as 2 L and 3 L options, suggesting that newer orals were exhausted in earlier LOTs and more complex combination regimens and older chemotherapy agents were reserved for later LOTs (consistent with guideline recommendations)Citation7,Citation8. Despite the economic burden reported in this study, which evaluated PPPM costs before and after progression to mCRPC and stratified by mCRPC LOT, the economic burden of patients with mCRPC may be underestimated due to the lack of representation of patients treated with ADT monotherapy. In fact, clinical trial data has shown that relative to patients treated with ARSIs, those treated with ADT monotherapy progress more rapidly to advanced disease stages and have shorter survivalCitation22,Citation23. Indeed the increased HRU and costs associated with PC disease progressionCitation11,Citation12,Citation24, combined with the high end of life care costsCitation25 over a shorter survival period could lead to higher PPPM costs for patients on ADT monotherapy relative to those treated with advanced therapies who benefit from delayed progression and extended survival.
The significant cost burden of mCRPC has been demonstrated in previous real-world studies using older data, with evidence of rising incremental costs as a patient progresses across stages of PC, including by LOT for mCRPCCitation11,Citation12,Citation24. In a US-based study by Appukkuttan et al.Citation12 using claims data from 2009 to 2015, the post-mCRPC progression costs are similar to the costs observed during 1 L mCRPC therapy in the current study, yet higher than those during the mCRPC period before the initiation of 1 L therapy in the current study. However, Appukkuttan et al. indexed at the date of metastases, as opposed to at the initiation of 1 L mCRPC therapy, as was done in this study. Interestingly, the proportion of all-cause costs attributable to pharmacy costs was notably different in the current study (50.1% during 1 L mCRPC therapy) relative to Appukkuttan et al.Citation12 (26.5% during the mCRPC period), which may be reflective of the exclusion of patients treated with ADT monotherapy in the current study, recent approvals in oral targeted therapies, and increased use of ARSIs. Indeed, leuprolide either alone or in combination, was used among two-thirds of patients and was the most common treatment post-mCRPC progression found in Appukkuttan et al.Citation12, while in the current study nearly two-thirds of patients initiated abiraterone acetate or enzalutamide as 1 L mCRPC therapy, among whom the majority were additionally treated with leuprolide. This increased use of ARSIs is consistent with guideline recommendations and clinical trial data demonstrating prolonged survival relative to ADT monotherapy among men with mCRPCCitation7,Citation22,Citation26. For instance, treatment with ADT + abiraterone acetate was associated with significantly longer median overall survival than ADT + placebo in the phase 3 COU-AA-302 clinical trial of patients with mCRPC (34.7 months vs 30.3 months; hazard ratio [95% confidence interval] = 0.81 [0.70-0.93]; p = 0.0033)Citation26.
In another US claims-based study by Freedland et al.Citation24 among Medicare Advantage fee-for-service patients with mCRPC between 2014 and 2019, all-cause total healthcare costs of $2,289 PPPM were observed in the year preceding mCRPC diagnosis, which is lower than costs observed in the current study during the pre-mCRPC progression periodCitation24. In addition, Freedland et al.Citation24 found increasing costs as patients progressed to subsequent LOTs, which was inconsistent with the current study findings that costs peaked during 1 L mCRPC therapy, driven predominantly by PC-related pharmacy costs and higher PC-related outpatient costs. The observed differences relative to this prior research may be explained in part by the fact that the current study did not include untreated patients or those treated with ADT monotherapy, whereas in Freedland et al. 22% of mCRPC patients did not receive life-prolonging therapy (i.e. ARSIs, chemotherapy, immunotherapy, radiotherapy, PARP inhibitors), despite clinical trials demonstrating inferior survival outcomes with ADT monotherapyCitation22,Citation24,Citation26. Furthermore, compared to Freedland et al.Citation24, the current study covers a more recent timeframe, includes patients who were on average 5 years younger, and patients’ treatment durations were shorter. Therefore, relative to Freedland et al.Citation24, the current study may reflect the evolving nature of the mCRPC treatment landscape, where ARSIs are increasingly used as 1 L therapy, and younger patients may be less likely to accrue additional costs in later LOTs favoring the use of more expensive or branded agents earlier. In addition, a notable difference was that the current study incorporated physician-reported clinical data for the identification of mCRPC, as opposed to reliance on claims-based algorithms which may be subject to miscoding and disease stage misclassification. The current study also included patients insured commercially and through Medicare Advantage. During baseline, costs were higher for commercially insured patients relative to those with Medicare Advantage insurance and across all lines of therapy, medical costs continued to be higher for patients with commercial relative to Medicare Advantage coverage. Despite the difference in age between these two populations, these trends are expected since a body of literature has reported that the cost of cancer care for commercially insured patients is generally higher than for those who are insured through Medicare Advantage, even for the same medical servicesCitation27–30.
In terms of HRU, a claims-based analysis by Wu et al.Citation11 found that in commercially and Medicare Advantage-insured patients, the number of all-cause hospitalizations and outpatient visits increased in the post-mCRPC progression period relative to the nmCRPC periodCitation11. This is consistent with the additional all-cause inpatient admissions and outpatient visits observed in the current study after initiation of 1 L therapy relative to the pre-mCRPC progression period. Interestingly, the number of PC-related outpatient visits was 2.7 times higher post-mCRPC progression relative to pre-mCRPC progression, decreasing following the initiation of 1 L mCRPC therapy, yet increasing with each successive LOT, suggesting increased HRU at times of disease progression. Since initiation of 1 L therapy in this study design is not suggestive of disease progression, but simply reflects treatment initiation following disease progression to mCRPC, the drop in PC-related outpatient visits after 1 L may be indicative of symptomatic improvement or delayed disease progression, while subsequent increases after 2 L/3L, as well as each LOT becoming successively shorter, may reflect disease progression.
With the approval of new medications, the guidelines for treating patients with mCRPC have evolved. Prior to the approval of ARSIs, docetaxel was the standard of care for mCRPCCitation3. The 2018 American Urological Association (AUA) Guidelines for the management of patients with CRPC aligned treatment recommendations for patients who had prior docetaxel use, or according to patient performance status. The ARSI agents, abiraterone + prednisone or enzalutamide, were cited as standard of care across patient categories, with docetaxel additionally recommended among those without prior treatment historyCitation8. Among patients previously treated with docetaxel with or without abiraterone + prednisone or enzalutamide, cabazitaxel is recommended for later LOTs based on superior survival relative to mitoxantrone in the TROPIC clinical trialCitation8,Citation31. In 2023, AUA guidelines were amended to reflect advancements in treatment, recommending clinicians offer germline and somatic genetic testing as well as treatment with a PARP inhibitor for patients with HRR-gene mutated mCRPC previously treated with enzalutamide or abiraterone, and/or a taxane-based chemotherapyCitation7. Among patients with mCRPC without a history of ARSI use, the amended guidelines continue to recommend continued ADT with abiraterone + prednisone, enzalutamide, or docetaxelCitation7. Real-world studies corroborate what is presented in guidelines, with findings documenting a shift to ARSIs in the 1 L, pushing chemotherapy to later LOTsCitation32–35. These findings align with the medication use observed in the current study where patients most commonly initiated abiraterone and enzalutamide as 1 L mCRPC therapy. However, the high proportion of patients initiating abiraterone and enzalutamide as 2 L therapy suggests that patients may be switching between these agents due to poor performance. Treatment patterns evolved to predominant use of docetaxel and cabazitaxel by 3 L. As genetic testing strategies evolve in PC disease management, future studies evaluating the integration of PARP inhibitors in the mCRPC treatment landscape are warranted. Indeed, the introduction of precision treatment will provide additional opportunities for outcome improvement in specific populations of patients while avoiding undue costs in populations with minimal expected benefit. To that end, the results of the current study provide an up-to-date characterization of the current HRU and cost burden in the US among patients with mCRPC over time as the disease progresses and various LOTs are initiated, which can help inform decision makers in clinical practice.
Limitations
Studies using various data sources have inherent limitations. First, the Flatiron algorithm for identifying CRPC status relied on physician report or observed rising PSA values and did not incorporate an evaluation of testosterone levels; as such, the evaluation of CRPC status may be subject to misclassification or reporting inaccuracies. However, this approach is superior to only leveraging claims data to identify CRPC status. The results presented in this study were obtained from two linked data sources including a database that represents the community and academic oncology perspective and an open-source healthcare claims database; therefore, results may not be representative of the entire population of patients with mCRPC in the US, which may limit the generalizability of the study. Indeed, when identifying the patient population, a notable proportion of patients did not have a claim in Komodo Health for the Flatiron oncologist-defined LOT, which may suggest an important proportion of patients were not evaluated in this study due to the absence of continuous insurance eligibility as reported by Komodo Health or patient refusal of prescribed medication – a phenomenon that is prevalent in patients using drugs with Medicare Advantage Part C benefit due to the heavy out of pocket burden associated with donut hole (the maximum patient co-pay)Citation36. Information on dual eligibility (i.e. patients who had dual insurance coverage from Medicaid and Medicare Advantage) was also not readily available in the linked databases. Furthermore, the Flatiron algorithm used to identify LOTs does not consider ADT, therefore patients treated with ADT monotherapy were not represented in this study. Moreover, results presented for the 2 L and 3 L therapy periods may have limited generalizability due to small sample size. In addition, although the Datavant Match method used for linking the two data sources has high precision, it does not guarantee perfect accuracy in correctly identifying all claims for a patient or all matches. An additional limitation is the use of administrative claims data, which depend on correct diagnosis, procedure, and drug codes. Coding inaccuracies may lead to case misidentification and cost imputation algorithms may be imprecise. Moreover, the use of administrative claims data may have resulted in the underreporting of mCRPC therapy use, if patients received treatment from other sources that were not captured in the data source (e.g. coupons or samples). In addition, all analyses were descriptive in nature and formal statistical testing would be necessary to confirm the observed numeric trends described across study periods. Furthermore, the study period overlapped with the COVID-19 pandemic which may have influenced the results; however, COVID-related HRU was low and did not appear to be a major driver of all-cause HRU and costs. Additionally, cost data is not always available in Komodo Health data, with an estimate of approximately 30% of claims having missing costs; imputation for missing costs was conducted by Komodo and costs may not represent true costs incurred by payers. Finally, this study described the HRU and direct healthcare costs of patients with mCRPC using claims data and did not incorporate an evaluation of indirect costs (e.g. lost productivity or caregiver costs), the inclusion of which would have resulted in a more comprehensive estimate of the economic burden of mCRPC.
Conclusions
In this descriptive study of patients with mCRPC treated in community oncology practices and academic centers, the economic burden was significant as patients progressed to mCRPC. The majority of the costs were driven by higher PC-related outpatient costs, which were four times higher post-mCRPC progression relative to the pre-mCRPC progression period. Furthermore, the increase in outpatient visits and chemotherapy use with each successive LOT further emphasize the humanistic burden faced by this patient population. Prior to the arrival of next-generation ARSIs, treatment costs may have been lower, but with a tradeoff for rapid progression without survival benefit. Despite the high costs associated with advanced therapies for mCRPC reported in this study, these therapies can be of high value by achieving substantial clinical benefit and delaying costly disease progression.
Transparency
Author contributions
LM, FK, AU, PL, and DP contributed to study conception and design, collection and assembly of data, and data analysis and interpretation. DRK, IK, EM, and DJG contributed to study conception and design, data analysis and interpretation. All authors reviewed and approved the final content of this manuscript.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Previous presentations
Part of the material in this manuscript was presented at the International Society for Pharmacoeconomics and Outcomes Annual Meeting from May 7-10, 2023 in Boston, MA, USA.
Data transparency
The authors declare that the data supporting the findings of this study are available within the article and its supplemental information files.
Supplemental Material
Download MS Word (48.5 KB)Acknowledgements
Medical writing assistance was provided by Jill Korsiak, PhD, and Loraine Georgy, PhD, MWC, employees of Analysis Group, Inc., a consulting company that has provided paid consulting services to Janssen Scientific Affairs, LLC., which funded the development and conduct of this study and manuscript.
Declaration of funding
This study was funded by Janssen Scientific Affairs, LLC.
Declaration of financial/other relationships
DRK is an assistant professor at Duke University School of Medicine and reports the following in the past 24 months: Janssen Scientific Affairs, LLC (consultancy). IK is an employee of Janssen Scientific Affairs, LLC and stockholder of Johnson & Johnson. At the time of this study, EM was an employee of Janssen Scientific Affairs, LLC and stockholder of Johnson & Johnson. LM, FK, AU, PL, and DP are employees of Analysis Group, Inc., a consulting company that has provided paid consulting services to Janssen Scientific Affairs, LLC, which funded the development and conduct of this study and manuscript. DJG is a professor at Duke University School of Medicine and reports the following in the past 24 months: has acted in leadership role for Capio Biosciences; has acted as a paid consultant for and/or as a member of the advisory boards of Bayer, Exelixis, Pfizer, Sanofi, Astellas Pharma, Innocrin Pharma, Bristol Myers Squibb, Genentech, Janssen, Merck Sharp & Dohme, Myovant Sciences, AstraZeneca, Michael J. Hennessy Associates, Constellation Pharmaceuticals, Physicians’ Education Resource, Propella Therapeutics, RevHealth, and xCures; has been a member of the speakers’ bureau of Sanofi, Bayer, and Exelixis; has received honoraria from Sanofi, Bayer, Exelixis, EMD Serono, OncLive, Pfizer, UroToday, Acceleron Pharma, American Association for Cancer Research, Axess Oncology, Janssen Oncology, and Millennium Medical Publishing; has received research funding from Exelixis, Janssen Oncology, Novartis, Pfizer, Astellas Pharma, Bristol Myers Squibb, Acerta Pharma, Bayer, Dendreon, Innocrin Pharma, Calithera Biosciences, and Sanofi/Aventis; and has received other research support (travel, accommodations, expenses) from Bayer, Exelixis, Merck, Pfizer, Sanofi, Janssen Oncology, and UroToday.
Data availability statement
Data that support the findings of this study were used under license from Flatiron Health, Inc. and Komodo Health Solutions. Restrictions apply to the availability of these data, which are not publicly available and cannot be shared. The data are available through request made directly to the data vendor, subject to the data vendor’s requirements for data access.
References
- Siegel RL, Miller KD, Wagle NS, et al. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48. doi: 10.3322/caac.21763.
- National Cancer Institute: surveillance Epidemiology and End Results. Cancer Stat Facts: prostate Cancer. 2022.
- Rebello RJ, Oing C, Knudsen KE, et al. Prostate cancer. Nat Rev Dis Primers. 2021;7(1):9. doi: 10.1038/s41572-020-00243-0.
- American Cancer Society. Hormone Therapy for Prostate Cancer. 2021.
- Karantanos T, Corn PG, Thompson TC. Prostate cancer progression after androgen deprivation therapy: mechanisms of castrate resistance and novel therapeutic approaches. Oncogene. 2013;32(49):5501–5511. doi: 10.1038/onc.2013.206.
- Moreira DM, Howard LE, Sourbeer KN, et al. Predictors of time to metastasis in castration-resistant prostate cancer. Urology. 2016;96:171–176. doi: 10.1016/j.urology.2016.06.011.
- Lowrance W, Dreicer R, Jarrard DF, et al. Updates to advanced prostate cancer: AUA/SUO guideline (2023). J Urol. 2023;209(6):1082–1090. doi: 10.1097/JU.0000000000003452.
- Lowrance WT, Murad MH, Oh WK, et al. Castration-resistant prostate cancer: AUA guideline amendment 2018. J Urol. 2018;200(6):1264–1272. doi: 10.1016/j.juro.2018.07.090.
- Chi KN, Rathkopf D, Smith MR, et al. Niraparib and abiraterone acetate for metastatic castration-resistant prostate cancer. J Clin Oncol. 2023;41(18):3339–3351. doi: 10.1200/JCO.22.01649.
- Clarke N, Armstrong A, Thiery-Vuillemin A, et al. Abiraterone and olaparib for metastatic castration-resistant prostate cancer. NEJM Evid. 2022;1(9). doi: 10.1056/EVIDoa2200043.
- Wu B, Li SS, Song J, et al. Total cost of care for castration-resistant prostate cancer in a commercially insured population and a medicare supplemental insured population. J Med Econ. 2020;23(1):54–63. doi: 10.1080/13696998.2019.1678171.
- Appukkuttan S, Tangirala K, Babajanyan S, et al. A retrospective claims analysis of advanced prostate cancer costs and resource use. Pharmacoecon Open. 2020;4(3):439–447. doi: 10.1007/s41669-019-00185-8.
- Janssen Pharmaceutical Companies of Johnson & Johnson. U.S. FDA approves expanded ZYTIGA® indication for treatment of metastatic castration-resistant prostate cancer. 2012.
- Astellas. U.S. FDA approves new indication for the use of XTANDI® (enzalutamide) capsules for patients with metastatic castration-resistant prostate cancer. 2014.
- AstraZeneca and Merck & Co. I. Lynparza approved in the US for HRR gene-mutated metastatic castration-resistant prostate cancer. 2020.
- Janssen Pharmaceutical Companies of Johnson & Johnson. U.S. FDA approves AKEEGA™ (niraparib and abiraterone acetate), the first-and-only dual action tablet for the treatment of patients with BRCA-positive metastatic castration-resistant prostate cancer. 2023.
- Ma X, Long L, Moon S, et al. Comparison of population characteristics in real-world clinical oncology databases in the US: flatiron health, SEER, and NPCR. medRxiv. 2020; 2020.03.16.20037143.
- Birnbaum B, Nussbaum N, Seidl-Rathkopf K, et al. Model-assisted cohort selection with bias analysis for generating large-scale cohorts from the EHR for oncology research. arXiv preprint arXiv:2001097652020. 2020.
- Datavant. Overview of Datavant’s de-identification and linking technology for structured data. 2020.
- Komodo Health Solutions. Imputing allowed amounts development and validation of an encounter-level allowed amount imputation model. 2023.
- US Department of Health and Human Services. 45 CFR 46: pre-2018 requirements. 2021.
- Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367(13):1187–1197. doi: 10.1056/NEJMoa1207506.
- Rathkopf DE, Smith MR, De Bono JS, et al. Updated interim efficacy analysis and long-term safety of abiraterone acetate in metastatic castration-resistant prostate cancer patients without prior chemotherapy (COU-AA-302). Eur Urol. 2014;66(5):815–825. doi: 10.1016/j.eururo.2014.02.056.
- Freedland S, Davis M, Epstein A, et al. Healthcare costs in men with metastatic castration-resistant prostate cancer: an analysis of US medicare Fee-For-service claims. Adv Ther. 2023;40(10):4480–4492. Online ahead of print. doi: 10.1007/s12325-023-02572-4.
- National Cancer Institute: cancer Trends Progress Report. Financial Burden of Cancer Care. 2023.
- Ryan CJ, Smith MR, Fizazi K, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015;16(2):152–160. doi: 10.1016/S1470-2045(14)71205-7.
- Congressional Budget Office. The prices that commercial health insurers and Medicare pay for hospitals’ and physicians’ services. 2022.
- Lopez E, Neuman T, Jacobson G, et al. How much more than Medicare do private insurers pay? A review of the literature. Issue brief. Kaiser Family Foundation; 2020.
- Meiselbach MK, Wang Y, Xu J, et al. Hospital prices for commercial plans are twice those for medicare advantage plans when negotiated by the same insurer. Health Aff (Millwood). 2023;42(8):1110–1118. doi: 10.1377/hlthaff.2023.00039.
- Tomicki S, Dieguez G, Latimer H, et al. Real-World cost of care for commercially insured versus medicare patients with metastatic pancreatic cancer who received guideline-recommended therapies. Am Health Drug Benefits. 2021;14(2):70–78.
- de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376(9747):1147–1154. doi: 10.1016/S0140-6736(10)61389-X.
- Wen L, Yao J, Valderrama A. Evaluation of treatment patterns and costs in patients with prostate cancer and bone metastases. J Manag Care Spec Pharm. 2019;25(3-b Suppl):S1–S11. doi: 10.18553/jmcp.2019.25.3-b.s1.
- Lavallee L, Morash C, Saad F, et al. Real-world management of metastatic castration-resistant prostate cancer (mCRPC): a national multicenter cohort study. J Clin Oncol. 2022;40(6_suppl):252–252. doi: 10.1200/JCO.2022.40.6_suppl.252.
- Shore ND, Ionescu-Ittu R, Laliberté F, et al. Beyond frontline therapy with abiraterone and enzalutamide in metastatic castration-resistant prostate cancer: a real-world US study. Clin Genitourin Cancer. 2021;19(6):480–490. doi: 10.1016/j.clgc.2021.07.009.
- George DJ, Sartor O, Miller K, et al. Treatment patterns and outcomes in patients with metastatic castration-resistant prostate cancer in a real-world clinical practice setting in the United States. Clin Genitourin Cancer. 2020;18(4):284–294. doi: 10.1016/j.clgc.2019.12.019.
- Medicare.gov. Costs in the coverage gap. 2023.