Abstract
Aims
This study described treatment patterns, healthcare resource utilization (HRU) and costs among advanced or metastatic non-small cell lung cancer (a/mNSCLC) patients with different epidermal growth factor receptor (EGFR) mutation types.
Materials and methods
This retrospective study leveraged NeoGenomics NeoNucleus linked with IQVIA PharMetrics Plus between 01 January 2016 to 30 April 2021 (study period). Patients with evidence of a/mNSCLC between 01 July 2016 to 31 March 2021 (selection window) with EGFR test results indicating exon 19 deletion (exon19del), exon 21 L858R (L858R), or exon 20 insertion (exon20i) mutations were included; date of first observed evidence of a/mNSCLC was the index date. Treatment patterns, all-cause HRU and costs during ≥1 month follow-up were reported for each cohort (exon19del, L858R, and exon20i).
Results
A total of 106 exon19del, 75 L858R, and 13 exon20i patients met the study criteria. The prevalence of hospitalization was highest in the exon20i cohort (76.9%), followed by L858R (62.7%) and exon19del (55.7%) cohorts. A higher proportion of patients had evidence of hospice/end-of-life care in the exon20i (30.8%) and L858R (29.3%) cohorts relative to the exon19del cohort (22.6%). The exon20i cohort had higher median total healthcare costs per patient per month ($27,069) relative to exon19del ($17,482) and L858R ($17,763). EGFR tyrosine kinase inhibitors (TKI) were the most frequently observed treatment type for exon19del and L858R cohorts, while chemotherapy was the most observed treatment in exon20i cohort.
Limitations
The sample size for the study cohorts was small, thus no statistical comparisons were conducted.
Conclusions
This is one of the first real-world studies to describe HRU and costs among a/mNSCLC patients by specific EGFR mutation type. HRU and costs varied between EGFR mutation types and were highest among exon20i cohort, potentially reflecting higher disease burden and unmet need among patients with this mutation.
PLAIN LANGUAGE SUMMARY
Patients with non-small cell lung cancer (NSCLC) in an advanced or metastatic stage (a/mNSCLC) where cancer has spread to other parts of the body have high chance of dying within five years. Treatment and management of a/mNSCLC also incurs significant healthcare resource utilization (HRU) and costs. Patients with a/mNSCLC may have their epidermal growth factor receptor (EGFR) gene mutated with different variations. Our study described what a/mNSCLC patients were treated with, their HRU and healthcare costs separately for the following three types of EGFR mutations: exon 19 deletion (exon19del), exon 21 L858R (L858R), or exon 20 insertion (exon20i). Our study found that patients with exon19del or L858R mutation were commonly treated with EGFR tyrosine kinase inhibitors (TKIs), while exon20i patients were mostly treated with chemotherapy due to lack of targeted treatment for exon20i during the time when the study was conducted. HRU and healthcare costs were highest for patients with exon20i, which shows that patients with exon20i face high burden and have a need for new treatment options.
Introduction
Lung cancer is the third most common cancer in the United States (US) and the leading cause of cancer mortality, with an estimated 238,340 new cases and 127,070 deaths in 2023, accounting for 12.2% of all new cancer cases and 20.8% of all cancer deathsCitation1. Non-small cell lung cancer (NSCLC) accounts for 80–90% of all lung cancers and is mostly diagnosed at an advanced stageCitation2,Citation3. In addition, the five-year survival rate is approximately 34% for patients initially diagnosed at regional stage and merely 7% for patients diagnosed at distant stageCitation4. In the US, approximately 24% of patients with NSCLC have a sensitizing mutation in the epidermal growth factor receptor (EGFR) gene that makes the tumor cells dependent on EGFR signaling and thus, a therapeutic target for molecular inhibition via TKIsCitation5. Previous studies have suggested that EGFR mutation was associated with improved overall survival compared to EGFR wild type partly due to availability of TKIs targeting EGFR and better clinicopathologic characteristics associated with EGFR mutationCitation6–8. To that end, the 2023 National Comprehensive Cancer Network (NCCN) guidelines recommend molecular testing for all advanced or metastatic NSCLC (a/mNSCLC) cases, especially those of adenocarcinoma subtype, to ensure the most appropriate treatment strategyCitation9.
The most common EGFR mutations are in-frame exon 19 deletion (exon19del) and exon 21 L858R point mutation (L858R), comprising approximately 45% and 44% of all EGFR mutations detected, respectivelyCitation10. The 2023 NCCN guidelines recommend EGFR TKIs for treatment of a/mNSCLC with EGFR exon19del or L858R mutationsCitation9. Previous studies have shown better survival outcomes and response rate for exon19del compared to L858R mutations after EGFR TKI therapyCitation11–13. EGFR exon 20 insertion mutations (exon20i) are more rare (5–12%)Citation14 and are associated with de novo resistance to TKIs, limiting the treatment options for these patientsCitation15.
Prior studies have shown that healthcare costs remain a cornerstone in oncologyCitation16,Citation17. In 2020, the estimated national expenditures for care of lung cancer was $23.8 billionCitation17, with attributed costs of $109,103 per NSCLC patient in the last year of lifeCitation17. A recent claims-based retrospective study found total all-cause costs of $25,499 per patient per month (PPPM) during the first line of therapy (1LOT), which consisted of drug-related costs ($15,356), other outpatient costs ($5400), inpatient costs ($4167), and other costs ($576) among EGFR mutated advanced NSCLC patientsCitation18. Similarly, there are many studies that describe cost-effectiveness of NSCLC treatment or associated healthcare costs among EGFR mutated NSCLC patients receiving treatmentsCitation19–21. Within EGFR mutated a/mNSCLC, differences in disease subtype based on EGFR mutation types or differences in responsiveness to available targeted therapies may lead to differences in HRU and healthcare costsCitation22; however, there is a lack of real-world evidence on the HRU and costs for specific EGFR mutation types.
This retrospective study leveraged a large claims database linked to genomic data to describe patient characteristics, treatment patterns, HRU and costs of a/mNSCLC patients by the specific EGFR mutation type: exon19del, L858R and exon20i.
Methods
Study design and data sources
This retrospective, descriptive cohort study leveraged laboratory-based genomic testing data from NeoGenomics NeoNucleus (NeoGenomics) linked to medical and pharmacy claims from IQVIA’s PharMetrics Plus database from 1 January 2016 to 30 April 2021 (study period; Supplementary S1). NeoGenomics is the largest cancer testing database in the US with approximately 1 million oncology tests performed per year. NeoGenomics includes data from >4400 practices across the US from all practice types and representation in all states. IQVIA PharMetrics Plus database comprises of fully adjudicated medical and pharmacy claims for more than 210 million unique enrollees and is representative of the commercially insured US population under 65 years old. The linkage of patient data between the two databases was achieved by a HIPAA-compliant deterministic matching algorithm which utilized de-identified data. An encryption algorithm was applied at the source to de-identify patient-level information by creating Patient IDs which were matched across datasetsCitation23.
Study population
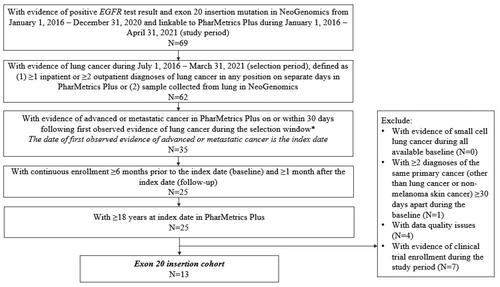
Three mutually exclusive study cohorts were created: exon19del, L858R, and exon20i. The exon19del and L858R cohorts included adult patients (≥18 years) with a/mNSCLC between 1 July 2016 to 31 March 2021 (selection window) in PharMetrics Plus with common EGFR mutation (exon19del or L858R) positive test results between 1 January 2016 and 31 December 2020 in NeoGenomics (). Patients were considered to have lung cancer if they had ≥1 inpatient or ≥2 outpatient diagnoses of lung cancer in any position of the claim on separate days in PharMetrics PlusCitation18. Patients were also required to have evidence of advanced or metastatic cancer, defined as ≥1 antineoplastic therapy or ≥1 diagnosis for metastatic cancer (Supplementary S2) in any position, on or within 30 days following the first lung cancer diagnosis. The date of earliest observed evidence of advanced or metastatic cancer was defined as the index date. All patients were required to have ≥6 months of continuous enrollment before index date and ≥1 month after the index date. Other inclusion and exclusion criteria are detailed in . Patients were stratified into exon19del or L858R cohorts based on their EGFR mutation type.
Figure 1. Patient selection for common EGFR mutation cohorts (exon19del and L858R). *Advanced or metastatic cancer was defined as evidence of ≥1 antineoplastic therapy or ≥1 diagnosis code for metastatic cancer in any position. Abbreviations. EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor.
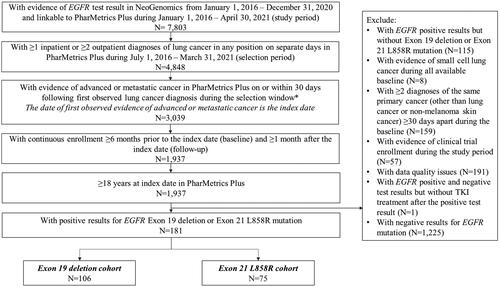
The exon20i cohort was selected separately to maximize sample size due to the low prevalence of this mutation type using similar eligibility criteria () yet al.so allowing for evidence of lung cancer to be defined as a sample collected from the lung in NeoGenomics.
Study measures
Patient demographic and baseline clinical characteristics were assessed during the 6-month baseline period; treatment patterns including LOT, all-cause HRU and healthcare costs, and location and number of incident metastatic sites were reported during ≥1 month follow-up for each cohort. EGFR testing patterns were also evaluated over the study period. The end of follow-up was defined as the earliest of end of continuous enrollment or end of the study period. LOT was defined by systemic treatment regimens using the dates of initiation and discontinuation of drugs of interest. Treatments started ≤30 days of another agent were counted within the same LOT (i.e. combination regimen). A new LOT was identified upon one of the following events: (1) switch or augmentation (i.e. additional therapies initiated after the first 30-day window within the LOT) or (2) evidence of treatment after discontinuation, defined as >60-day gap in treatment sessions after the date of last claim plus the days’ supply for oral drugs, and the last date of administration + 28 days for injectable drugs.
Statistical analysis
Study measures were described for each cohort using descriptive statistics. Categorical variables were reported in frequency (n) and percentage (%), while continuous variables were reported with mean, standard deviation (SD), median, first quartile (Q1), third quartile (Q3), minimum (min) and maximum (max). All costs included the amount paid by the insurer/payers and out-of-pocket costs by patients and were adjusted to 2021 US dollar using the medical care component of the US Consumer Price Index and reported PPPM over the follow-up for each cohortCitation24. All analyses were conducted using SAS Release 9.4 (SAS Institute Inc., Cary, NC).
Results
Study sample
A total of 106 patients with a/mNSCLC had positive results for exon19del mutation (i.e. exon19del cohort), 75 for L858R mutation (i.e. L858R cohort) (), and 13 for exon20i mutation (i.e. exon20i cohort) ().
Patient characteristics
On average, patients in L858R cohort were older (mean [SD] 63.3 [8.3] years) relative to exon19del (59.9 [8.6]) or exon20i (58.0 [10.7]) cohorts (). Patients were predominantly female across cohorts, ranging from 62.3% in exon19del cohort to 76.9% in exon20i cohort. For all cohorts, the majority of patients were located in the South or West region of the US (69.2–73.6%); the exon20i cohort had higher proportion of patients located in the Northeast (23.1%) relative to exon19del (7.6%) and L858R (5.3%) cohorts. The most prevalent payer type was commercial payers (61.5–68.9%) across the cohorts.
Table 1. Baseline characteristics.
The exon20i cohort had a higher proportion of patients with Charlson Comorbidity Index (CCI) score (excluding cancer) of ≥3 (15.4%) relative to the L858R (10.7%) or the exon19del (4.7%) cohorts. The most commonly observed baseline comorbidities/conditions across cohorts were infection (60.0%–76.9%), hypertension (42.5–46.7%) and chronic pulmonary disease (23.1–25.3%). There was a higher proportion of patients in the exon20i cohort with evidence of fatigue/asthenia (46.2%) relative to exon19del (17.0%) and L858R (22.7%); a similar pattern was observed for obesity (exon20i: 30.8%; exon19del: 16.0%; and L858R: 10.7%).
During baseline, bone and bone marrow was the most common location of metastasis across all cohorts, with the highest prevalence observed in the exon20i (53.8%), followed by L858R (44.0%) and exon19del (42.5%) cohorts (). Other common locations of metastasis at baseline included central nervous system (CNS; 15.4–33.0%), lymph nodes (15.1–30.7%) and the respiratory system (7.7–33.0%). There was a higher proportion of patients having ≥2 metastasis sites during baseline in the exon19del (43.4%) and L858R (40.0%) cohorts than the exon20i cohort (30.8%).
Table 2. Metastasis during baseline and follow-up.
During ≥1 month of follow-up, the prevalence of incident metastasis was higher in the exon20i cohort (92.3%) than the exon19del (67.0%) and L858R (66.7%) cohorts. Respiratory system was the most common incident metastasis site over follow-up for all cohorts (exon19del: 26.4%; L858R: 30.7%; exon20i: 61.2%), followed by bone and bone marrow for exon19del (25.5%) and L858R (22.7%) and CNS in exon20i (46.2%).
EGFR testing patterns
All patients in the exon20i cohort and the majority of L858R (86.7%) and exon19del (69.8%) cohorts had EGFR test results within one month after the index date or before the index date. Among patients with systemic therapies observed during follow-up, all patients in exon20i cohort and most (89.4%) in L858R cohort had EGFR test results before initiation of first post-index therapy, while one-third of patients in exon19del cohort (32.0%) had EGFR test results after the initiation of first post-index therapy ().
Table 3. EGFR testing and treatment patterns during the follow-up.
Treatment patterns
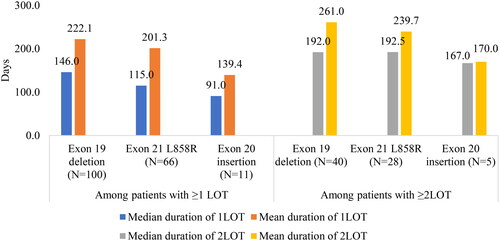
The median (Q1–Q3) duration of follow-up was 11.5 (6.0–24.1) months for exon19del, 9.7 (5.6–20.1) months for L858R, and 10.9 (3.3–16.9) months for exon20i cohorts (). The exon20i cohort had the highest proportion of patients with ≥3 LOTs observed during follow-up (23.1%) relative to exon19del (13.2%) and L858R (12.0%). The most commonly observed drug class during follow-up in the exon19del and L858R cohorts was EGFR TKI (84.9% and 80.0%, respectively). Osimertinib was the most commonly used EGFR TKI for exon19del cohort (61.3%) and L858R cohort (64.0%), followed by erlotinib (exon19del: 29.2%; L858R: 25.3%) and afatinib (exon19del: 14.2%; L858R: 9.3%). The only EGFR TKI used in the exon20i cohort was osimertinib. In the exon20i cohort, non-platinum-based chemotherapy (69.2%) and platinum-based chemotherapy (61.5%) were the most commonly observed classes, while EGFR TKI was used by 30.8% of patients. Among patients with ≥1LOT (94.3% of exon19del, 88.0% of the L858R, and 84.6% of the exon20i), the majority of patients had EGFR TKI as 1LOT in the exon19del and L858R (75.0% and 72.7%, respectively) cohorts while combination therapy of non-platinum and platinum-based chemotherapy was the most frequently observed (36.4%) 1LOT for the exon20i cohort. Similar patterns were observed for 2LOT (). The median duration of 1LOT and 2LOT was shorter in the exon20i cohort (91.0 days and 167.0 days, respectively) relative to exon19del (146.0 days and 192.0 days, respectively) and L858R (115.0 days and 192.5 days, respectively) cohorts ().
All-cause HRU and healthcare costs
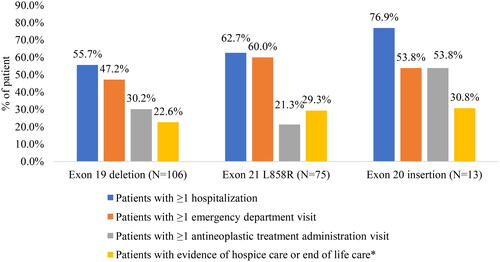
Overall, HRU was higher in the exon20i cohort. The proportion of patients with all-cause hospitalization during follow-up was higher in the exon20i cohort (76.9%) relative to exon19del (55.7%) and L858R (62.7%) cohorts (). Approximately half of patients in exon20i (53.8%) and exon19del (47.2%) cohort and 60.0% of the L858R cohort had ≥1 all-cause ED visit during follow-up, and the proportion of patients with ≥1 office visit associated with antineoplastic treatment administration was higher in exon20i cohort (53.8%) relative to exon19del (30.2%) and L858R (21.3%). Lastly, evidence of hospice care or end of life care was also higher in the exon20i (30.8%) and L858R (29.3%) cohorts than the exon19del cohort (22.6%). The frequency of all-cause HRU per person was also relatively higher in the exon20i cohort across different types of HRU categories (Supplementary S3).
Figure 4. All-cause healthcare resource utilization during follow-up. Hospice care was defined using place of service codes and does not include home visits with a hospice nurse; end-of-life care was identified using an exploratory claims-based proxy, with evidence of any of the following during the last month in which medical and pharmacy claims were available prior to disenrollment: cerebral death, cardiac event including resuscitation, cardiac arrest/failure, respiratory arrest, hepatic coma, acute myocardial infarction, intensive care unit, critical care, do not resuscitate status, palliative care or death.
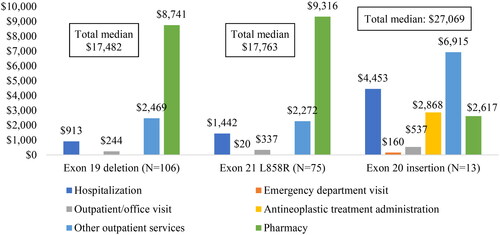
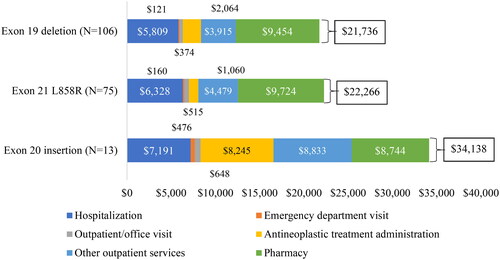
The exon20i cohort had the highest median (Q1–Q3) total all-cause healthcare costs PPPM of $27,069 ($16,206–$39,662), followed by $17,763 ($11,537–$25,748) in L858R and $17,482 ($9557–$27,521) in exon19del cohorts (). The mean (SD) total healthcare costs and breakdown of cost components are displayed in and Supplementary S4. In the exon20i cohort, 74.4% of the total mean healthcare costs were medical services, including antineoplastic treatment administration (24.2%), hospitalization (21.1%) and other outpatient services (25.9%). In the exon19del and L858R cohorts, the main drivers of total healthcare costs were pharmacy costs (43.5% and 43.7%, respectively), followed by hospitalizations (26.7% and 28.4%).
Discussion
To our knowledge, this is one of the first claims-based real-world studies to describe patient characteristics, treatment patterns, and economic burden among a/mNSCLC patients by specific EGFR mutation types, which are known to have important implications on treatment decisions and disease outcomesCitation9. Although a/mNSCLC typically affects older patients (mean age at NSCLC diagnosis 70 yearsCitation25), the mean age of our study cohorts was around 60 years old; reflective of the PharMetrics Plus study database which includes a younger, commercially insured US population. The majority of patients in our study were female (across all cohorts), with the highest proportion observed in the exon20i cohort (76.9%). Our findings align with previous studies by Arcila et al. which reported median age of 66 years and 66.7% females, and by Oxnard et al. which reported median age of 60 years and 70.4% female among NSCLC patients with exon20iCitation26,Citation27. Similarly, a systematic literature review found that pooled prevalence of EGFR mutation was more common among female (43.7%) compared to male patients (24.0%)Citation5. In our study, the exon20i cohort had a higher comorbidity burden relative to the other cohorts with common EGFR mutations, with higher prevalence of certain comorbidities including fatigue/asthenia and obesity. Across all cohorts in our study, the most commonly observed location of metastasis during baseline was bone and bone marrow. Multiple previous retrospective database studies have also shown bone and brain as the common location of metastasis among NSCLC patients at baselineCitation28–30.
Our study found that the majority of patients in all cohorts had EGFR test results within one month after the first evidence of a/m NSCLC and before the initiation of first systemic therapy post-index. This finding is aligned with the 2023 NCCN treatment guideline where EGFR test result plays an important role in treatment decision makingCitation9. However, despite all patients in the exon20i cohort having their EGFR mutation test results before initiating therapy, nearly one-third of patients continued to be treated with EGFR TKIs not indicated for exon20i mutation during the study period. This treatment decision underscores the lack of other targeted therapy options for patients with exon20i mutations where chemotherapies were the most commonly observed treatment over the follow-up, which could have contributed to higher total healthcare costs from potential adverse events. Amivantamab (a bispecific EGFR-directed and MET receptor-directed antibody) and mobocertinib (an irreversible TKI), have been approved by FDA for treatment of a/mNSCLC with EGFR exon20i mutation in 2021 and therefore, were not included within the scope of current studyCitation31. However, mobocertinib has been withdrawn from the market following failure to meet primary endpoints in the Phase 3 EXCLAIM-2 study in 2023Citation32.
In our study, the majority of patients in exon19del and L858R cohorts had EGFR TKI as 1LOT (75.0% and 72.7%, respectively) and as 2LOT (65.0% and 50.0%), which is consistent with a previous retrospective real-world study that found 82.2% and 61.1% of EGFR positive advanced NSCLC patients had EGFR TKI utilization in their 1LOT or 2LOT, respectivelyCitation33. On the other hand, non-platinum and platinum-based chemotherapy were the most commonly observed treatment classes for the exon20i cohort and TKIs were only observed in 18.2% of patients’ 1LOT and 20.0% of the 2LOT. This aligns with the NCCN treatment guidelines where systemic therapy serves as the 1LOT for exon20i mutation before subsequent targeted therapy, given the general lack of response to EGFR TKI therapyCitation9. This finding also resonates with a previous systematic literature review assessing clinical burden of exon20i mutation, which found a trend of greater overall survival and response for chemotherapy compared with TKIs as 1LOTCitation14. Furthermore, the shorter treatment duration observed for 1LOT and 2LOT in the exon20i cohort relative to the exon19del and L858R cohorts may indicate the lack of effective targeted treatment during the study period and worse prognosis among patients with exon20i mutation compared to the common EGFR mutations, which has been suggested in previous studiesCitation34,Citation35. Exon19del and L858R cohorts had similar use of EGFR TKIs, which aligns with a prior study assessing treatment patterns by EGFR mutation types; the higher prevalence of osimertinib use observed in our study (61.3–64.0%) compared to the prior study (24.8% and 35.2% in 2LOT and 3LOT, respectively) may be due to our study including more recent years of data as osimertinib was approved as a 1LOT for mNSCLC with EGFR exon19del or L858R mutation in 2018Citation35,Citation36. Osimertinib was the only EGFR TKI used in the exon20i cohort in our study, which is different from the previously published studies where other EGFR TKIs (afatinib or erlotinib) were observed; this may be due to differences in study time period and the small sample size of exon20i cohort in our study (n = 13)Citation34,Citation35.
To that end, in our study, the exon20i cohort had the highest proportion of patients with incident metastasis during follow-up, particularly in respiratory system and CNS. The higher proportion of patients with multiple incident metastatic sites observed during follow-up in this cohort may be one of the contributing factors of poorer prognosis among patients with exon20i mutation compared to the common EGFR mutationsCitation37. Along these lines, we found that the exon20i cohort had higher prevalence of hospitalizations and evidence of hospice or end of life care relative to the exon19del and L858R cohorts; total all-cause healthcare costs PPPM were also higher in the exon20i cohort relative to exon19del and L858R cohorts. Within the common EGFR mutation cohorts in our study, HRU for the L858R cohort was higher relative to that observed for the exon19del cohort, including hospitalization and hospice/end of life care, which aligns with previous studies reporting worse clinical outcomes for patients with L858R mutation compared to those with exon19del mutationCitation5,Citation38.
While prior studies have demonstrated HRU and costs among mNSCLC patients with EGFR mutationsCitation39, our study is one of the first claims-based studies to describe economic burden by different mutation types. A recent retrospective claims database study reported mean total all-cause healthcare costs of $25,499 PPPM among mNSCLC patients with EGFR mutation, of which pharmacy costs account for nearly half of the total costs ($11,752)Citation18. Our study found similar results for exon19del and L858R cohorts, where pharmacy costs were the main driver of total healthcare costs, which may be associated with use of EGFR TKIs in over 80% of these cohorts. In contrast, the main cost driver for the exon20i cohort was medical service costs, particularly costs associated with antineoplastic treatment administration and hospitalization, which reflect the high proportion of exon20i cohort receiving chemotherapy along with the high prevalence of hospitalization observed.
The strengths of our study include use of fully adjudicated medical and pharmacy claims from PharMetrics Plus linked to NeoGenomics data. The PharMetrics Plus database provides comprehensive capture of HRU and healthcare costs across all settings of care. Additionally, while most previously published studies that used administrative claims databases in the NSCLC population relied on a combination of lung cancer diagnoses and initiation of NSCLC-related treatments to proxy patients with EGFR mutationCitation21,Citation40, the NeoGenomics data provides confirmed EGFR mutation status and mutation types; a novelty of the study at-hand.
Our study has limitations inherent to a retrospective study using claims data, including potential misclassification of diagnosis records, data entry error and lack of social determinants of health or clinical data to confirm disease stage. However, evidence of cancer treatment and diagnosis for metastatic disease were used as a proxy for advanced or metastatic cancer stage. Future studies including social determinants of health, such as race/ethnicity and education level, may provide more insight into potential disparities in healthcare costs and HRU among this patient population. Similarly, data on mortality was not available in the claims database. To address this limitation, proportion of patients with evidence of hospice or end-of-life care were reported, although this is likely to be underreported. While costs were reported PPPM, the interpretation of the HRU over the follow-up period should also consider different duration of follow-up between cohorts. In addition, the study population is primarily commercially insured and therefore the findings may not be generalizable to patients with a/mNSCLC who are uninsured or are covered by Medicaid or traditional Medicare. Finally, sample sizes for the study cohorts were small, and findings should be reassessed as more patients with longer follow-up become available. Future studies with larger sample size, including recently approved targeted therapy for exon20i (i.e. amivantamab), are warranted to confirm our findings and understand the impact of amivantamab on HRU and costs.
Conclusions
This is one of the first real-world studies to describe treatment patterns, HRU and healthcare costs among a/mNSCLC patients with specific EGFR mutation types. Patients with EGFR exon20i mutations had higher HRU, healthcare costs and evidence of disease progression relative to the more common EGFR mutations (i.e. exon19del and L858R), suggesting worse prognosis and higher disease burden. Furthermore, while EGFR TKIs approved for exon19del and L858R mutations are not recommended for the treatment of exon20i mutation, nearly one-third of exon20i patients were treated with EGFR TKIs, highlighting the need for therapy options targeting exon20i and the importance of understanding physician treatment decisions in this group of patients.
Transparency
Declaration of funding
This study was supported by Janssen Scientific Affairs, LLC (Horsham, PA, USA).
Declaration of financial/other relationships
PV, DW and JV are employees of Janssen Scientific Affairs10.13039/100017183, LLC. IL, JC, DT and AN are employees of IQVIA, which received funding for this study from Janssen Scientific Affairs10.13039/100017183, LLC. DL is employee of NeoGenomics, which received part of the research funding Janssen provided to IQVIA. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article and take responsibility for the integrity of the work as a whole. IL and JC drafted the manuscript, and all authors critically revised the manuscript. All authors read and approved the final manuscript.
Previous presentations
Part of this research was presented at the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) 2023.
Supplemental Material
Download MS Word (75 KB)Acknowledgements
No assistance in the preparation of this article is to be declared.
Data availability statement
The original de-identified data used in this analysis were obtained from and are the property of IQVIA and NeoGenomics. IQVIA and NeoGenomics have restrictions prohibiting the authors from making the data set publicly available. Interested researchers may contact IQVIA/NeoGenomics to apply to gain access to the study’s data in the same way the authors obtained the data (see https://www.iqvia.com/contact/sf or https://neogenomics.com/contact-us).
References
- Cancer Stat Facts: Lung and Bronchus Cancer. National Cancer Institute: surveillance, epidemiology and end results program [accessed 2023 Jul]. Available from: https://seer.cancer.gov/statfacts/html/lungb.html
- Planchard D, Popat S, Kerr K, et al. Metastatic non-small cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up†. Ann Oncol. 2018;29(Suppl 4):iv192–iv237. doi: 10.1093/annonc/mdy275.
- Besse B, Adjei A, Baas P, et al. 2nd ESMO consensus conference on lung cancer: non-small-cell lung cancer first-line/second and further lines of treatment in advanced disease. Ann Oncol. 2014;25(8):1475–1484. doi: 10.1093/annonc/mdu123.
- American Cancer Society. Lung cancer survival rates; [accessed 2023 April 5]. Available from: https://www.cancer.org/cancer/lung-cancer/detection-diagnosis-staging/survival-rates.html
- Zhang Y-L, Yuan J-Q, Wang K-F, et al. The prevalence of EGFR mutation in patients with non-small cell lung cancer: a systematic review and meta-analysis. Oncotarget. 2016;7(48):78985–78993. doi: 10.18632/oncotarget.12587.
- Li WY, Zhao TT, Xu HM, et al. The role of EGFR mutation as a prognostic factor in survival after diagnosis of brain metastasis in non-small cell lung cancer: a systematic review and meta-analysis. BMC Cancer. 2019;19(1):145. doi: 10.1186/s12885-019-5331-z.
- Fang S, Wang Z. EGFR mutations as a prognostic and predictive marker in non-small-cell lung cancer. Drug Des Devel Ther. 2014;8:1595–1611. doi: 10.2147/dddt.S69690.
- Suda K, Mitsudomi T, Shintani Y, et al. Clinical impacts of EGFR mutation status: analysis of 5780 surgically resected lung cancer cases. Ann Thorac Surg. 2021;111(1):269–276. doi: 10.1016/j.athoracsur.2020.05.041.
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: non-small cell lung cancer; [updated 2023 Apr 13; accessed 2023 Jul]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
- Graham RP, Treece AL, Lindeman NI, et al. Worldwide frequency of commonly detected EGFR mutations. Arch Pathol Lab Med. 2017;142(2):163–167. doi: 10.5858/arpa.2016-0579-CP.
- Ullas B, Bivas B, Kumar P, et al. Differential clinicopathological features, treatments and outcomes in patients with exon 19 deletion and exon 21 L858R EGFR mutation-positive adenocarcinoma non-small-cell lung cancer. BMJ Open Respir Res. 2023;10(1):e001492. doi: 10.1136/bmjresp-2022-001492.
- Sheng M, Wang F, Zhao Y, et al. Comparison of clinical outcomes of patients with non-small-cell lung cancer harbouring epidermal growth factor receptor exon 19 or exon 21 mutations after tyrosine kinase inhibitors treatment: a meta-analysis. Eur J Clin Pharmacol. 2016;72(1):1–11. doi: 10.1007/s00228-015-1966-0.
- Jiang H, Zhu M, Li Y, et al. Association between EGFR exon 19 or exon 21 mutations and survival rates after first‑line EGFR‑TKI treatment in patients with non‑small cell lung cancer. Mol Clin Oncol. 2019;11(3):301–308. doi: 10.3892/mco.2019.1881.
- Burnett H, Emich H, Carroll C, et al. Epidemiological and clinical burden of EGFR exon 20 insertion in advanced non-small cell lung cancer: a systematic literature review. PLoS One. 2021;16(3):e0247620. doi: 10.1371/journal.pone.0247620.
- Vyse S, Huang PH. Targeting EGFR exon 20 insertion mutations in non-small cell lung cancer. Signal Transduct Target Ther. 2019;4(1):5. doi: 10.1038/s41392-019-0038-9.
- Bonetti A, Giuliani J. Implications of drugs with rebate in Europe. Lancet Reg Health Eur. 2021;3:100060. doi: 10.1016/j.lanepe.2021.100060.
- National Cancer Institute. Financial burden of cancer care; [accessed 2023 May 3]. Available from: https://progressreport.cancer.gov/after/economic_burden
- Vanderpoel J, Emond B, Ghelerter I, et al. Healthcare resource utilization and costs in patients with EGFR-Mutated advanced non-small cell lung cancer receiving first-Line treatment in the United States: an insurance claims-based descriptive analysis. Pharmacoecon Open. 2023;7(4):617–626. doi: 10.1007/s41669-023-00407-0.
- Albaba H, Lim C, Leighl NB. Economic considerations in the use of novel targeted therapies for lung cancer: review of current literature. Pharmacoeconomics. 2017;35(12):1195–1209. doi: 10.1007/s40273-017-0563-8.
- Shen C, Holguin RAP, Schaefer E, et al. Utilization and costs of epidermal growth factor receptor mutation testing and targeted therapy in Medicare patients with metastatic lung adenocarcinoma. BMC Health Serv Res. 2022;22(1):470. doi: 10.1186/s12913-022-07857-y.
- Samuelsen C, Lim J, Golembesky A, et al. Healthcare resource utilization and costs associated with patients prescribed afatinib or erlotinib as first-line therapy for EGFR mutation-positive NSCLC in the United States. J Med Econ. 2020;23(1):48–53. doi: 10.1080/13696998.2019.1645681.
- Hou J, Li H, Ma S, et al. EGFR exon 20 insertion mutations in advanced non-small-cell lung cancer: current status and perspectives. Biomark Res. 2022;10(1):21. doi: 10.1186/s40364-022-00372-6.
- Huse D, Russo P, Vasey J. Expanding the evidence base in outcomes research: linking electronic medical records and claims data. ISPOR Connect. 2013;19(3):5–7.
- US Bureau of Labor Statistics. Medical care in U.S. city average, all urban consumers, seasonally adjusted. 2023 [accessed 2023 Aug 15]. Available from: https://data.bls.gov/timeseries/CUSR0000SAM
- ASCO. Lung cancer - non-small cell: statistics; [accessed 2022 Sept 30]. Available from: https://www.cancer.net/cancer-types/lung-cancer-non-small-cell/statistics
- Arcila ME, Nafa K, Chaft JE, et al. EGFR exon 20 insertion mutations in lung adenocarcinomas: prevalence, molecular heterogeneity, and clinicopathologic characteristics. Mol Cancer Ther. 2013;12(2):220–229. doi: 10.1158/1535-7163.Mct-12-0620.
- Oxnard GR, Lo PC, Nishino M, et al. Natural history and molecular characteristics of lung cancers harboring EGFR exon 20 insertions. J Thorac Oncol. 2013;8(2):179–184. doi: 10.1097/JTO.0b013e3182779d18.
- Tamura T, Kurishima K, Nakazawa K, et al. Specific organ metastases and survival in metastatic non-small-cell lung cancer. Mol Clin Oncol. Jan 2015;3(1):217–221. doi: 10.3892/mco.2014.410.
- Carroll R, Bortolini M, Calleja A, et al. Trends in treatment patterns and survival outcomes in advanced non-small cell lung cancer: a Canadian population-based real-world analysis. BMC Cancer. 2022;22(1):255. doi: 10.1186/s12885-022-09342-5.
- Chen Y, Deng J, Liu Y, et al. Analysis of metastases in non-small cell lung cancer patients with epidermal growth factor receptor mutation. Ann Transl Med. 2021;9(3):206–206. doi: 10.21037/atm-20-2925.
- Olivier T, Prasad V. Amivantamab and mobocertinib in exon 20 insertions EGFR mutant lung cancer, challenge to the current guidelines. Transl Oncol. 2022;23:101475. doi: 10.1016/j.tranon.2022.101475.
- Takeda Press Releases. Takeda provides update on EXKIVITY® (mobocertinib); [accessed 2023 Oct]. Available from: https://www.takeda.com/newsroom/newsreleases/2023/Takeda-Provides-Update-on-EXKIVITY-mobocertinib/
- Riely GJ, Lovly CM, Messina CG, et al. Real-world utilization of EGFR TKI therapies and treatment outcomes in patients with advanced EGFR-sensitizing mutation-positive NSCLC. J Clin Oncol. 2019;37(15_suppl):9062–9062. doi: 10.1200/JCO.2019.37.15_suppl.9062.
- Chelabi S, Mignard X, Leroy K, et al. EGFR exon 20 insertion in metastatic non-small-cell lung cancer: survival and clinical efficacy of EGFR tyrosine-kinase inhibitor and chemotherapy. Cancers. 2021;13(20):5132. doi: 10.3390/cancers13205132.
- O'Sullivan DE, Jarada TN, Yusuf A, et al. Prevalence, treatment patterns, and outcomes of individuals with EGFR positive metastatic non-small cell lung cancer in a Canadian real-world setting: a comparison of exon 19 deletion, L858R, and exon 20 insertion EGFR mutation carriers. Curr Oncol. 2022;29(10):7198–7208. doi: 10.3390/curroncol29100567.
- Food and Drug Administration. Supplement approval: NDA 208065/S-008; [accessed 2023 Sept 21]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2018/208065Orig1s008ltr.pdf
- Gibson AJW, Li H, D'Silva A, et al. Impact of number versus location of metastases on survival in stage IV M1b non-small cell lung cancer. Med Oncol. 2018;35(9):117. doi: 10.1007/s12032-018-1182-8.
- Yang W, Gao Y, Li X, et al. Postoperative survival of EGFR-TKI-targeted therapy in non-small cell lung cancer patients with EGFR 19 or 21 mutations: a retrospective study. World J Surg Oncol. 2017;15(1):197. doi: 10.1186/s12957-017-1251-z.
- Giuliani J, Bonetti A. Pharmacologic costs of tyrosine kinase inhibitors in first-Line therapy for advanced non-small-cell lung cancer with activating epidermal growth factor receptor mutations: a review of pivotal phase III randomized controlled trials. Clin Lung Cancer. 2016;17(2):91–94. doi: 10.1016/j.cllc.2015.12.005.
- Choi YC, Zhang D, Tyczynski JE. Comparison between health insurance claims and electronic health records (EHRs) for metastatic non-small-cell lung cancer (NSCLC) patient characteristics and treatment patterns: a retrospective cohort study. Drugs Real World Outcomes. 2021;8(4):577–587. doi: 10.1007/s40801-021-00269-0.