Abstract
Background
Ebola virus disease (EVD) continues to be a major public health threat globally, particularly in the low-and-middle-income countries (LMICs) of Africa. The social and economic burdens of EVD are substantial and have triggered extensive research into prevention and control. We aim to highlight the impact and economic implications, identify research gaps, and offer recommendations for future economic studies pertaining to EVD.
Method
We conducted a comprehensive librarian-led search in PubMed/Medline, Embase, Google Scholar, EconLit and Scopus for economic evaluations of EVD. After study selection and data extraction, findings on the impact and economics of EVD were synthesized using a narrative approach, while identifying gaps, and recommending critical areas for future EVD economic studies.
Results
The economic evaluations focused on the burden of illness, vaccine cost-effectiveness, willingness-to-pay for a vaccine, EVD funding, and preparedness costs. The estimated economic impact of the 2014 EVD outbreak in Guinea, Liberia, and Sierra Leone across studies ranged from $30 billion to $50 billion. Facility construction and modification emerged as significant cost drivers for preparedness. The EVD vaccine demonstrated cost-effectiveness in a dynamic transmission model; resulting in an incremental cost-effectiveness ratio of about $96 per additional disability adjusted life year averted. Individuals exhibited greater willingness to be vaccinated if it incurred no personal cost, with a minority willing to pay about $1 for the vaccine.
Conclusions
The severe impact of EVD puts pressure on governments and the international community for better resource utilization and re-allocation. Several technical and methodological issues related to economic evaluation of EVD remain to be addressed, especially for LMICs. We recommend conducting cost-of-sequelae and cost-of-distribution analyses in addition to adapting existing economic analytical methods to EVD. Characteristics of the affected regions should be considered to provide evidence-based economic plans and economic-evaluation of mitigations that enhance resource allocation for prevention and treatment.
PLAIN LANGUAGE SUMMARY
Ebola virus disease (EVD) is a serious health problem, not only in Africa where there have been outbreaks but in other parts of the world as well. In addition to its severe health implications and resultant death, EVD also poses significant impact across several sectors, including food and agriculture, transportation, education, among others, ultimately impacting the economies of affected countries. While some studies have estimated the economic burden of EVD, there remains questions that need addressing. We conducted a review of published studies to estimate what is known about the economic burden of EVD, identified research gaps. Studies looked at how much money EVD costs in terms of prevention and treatment, while others reported on people’s willingness to pay for a vaccine. The estimated economic impact of the 2014 EVD outbreak in Guinea, Liberia, and Sierra Leone ranged from approximately $30 billion to $50 billion across studies. Healthcare facility construction and modification were significant cost factors for response preparedness for EVD outbreaks. While the EVD vaccine showed cost-effectiveness, surveys of people across various regions revealed that more individuals were willing to get vaccinated if it was free, with a minority willing to pay a median of about $1 for the vaccine. The severe impact of EVD puts pressure on governments and the international community to use resources more efficiently. We recommend conducting analyses on the costs of long-term effects of EVD and costs of vaccine and treatment distribution, as well as adapting existing economic methods to the specific characteristics of affected regions. This would help create evidence-based economic plans and evaluations of strategies to enhance resource allocation for EVD prevention and treatment.
Introduction
Ebola virus disease (EVD), a severe viral infection with a mean case fatality of 50% (range 25% to 90%), has affected the world since the virus was isolated in 1976.Citation1 The first two outbreaks occurred simultaneously in Nzara, Sudan, where the index case was traced to a rural cotton factory, and the northern part of the Democratic Republic of Congo (DRC) (formerly Zaire), where a male patient being treated for malaria presented with mysterious symptoms. In DRC, the disease, initially thought to be yellow fever, typhoid, or malaria, occurred at the Yambuku Mission Hospital (YMH), run by Belgian nuns, in a village near the 240-km-long Ebola River. Within a week, several of his contacts, some staff of YMH, as well as those who received treatment at YMH suffered similar symptoms, all of whom died from the disease. One of the nuns at YMH who had contracted the disease was persuaded by Jean-Jacques Muyembe Tanfum, a Belgian-trained virologist physician and member of a team of researchers studying the strange disease, to be taken to Kinshasa so blood samples could be drawn and analyzed for the culprit organism. The blood specimens were sent to the Institute of Tropical Medicine in Antwerp, where the virus was found to be morphologically similar to but immunologically distinct from the Marburg virus which had earlier been isolated.Citation2 The virus was named Ebola virus after the River.Citation3,Citation4 In these first outbreaks of what is now known as ebola virus disease (EVD), 284 and 318 cases associated with case fatalities of 53% and 88%, respectively, were reported in Sudan and DRC. After the emergence of EVD and prior to 2014, about 2345 cases of EVD were confirmed with about 1546 resultant deaths.Citation2 The virus was named Ebola virus after the River.Citation3,Citation4
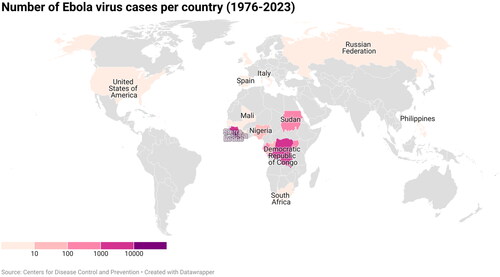
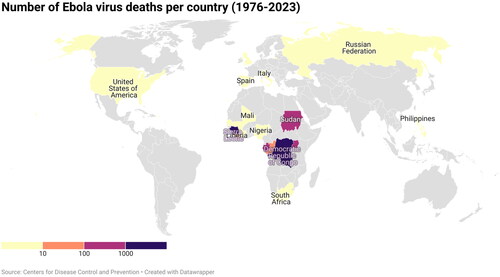
The largest EVD outbreak occurred between March 2014 and June 2016 and affected over 28,000 people in Guinea, Liberia, and Sierra Leone, with a few isolated cases in DRC, Nigeria, Mali, Senegal, United States, United Kingdom and Italy.Citation5 Since its discovery in 1976, 15 outbreaks have affected the DRC, last 8 of which occurred over the past 6 years ( and ). The 2018–2020 outbreak in DRC, thought to be the world’s second deadliest, affected about 3470 people, with a case fatality rate of 66%. Although on the 25th of June 2020, the government of DRC together with the World Health Organization (WHO) announced the end of the outbreak in the eastern part of the country,Citation6 a new epidemic erupted again in its western Équateur province in August 2022.Citation7,Citation8 This outbreak was compounded by the largest outbreak of measles recorded in DRC,Citation9 as well as the COVID-19 pandemic.Citation10 On 20 September 2022, following the isolation and identification of a patient with suspected viral hemorrhagic fever at Mubende Regional Referral Hospital in Uganda, an outbreak was declared by its Ministry of Health marking the 6th outbreak in the country. The outbreak was declared over on 11 January 2023, with a total of 142 confirmed cases and 55 confirmed deaths.Citation10 On 20 September 2022, following the isolation and identification of a patient with suspected viral hemorrhagic fever at Mubende Regional Referral Hospital in Uganda, an outbreak was declared by its Ministry of Health marking the 6th outbreak in the country. The outbreak was declared over on 11 January 2023, with a total of 142 confirmed cases and 55 confirmed deaths.Citation1
The ebola virus is primarily transmitted from person to person through direct physical contact with blood and other body fluids such as urine, sweat, feces, saliva, semen, and breast milk. Percutaneous injury resulting in broken skin and exposure to the mucosal membranes in the eyes, nose or mouth are the most likely media of viral entry into the human body. Infection with the virus may also occur indirectly by contact with previously contaminated surfaces and objects.Citation11–13 Studies also found that direct exposure to the remains of an infected person without appropriate personal protective equipment, as well as contact with infected bats, rodents and primates could lead to transmission of EVD.Citation11,Citation13 The transmission dynamics of EVD differ significantly from those of other pathogens, such as COVID-19, as contagion only occurs when infected individuals display symptoms. The limited transmission during symptomatic phases is attributed to the virus’s reliance on direct contact with the infected person’s bodily fluids, such as blood, sweat, and urine, in contrast to the airborne transmission observed in respiratory particles during sneezing or speaking. Furthermore, the distinctive and severe symptoms of EVD, including fever, fatigue, vomiting, and diarrhea, facilitate the prompt identification of the disease, ensuring that infected individuals not only refrain from spreading the virus until the onset of illness but also display noticeable symptoms for timely recognition and intervention. Individuals at the highest risk of contracting EVD are those closely involved in the care of severely ill patients, irrespective of whether such care takes place in a domestic or hospital setting.Citation14 Swift identification of patients, their immediate isolation, and proactive follow-up measures have proven effective in promptly curbing the potential spread of the disease. The effective containment and limited global spread of EVD can be attributed to the success of isolating cases.Citation14
Two treatments have gained approval from the U.S. Food and Drug Administration for the treatment of EVD caused by Zaire ebolavirus in adults and children. In October 2020, Inmazeb™ was the first drug approved, constituting a combination of three monoclonal antibodies. Subsequently, Ebanga™, a single monoclonal antibody, received approval in December 2020. Both treatments demonstrated significantly higher overall survival rates in a randomized controlled trial conducted during the 2018–2020 Ebola outbreak in DRC. Currently, however, these treatments’ efficacy against species other than Zaire ebolavirus remains unassessed.Citation1 The urgency to address the severe EVD outbreak in West Africa from 2013 to 2016 significantly expedited the progression towards licensed vaccines. At present, Ervebo (rVSV-ZEBOV) and a dual-dose combination of Zabdeno (Ad26.ZEBOV) and Mvabea (MVA-BN-Filo), have successfully obtained licenses and are currently in use. Additionally, three vaccines have either completed trials up to Phase 1 or are currently undergoing such trials. Furthermore, two vaccines are in the Phase 2 stage of trials and a final candidate has successfully completed Phase 3 trials.Citation15 Reports highlight frequent mutations in the genome of the EVD, resulting in the emergence of new strains which presents a challenge to the development of novel therapeutic interventions capable of addressing these evolving viral strains.Citation16,Citation17 Sequencing studies identified mutations in the genetic sequence encoding EVD glycoprotein, which are associated with increased infectivity in human cells.Citation16,Citation17 While this mutation did not significantly impact disease progression, pathogenicity, or virus shedding in rhesus macaques, the findings highlight the potential implications of EVD GP mutations for anti-GP-based interventions, such as vaccines and immunotherapies, due to their potential to affect antibody binding and lead to the emergence of escape mutants.Citation18
The considerable harm inflicted by EVD on individuals, communities, and nations, particularly in low- and middle-income countries (LMICs) in Africa (Supplement I), underscores the urgent need for epidemiological and clinical evidence. Despite resource constraints and competing needs in this region, the notable success in preventing the global spread of EVD emphasizes the effectiveness of containment measures. Swift patient identification, immediate isolation, and proactive follow-up measures have proven highly successful, showcasing the resilience and efficacy of public health efforts in Africa, and contributing significantly to the localized nature of EVD outbreaks.Citation14 This achievement not only highlights the unique challenges faced in LMICs but also demonstrates their ability to implement strategies that mitigate the impact of EVD on a global scale, given the necessary resources and global support. Countries in which outbreaks have erupted have suffered various degrees of financial and other economic effects and have depended largely on global funds for fighting EVD. Though geographically centered in sub-Saharan Africa, EVD remains a public health issue of global concern. Efforts are continuously being made towards containing outbreaks and spread, developing, and testing efficacious and effective vaccines and treatments, educating communities, putting in place infrastructure and processes – all aiming at complete eradication of EVD.
Although a lot has been learnt about the impact, cost, and economics of EVD since its emergence, most of the knowledge has accrued during and after the 2004 outbreaks. We aim to review here studies examining various aspects of the economics of EVD. We also identify several areas of needed economic analysis, including EVD and viral disease specific methods and analytics, that will enhance informed resource planning and allocation. While the focus of this paper is on EVD as this disease will continue to be a threat, several of the issues translate to COVID-19 outbreaks while aiming to provide a broader foundation to examine the economics of viral disease outbreaks and epidemics. As such, this paper aims to call attention to the economic burden of EVD, identify domains that can be further explored in research, and make recommendations for future EVD economic studies.
Method
A published literature search was conducted in PubMed/Medline (National Library of Medicine), Embase (Elsevier), Google Scholar (Alphabet), EconLit (American Economic Association) and Scopus (Elsevier) using keywords and controlled vocabulary including but not limited to, “ebola virus disease”, “cost”, “cost analysis”, “cost benefit analysis”, “economic evaluation”, and “cost effectiveness analysis” (Supplement II). All articles published on the subject since 1976 through present were included. We excluded articles that did not report on EVD nor economic evaluations in EVD, as well as unpublished and grey literature. No language limits were applied in our search. Two independent reviewers initially selected articles and screened titles and abstracts for eligibility. Disagreements were resolved by consensus. Data was abstracted using a data abstraction form which was subsequently employed in the evidence synthesis. We report our findings employing a narrative strategy, shedding light on the challenges confronting the conduct of economic evaluations of EVD. Additionally, we put forth recommendations for technical and methodological improvements.
Results
Our search yielded 1257 results and after initial reviews, 34 studies that reported some form of economic evaluation were included in the final evaluation (). The findings of the review encompassed a range of economic evaluations, including 13 studies focusing on the costs or burden of illness,Citation19–31 5 studies investigating willingness-to-pay for an EVD vaccine,Citation32–36 1 study centered on cost-effectiveness,Citation37 1 study was a cost-benefit analysis of early response to EVD,Citation38 12 studies examining the expenses related to preparedness for EVD,Citation38–49 and 3 studies exploring funding aspects related to EVD.Citation50–52
Table 1. Burden of disease.
Table 2. Willingness to pay for EVD vaccine.
Table 3. Cost-effectiveness analysis.
Table 4. Cost-benefit analysis.
Table 5. Cost of preparedness.
Table 6. Funding.
Cost/burden of illness
Among the studies aimed at estimating the costs or burden of EVD, four assessed the direct and indirect costs; typically, the costs of medications, laboratory examinations, and personal protective equipment; personnel costs; and productivity losses.Citation19,Citation22,Citation24,Citation31 Seven studies also estimated the costs of EVD deaths, non-EVD deaths, long term EVD sequelae, social factors, direct economic losses, and the decline in economic activities in the affected countries.Citation22–27,Citation29 Additionally, one study examined the total welfare cost associated with EVD-related mortality.Citation21 Of the above studies, 7 focused mainly on the 2014 EVD outbreak and were mainly conducted in Liberia, Sierra Leone, Guinea, and Nigeria ().
Huber et al. in their systematic review of EVD, pooled together existing estimates, determined areas that had not been covered, and estimated the comprehensive cost of the 2014 EVD outbreak in Guinea, Liberia, and Sierra Leone to be $53.2 billion.Citation23 The greatest cost factor was the cost attributed to death from non-EVD causes. Healthcare worker deaths, along with its diminished pool and healthcare resources diverted from non-EVD care, led to an estimated 3.5 million untreated cases of malaria and an additional 10,623 deaths attributed to HIV/AIDS, tuberculosis, and malaria between March 2014 and March 2015.Citation23 In a study using a mathematical model to estimate the cost of a single case of EVD in Guinea, Liberia, and Sierra Leone, Bartsch et al. reported a wide range between about $500 and $18000 depending on the severity and the outcome.Citation19 Similar to findings by Huber et al.,Citation23 Bartsch and colleagues employing a monte carlo simulation model to estimate the productivity losses,Citation19 as well as Kirigia et al. who used a human capital approach to determine the monetary value of lives lost to EVD,Citation25 also found mortality to be the highest component of the cost incurred, together with the resultant loss of productivity. Zeng and colleagues used a top-down costing approach and data on budget and expenditure from WHO and GAVI to estimate the unit cost of various EVD interventions employed in the DRC during the 2018–2020 EVD outbreak. They estimated the unit costs of $66,182 for maintaining a rapid response team per month, US$4,435 for contact tracing and surveillance per identified EVD case, US$1,464 for EVD treatment per case, US$59.4 per EVD laboratory test, US$120.7 per vaccinated individual against EVD, and US$175.0 for mental health and psychosocial support per beneficiary.Citation31
Willingness-to-pay for Ebola virus vaccine
Willingness-to-pay studies assessing the trade-offs that people in the general population are willing to make with regards to their personal wealth versus their perceived risk of disease and possible death were conducted in Sierra Leone,Citation32 Guinea,Citation33 Indonesia,Citation34 USACitation35 and NigeriaCitation36 (). These reported on surveys regarding participants’ readiness to be vaccinated against EVD and how much they were prepared to pay for the vaccine if it was not to be given for free. The prevailing preference was for vaccination against ebola virus to be provided free of charge by participants’ respective governments. While a fair proportion of people were willing to be vaccinated free of charge (55%-80%), this number declined significantly where respondents were required to pay for the vaccine (27–60%). Across the latter studies, respondents were willing to pay a median of $1 to $2 for an EVD vaccineCitation33–36 and this was associated with international travel, monthly income, and the perceived efficacy of the vaccine.Citation35,Citation36 In the USA in 2018, a national survey conducted using the GfK Group’s KnowlegePanel® showed that while 40.3% of study respondents were unwilling to pay for an EVD vaccine, 59.7% would pay at least $1, a majority of whom (66%) were willing to pay up to $50 for the vaccine.Citation35 Similarly in Indonesia, 288 participants out of 311 were willing to pay a mean of $2.08 for the vaccine in 2019Citation34 and in Nigeria, majority of the respondents were of the opinion that the EVD vaccine must be free.Citation36 A need to increase awareness and knowledge of EVD and the possible benefits of the vaccine, as well as an advocacy for governments to absorb the cost of vaccination, were considered critical to improved vaccine acceptability and uptake.Citation32–36
Cost effectiveness analysis
One cost effectiveness analysis of an EVD vaccine was identified. In this study, Obeng-Kusi et al. assessed the cost-effectiveness of an EVD vaccine within a hypothetical population of 1,000 individuals using a dynamic transmission modeling approach based on epidemiological data from EVD outbreaks in DRC (). The study utilized an estimate of the comprehensive vaccination cost sourced from the World Health Organization (WHO) and the United Nations Children’s Emergency Fund (UNICEF). An SEIR-D model was employed, considering unvaccinated and uninfected individuals as susceptible and at risk. These individuals were then transitioned through exposed, infected, recovered, or dead states, with disease transition probabilities determined by epidemiological parameters extracted from published literature on past EVD outbreaks DRC. The findings indicated that vaccinating 50% of the at-risk population in DRC averted approximately 99% of EVD cases, deaths, and disability-adjusted life years (DALYs). The vaccine demonstrated cost-effectiveness, evidenced by an incremental cost-effectiveness ratio (ICER) of $95.63 per DALY averted. Additionally, factors such as vaccination coverage, population size, case fatality rate, as well as vaccine efficacy were found to be proportionally correlated with vaccine cost effectiveness.Citation37
Cost benefit analysis
Kellerborg and colleagues investigated the costs and health benefits of earlier interventions during the 2014–2016 Ebola virus disease (EVD) outbreak in West Africa, the largest recorded outbreak of its kindCitation38 (). Using a deterministic and stochastic compartment model based on various data sources, they estimated that interventions implemented four weeks earlier in Sierra Leone could have prevented 10,257 additional cases and 8,835 additional deaths, resulting in incremental savings of 456,000 DALYs and $203 million, largely due to the averted mortality. In this evaluation, the authors determined that rapid responses to EVD were cost-effective and provided value for money, highlighting the need for such investments in healthcare systems when needed.Citation38
Cost of preparedness studies
Twelve studies described efforts to assess preparedness activities, such as construction and modification of treatment and isolation facilities, direct supply of medications and vaccines, laboratory testing, human resource training, among others, as well as the associated costs of such preparations in anticipation of possible EVD cases. While most of the studies were conducted in the healthcare setting, one study was conducted in a steel extraction firm to estimate the cost attributable to its internal preventive measures. These measures included the establishment of an EVD treatment center, on-site screening of employees, compensation for consultants, and the provision of EVD education and training.Citation48 Although the majority of studies were conducted in the United States, a limited number were centered in Sierra Leone,Citation38 the Netherlands,Citation47 and selected regions within West AfricaCitation48 ().
Hospital preparedness was found to require extraordinary resources which needed to be diverted from existing infection prevention and control activities.Citation40,Citation45,Citation49 Costs of preparedness varied widely across different settings depending on the cost elements included.
Notably, construction and modification accounted for a huge part of the cost of preparedness in most cases.Citation43,Citation44 Following the identification of the initial case of EVD in the US in 2014 and the directive from the Centers for Disease Control and Prevention (CDC) that hospitals nationwide should be equipped to manage individuals with EVD, efforts for resource allocation were initiated in anticipation of potential cases. A cross-sectional survey assessing the costs, benefits, and challenges, as well as the perceived value of EVD preparedness activities across the US found that the financial impact of preparedness activities for acute-care hospitals in the US exceeded $360 million.Citation46 Kellerborg and colleagues, in their evaluation of the costs and health effects of early response to EVD, determined that such responses are cost-effective, that such investments in healthcare systems are needed, and provide value for money.Citation38 Of significance also, a total cost of $10.58–11.11 million was incurred by ArcelorMittal, the steel extraction firm in West Africa, for its adoption of preventive measures - about 30–31% of which was attributable to its internal measures, about 11–12% accounting for their external donations supporting humanitarian response and about 7–12% being spent on relational costs.Citation48
Funding for Ebola virus disease
Three reports reviewed EVD-related funding, including research funding, funding in support of various interventions, and funding for public health surveillance and modelingCitation50–52 (). Several public and philanthropic sources contributed to funding for research, outbreak, containment, and management of EVD.Citation50,Citation51,Citation53,Citation54
In an issue brief, Boddie evaluated US federal funding in support of research and development for EVD. Funding totaling $1.1 billion for the development of EVD countermeasures (drugs, vaccines, and diagnostics) were mainly supported by the NIH and the US Department of Defense. Boddie also highlighted the 2015 Consolidated and Further Continuing Appropriation Act, which set aside $5.4 billion in emergency funding to help the US in their EVD response.Citation50
A systematic review by Fitchett et al. estimated total EVD research funding from 1997 to 2015 at $1.035 billion. Notably, the authors point out that 42% of this funding was awarded globally to diverse entities, including publicly funded institutions, private for-profit organizations, and philanthropic non-profit institutions during the outbreak in 2014. These funds were raised from several public, private, and philanthropic funders including the NIH, European Commission, CDC, Gates Foundation, public and private universities, and biopharmaceutical companies. Ebola research funds have supported clinical trials, product development, surveillance, modeling, and other innovative steps such as bioinformatics and operational research to find solutions to the disease.Citation51
Shimizu and colleagues examined disparities in equitable development assistance for health (DAH) with disease burden in EVD-affected countries using estimates from the Global Burden of Disease (GBD) Study 2017 and the DAH Database 1990–2019 during 2005–2017.Citation52 Analyzing DALYs across disease categories, including vaccine-preventable diseases (VPDs), HIV/AIDS, malaria, tuberculosis, and EVD, they found that during the West African EVD outbreak (2013–2016), DAH/DALYs surged for EVD, showing fluctuations in HIV/AIDS and malaria. The authors concluded that to effectively mitigate risks during and post-health emergencies, prudent pre-emergency funding allocation based on disease burden is essential, supplemented by real-time assessment of immediate health needs in crisis situations.Citation52
According to a report by the United Nations Special Envoy on Ebola, of the $8.9 billion pledged by individuals, organizations, and governments towards containing, preventing, and treating the disease, $5.9 billion had been disbursed as of October 2015.Citation55 In addition, affected countries invested considerably in healthcare and disease management plans during the epidemic.Citation23
The World Health Organization Ebola Response Fund reported receipt of over $459 million in contributions from over 60 donors during the 2014–2016 outbreak, including countries, private organizations, not-for-profit organizations, and the United Nations.Citation56 In its bid to offer relief from the current EVD outbreak in DRC, the World Bank announced a $300 million emergency fund toward EVD managementCitation53 while the Vaccine Alliance (Gavi) offered over $9 million to fund vaccination in the DRC.Citation54
Discussion
Our review on the economics of EVD identified several areas of prior research. These concern EVD cost estimates, willingness-to-pay for a vaccine, cost of preparedness and funding. The estimated costs across the various studies examined were high and varied widely. This may be attributable to the absence of standardized methods, perspectives, outcome metrics, and types of analyses. Studies revealed willingness to pay a nominal amount for the vaccine, possible reasons for which could be the perceived low EVD risk, therefore, limited motivation to pursue vaccination actively, and participants’ desire for assurance of a vaccine’s safety and effectiveness before expressing interest. Another potential explanation for this could be the hypothetical nature of the questions and unmeasured factors, such as vaccine characteristics and timeliness that could have influenced participants’ responses regarding vaccine interest and willingness to pay.Citation35,Citation36 A need to increase awareness and knowledge of EVD and the possible benefits of the vaccine, as well as an advocacy for governments to absorb the cost of vaccination, are considered critical to improved vaccine acceptability and uptake.Citation32–36 Most cost-of-preparedness studies were conducted in high-income countries (HIC) and do not accurately reflect the situation in LMICs, especially in the regions of Africa that have been most impacted by the virus. In spite of having received donations to the tune of about $276 million from August 2018 to present for the mitigation of EVD, WHO Ebola Response Funding reported a need of an additional $28.5 million to ensure continuity of EVD response activities and to avoid cashflow shortages.Citation57
We also identified areas related to the economic burden of EVD that had not extensively been explored. Whereas the indirect burden of the disease has been discussed in some research findings, the long-term effects of these have not been extensively deliberated. The long-term burden related to disrupted functioning of various systems in affected countries, including impact on human resources, health, travel, commerce, tourism, social, cultural, and economic activities, and the effect it would have on the reorganization of their economies remain poorly understood. While this burden will be mainly regional and not reach the scale of the 2003 SARS and the 2019 COVID-19 pandemics, the overall impact on the already impoverished economies of regions with EVD outbreaks is of such a magnitude that it majorly affects the productivity, health, and wellbeing of individuals, as well as communities collectively. Also, with the introduction of the EVD vaccine, there is a need to have better insights into the transparency of vaccine pricing and the impact that free vaccination will have on the budgets of countries that opt to make this available to its citizens. Such research will inform decisions as to whether to mass vaccinate, as in the case of COVID-19, or vaccinate according to the individual and community levels of relative risk for rapid spread, morbidity, and mortality, using established methods of epidemiological practice. In situations like EVD, it is critical to consider proximity to and number of infected persons, which ultimately influences the force of infection and thus, the spread of EVD. In turn, this will also help in the mobilization of funds for such programs. Economic evaluations and cost-effectiveness studies in particular are needed to adopt pan-African and national vaccination policies in EVD-endemic regions. Despite the significant funds invested in EVD, large gaps remain in financial support towards fighting the disease. There are calls for global commitment to funding in support of managing the disease, along with research and development, as well as global financing mechanisms towards other pandemics that may arise in the future.
Fully comprehending the burden of EVD and appropriately estimating the value of potential healthcare programs and services for the prevention and control of EVD, will require foundational research and technical development. The overarching objective is to recommend harmonized approaches to enable rigorous, epidemiologically justified, and regionally relevant economic evaluations of EVD prevention and control. Note that vaccinations protect against the impact of exposure to a virus and are likely to reduce the intensity of a person’s response to exposure. However, as polio vaccination has shown globally, it is the collective effect of broad vaccination that may eliminate the emergence of disease despite the continued existence of the virus. In addition to adapting the existing analytics and classical pharmacoeconomic methods (Table AI) to evaluate and inform EVD economic decision-making,Citation58,Citation59 several other useful evaluations, such as the cost of long-term sequelae, and costs of distribution can be considered. Studies have found some EVD-related sequelae that persist for more than a year after hospital discharge, the most common of which are headache, myalgia, joint pain, ocular complications, and abdominal pains.Citation60–62 Costing the EVD-related sequelae helps to elucidate the incremental economic burden these complications present and to plan for their financial management. This assessment also helps provide a more wholesome assessment of the cost implications of EVD over the long term. Another evaluation that could be valuable is the cost-of-distribution model which develops a comprehensive plan of authorization and distribution channels, along with required logistics for vaccine or therapeutics distribution under consideration of regional and local logistics and infrastructure.Citation63 Such a model needs to account for approval of the vaccine or therapeutic within the particular national market, demographics, distribution systems, modes of population or patient access, dose of vaccines and therapeutics thus number of either required, type of vaccine or therapeutic procurement (government-, private sector- or donor-purchased), as well as cold chain requirements for storage and maintenance. These strategies consider the health system, funding and means of monitoring successes, in addition to the logistics.Citation64 A well-developed cost-of-distribution model will ensure widespread access to vaccines and therapeutics and extensive coverage of the population that needs the vaccine or medicine while optimizing both outcomes and costs.Citation63 Additional analytic methods such as the constrained optimization modeling which employs a mathematical objective function, e.g. reducing disease cases; to optimize interventions, within predefined constraints for a target population and the fiscal health modeling which measures changes in the net present value of government revenues and expenditures that result in specified disease outcomes have been recommended by the International Society for Pharmacoeconomics and Outcomes Research.Citation58
Given that the primary objective of economic evaluation is to inform quality decision making and resource allocation, the methodology for economic evaluation should be consistent with the needs and setting of the decision-maker. Guidelines on economic evaluation of vaccination programs support important considerations including but not limited to the characteristics of the target population, the impact of the vaccination program on disease cases over time using dynamic transmission epidemiologic models, the costs associated with implementing and operating the vaccination program, and the alterations in costs and health outcomes related to the targeted disease need to inform the choice of evaluation methodCitation58,Citation59,Citation65,Citation66 (Table A2). Particularly in LMICs settings, the societal perspective is preferred as most of health is financed either by governments or through out-of-pocket means.Citation67 With little enrollment in insurance or healthcare management organizations, the payer perspective is of limited relevance in LMICs.Citation68 The choice of outcomes measures is critical since they quantify the usefulness of the healthcare program being assessed. HIC measures of health may be too narrow in LMICs to reflect the entire picture, eliminate some critical dimensions of the effects of the intervention being assessed, and thus may underestimate or undervalue the intervention.Citation67 Benchmarks for determining cost-effectiveness in LMICs need to be chosen deliberately to reflect the income group of the population being assessed. Setting these levels too high and out of proportion may portray all interventions as “cost-effective” and impair educated priority setting.Citation68 The levels used in HICs do not account for the HICs-to-LMICs inequities in health and wealth. For LMICs, country- or income group-specific thresholds should be considered to account for these differences and to align cost-effectiveness thresholds with affordability.
Conclusion
The resultant pressure on governments for better resource utilization and re-allocation underscores the need for evidence-based regionally relevant economic plans to handle present and prevent and respond to future infectious disease outbreaks. Although viral diseases differ in biology and immunology, their effects on the affected population, varied in magnitude though they may be, are essentially the same: mortality and morbidity, social and societal impacts, and economic implications. Lessons learnt from the management of any of these outbreaks are valuable and transferable to others. In the DRC, for instance, public health response to COVID-19 and coincident measles has been guided largely by the experience in combatting EVD. There is evidence that alignment of the responses for these outbreaks has made DRC more responsive and lowers morbidity and mortality.Citation10 In this same vein, lessons derived from the economics of one outbreak will be invaluable for preparing towards and financing the prevention of and response to future outbreaks. With several decades of experience in dispensing and utilizing funds for a fairly successful global EVD mitigation campaign, capacities built in EVD economics and funds management can be applied to COVID-19 and any other epidemics that may occur in the future. There is evidence that alignment of the responses for these outbreaks has made DRC more responsive and lowers morbidity and mortality.Citation10 In this same vein, lessons derived from the economics of one outbreak will be invaluable for preparing towards and financing the prevention of and response to future outbreaks.
The burden of EVD globally and particularly in the countries that have been directly affected is enormous epidemiologically, clinically, socially, and economically; putting pressure on governments and the international community for better resource utilization and re-allocation to address both the immediate clinical needs but also the ensuing longer-term social and economic consequences. There is urgent need for territory-specific evidence-based economic plans and economic evaluation of mitigations to enhance resource allocation for EVD prevention and treatment. This is also important as the ebola virus has been shown to mutate - evading vaccine protection and making antibody treatments ineffective, as noted earlier. This may change, at least partially, how vaccines are used and how regional outbreaks and disease spread may require adaptations of vaccines and vaccinations.
Transparency
Declaration of funding
No funding was received to produce this article.
Declaration of financial/other relationships
No potential conflict of interest was reported by the author.
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplemental Material
Download MS Word (37.9 KB)Acknowledgements
None stated.
References
- Centers for Disease Control and Prevention. Outbreaks Ebola (Ebola Virus Disease). 2023 https://www.cdc.gov/vhf/ebola/outbreaks/index-2018.html
- World Health Organization. Ebola haemorrhagic fever in Zaire, 1976. Bulletin of the World Health Organization. 1978;56:271–293.
- Centers for Disease Control and Prevention. Ebola (Ebola Virus Disease) | CDC. Ebola (Ebola Virus Disease). 2018. p. 1. https://www.cdc.gov/vhf/ebola/index.html.
- Weyer J, Grobbelaar A, Blumberg L. Ebola virus disease: history, epidemiology and outbreaks. Curr Infect Dis Rep. 2015;17(5)Current Medicine Group LLC:480. doi:10.1007/s11908-015-0480-y.
- Wiwanitkit V. Ebola virus disease. J Chin Med Assoc. 2015;78(4):264. https://www.sciencedirect.com/science/article/pii/S1726490115000593.
- Maxmen A. World’s second-deadliest ebola outbreak ends in democratic republic of the Congo. Nature. 2020; doi:10.1038/d41586-020-01950-0.
- Harris M, Boakye-Agyemang C. New Ebola outbreak detected in northwest Democratic Republic of the Congo; WHO surge team supporting the response. World Health Organization. 2020. https://www.who.int/news-room/detail/01-06-2020-new-ebola-outbreak-detected-in-northwest-democratic-republic-of-the-congo-who-surge-team-supporting-the-response
- Centers for Disease Control and Prevention. 2020 Democratic Republic of the Congo, Equateur Province (ongoing) | Democratic Republic of Congo | Outbreaks | Ebola (Ebola Virus Disease) | CDC. 2020. https://www.cdc.gov/vhf/ebola/outbreaks/drc/2020-june.html
- Measles: the never-ending fight against one of the world’s most contagious diseases - Democratic Republic of the Congo | ReliefWeb. 2023. https://reliefweb.int/report/democratic-republic-congo/measles-never-ending-fight-against-one-worlds-most-contagious-diseases
- Nachega JB, Mbala-Kingebeni P, Otshudiema J, et al. The colliding epidemics of COVID-19, ebola, and measles in the democratic republic of the Congo. Lancet Glob Health. 2020;8(8):e991–e992. doi:10.1016/S2214-109X(20)30281-3.
- Judson S, Prescott J, Munster V. Understanding ebola virus transmission. Viruses. MDPI AG. 2015;7(2):511–521. doi:10.3390/v7020511.
- World Health Organization. What we know about transmission of the ebola virus among humans. Media centre; news releases. 2014. p. 2014–2016. https://www.who.int/mediacentre/news/ebola/06-october-2014/en/
- Rewar S, Mirdha D. Transmission of ebola virus disease: an overview. Ann Glob Health. 2014;80(6):444–451. Elsevier USA doi:10.1016/j.aogh.2015.02.005.
- Wilder-Smith A. COVID-19 in comparison with other emerging viral diseases: risk of geographic spread via travel. Trop Dis Travel Med Vaccines. 2021;7(1):3. doi:10.1186/s40794-020-00129-9.
- Tomori O, Kolawole MO. Ebola virus disease: current vaccine solutions. Curr Opin Immunol. 2021; 71:27–33. https://www.sciencedirect.com/science/article/pii/S0952791521000285.
- Gire SK, Goba A, Andersen KG, et al. Genomic surveillance elucidates ebola virus origin and transmission during the 2014 outbreak. Science. 2014;345(6202):1369–1372. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4431643/.
- Baize S, Pannetier D, Oestereich L, et al. Emergence of Zaire ebola virus disease in Guinea. N Engl J Med. 2014;371(15):1418–1425. doi:10.1056/NEJMoa1404505.
- Haque A, Hober D, Blondiaux J. Addressing therapeutic options for ebola virus infection in current and future outbreaks. Antimicrob Agents Chemother. 2015;59(10):5892–5902. doi:10.1128/AAC.01105-15.
- Bartsch SM, Gorham K, Lee BY. The cost of an ebola case. Pathog Glob Health. 2015;109(1):4–9. http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L603981462.
- Bowles J, Hjort J, Melvin T, et al. Ebola, jobs and economic activity in Liberia. J Epidemiol Community Health. 2016;70(3):271–277. http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L614446481. doi:10.1136/jech-2015-205959.
- Da Costa S. The impact of the ebola crisis on mortality and welfare in Liberia. Health Econ. 2020;29(12):1517–1532. doi:10.1002/hec.4150.
- Elmahdawy M, Elsisi GH, Carapinha J, et al. Ebola virus epidemic in west africa: global health economic challenges, lessons learned, and policy recommendations. Value Health Reg Issues. 2017;13:67–70. http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L618232240. doi:10.1016/j.vhri.2017.08.003.
- Huber C, Finelli L, Stevens W. The economic and social burden of the 2014 ebola outbreak in west africa. J Infect Dis. 2018;218(suppl_5):S698–S704. https://academic.oup.com/jid/article-abstract/218/suppl_5/S698/5129071. doi:10.1093/infdis/jiy213.
- Kirigia JM, P A, Masiye F, et al. Indirect costs associated with deaths from the ebola virus disease in west africa. Infect Dis Poverty. 2015;4(1):45. http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L606662254. doi:10.1186/s40249-015-0079-4.
- Kirigia JM, Muthuri RNDK, Muthuri NG. The monetary value of human lives lost through ebola virus disease in the democratic republic of Congo in 2019. BMC Public Health. 2019;19(1):1218. doi:10.1186/s12889-019-7542-2.
- Kum FV, Olayiwola S, Aloysius NM. The impact of ebola virus disease on government expenditure in Sierra Leone. In: Socio-cultural dimensions of emerging infectious diseases in Africa. Springer Nature Switzerland AG; 2019. p. 75–90. https://www.worldbank.org/en/topic/macroeconomics/publication/2014-2015-west-africa-ebola-crisis-impact-update
- Morrison IDL, Anderson BI, Brower AI, et al. Macroeconomic impact of ebola outbreaks in Sub-Saharan africa and potential mitigation of GDP loss with prophylactic ebola vaccination programs. PLOS ONE. 2023;18(4):e0283721. doi:10.1371/journal.pone.0283721.t001.
- Olugasa BO, Oshinowo OY, Odigie EA. Preventive and social cost implications of ebola virus disease (EVD) outbreak on selected organizations in Lagos state, Nigeria. Pan Afr Med J. 2015;22(Supp 1):20–20. http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L616010262. doi:10.11604/pamj.supp.2015.22.1.6673.
- Onyekuru NA, Ihemezie EJ, Ezea CP, et al. Impacts of ebola disease outbreak in west africa: implications for government and public health preparedness and lessons from COVID-19-NC-ND license. Sci Afr. 2023;19:e01513. (http://creativecommons.org/licenses/by-nc-nd/4.0/). doi:10.1016/j.sciaf.2022.e01513.
- Zacharowski K, Brodt HR, Wolf T. Medical treatment of an ebola-infected doctor - Ethics over costs? Lancet. 2015;385(9969):685–685. doi:10.1016/S0140-6736(15)60279-3.
- Zeng W, Samaha H, Yao M, et al. The cost of public health interventions to respond to the 10th ebola outbreak in the democratic republic of the Congo. BMJ Glob Health. 2023; 8(10):e012660. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10583089/. doi:10.1136/bmjgh-2023-012660.
- Huo X, Shi G, Li X, et al. Knowledge and attitudes about ebola vaccine among the general population in Sierra Leone. Vaccine. 2016;34(15):1767–1772. doi:10.1016/j.vaccine.2016.02.046.
- Kpanake L, Sorum PC, Mullet É. Willingness to get vaccinated against ebola: a mapping of guinean people positions. Hum Vaccin Immunother. 2018;14(10):2391–2396. doi:10.1080/21645515.2018.1480236.
- Mudatsir M, Anwar S, Karunia Fajar J, et al. Willingness-to-pay for a hypothetical ebola vaccine in Indonesia: a cross-sectional study in Aceh. F1000Res. 2019;8:1441. doi:10.12688/f1000research.20144.1.
- Painter JE, von Fricken ME, Viana de O, et al. Willingness to pay for an ebola vaccine during the 2014–2016 ebola outbreak in west africa: results from a U.S. National sample. Hum Vaccin Immunother. 2018;14(7):1665–1671. doi:10.1080/21645515.2018.1423928.
- Ughasoro MD, Esangbedo DO, Tagbo BN, et al. Acceptability and willingness-to-pay for a hypothetical ebola virus vaccine in Nigeria. PLoS Negl Trop Dis. 2015;9(6):e0003838. http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L605059981. doi:10.1371/journal.pntd.0003838.
- Obeng-Kusi M, Habila MA, Roe DJ, et al. Economic evaluation using dynamic transition modeling of ebola virus vaccination in lower-and-Middle-income countries. J Med Econ. 2021;24(sup1):1–13. doi:10.1080/13696998.2021.2002092.
- Kellerborg K, Brouwer W, Van Baal P. Costs and benefits of early response in the ebola virus disease outbreak in Sierra Leone. Cost Eff Resour Alloc. 18(1):13. doi:10.1186/s12962-020-00207-x.
- Abraham N, Jain A, Harrison D, et al. 70 A cost analysis of a county hospital emergency department’s ebola virus disease preparedness. Ann Emerg Med. 2015;66(4):S25. doi:10.1016/j.annemergmed.2015.07.102.
- Herstein JJ, Biddinger PD, Kraft CS, et al. Initial costs of ebola treatment centers in the United States. Emerg Infect Dis. 2016;22(2):350–352. doi:10.3201/eid2202.151431.
- Herstein JJ, Le AB, McNulty LA, et al. Update on ebola treatment center costs and sustainability, United States, 2019. Emerg Infect Dis. 2020;26(5):1007–1009. doi:10.3201/eid2605.191245.
- Mccullough JM, Fowle N, Sylvester T, et al. Cost analysis of 3 concurrent public health response events: financial impact of measles outbreak, super bowl surveillance, and ebola surveillance in maricopa county. J Public Health Manag Pract. 2019;25(4):357–365. doi:10.1097/PHH.0000000000000818.
- Morgan DJ, Braun B, Milstone AM, et al. Lessons learned from hospital ebola preparation. Infect Control Hosp Epidemiol. 2015;36(6):627–631. doi:10.1017/ice.2015.61.
- Ripper J, Nagurka R, Patel M, et al. 98 The cost of safety: implementation, maintenance, and patient care expenses of an emergency department ebola extended treatment area. Annals of Emergency Medicine. 2015;66(4):S34–S35. doi:10.1016/j.annemergmed.2015.07.130.
- Simonsen KA, Phipps AR, Hall M, et al. Costs associated with ebola preparedness at a freestanding pediatric assessment center. Infect Control Hosp Epidemiol. 2017;38(11):1367–1369. doi:10.1017/ice.2017.193.
- Smit MA, Rasinski KA, Braun BI, et al. Ebola preparedness resources for acute-care hospitals in the United States: a cross-sectional study of costs, benefits, and challenges. Infect Control Hosp Epidemiol. 2017;38(4):405–410. doi:10.1017/ice.2017.6.
- Suijkerbuijk AWM, Swaan CM, Mangen MJJ, et al. Ebola in The Netherlands, 2014–2015: costs of preparedness and response. Eur J Health Econ. 2018;19(7):935–943. doi:10.1007/s10198-017-0940-4.
- Tariq H, Emes DT, Boo YY, et al. Economic impact of ebola virus disease outbreak on an extractive firm: a case study. UCL Open Environ. 2020;2:e007. doi:10.14324/111.444/ucloe.000007.
- Yacisin K, Balter S, Fine A, et al. Ebola virus disease in a humanitarian aid worker—New York city, october 2014. Mortality Weekly Report. 2015;64:321–323.
- Boddie C. Federal funding in support of ebola medical countermeasures R&D. Health Secur. 2015;13(1):3–8. doi:10.1089/hs.2015.0001.
- Fitchett JRA, Lichtman A, Soyode DT, et al. Ebola research funding: a systematic analysis, 1997-2015. J Glob Health. 2016;6(2):020703. doi:10.7189/jogh.06.020703.
- Shimizu K, Checchi F, Warsame A. Disparities in health financing allocation among infectious diseases in ebola virus disease (EVD)-affected countries, 2005–2017. Healthcare (Switzerland). 2022;10(2):179. doi:10.3390/healthcare10020179.
- World Bank Group. World Bank Mobilizes US$300 Million to Finance the Ebola Response in Democratic Republic of Congo. World Bank Fiscal Report. 2019 cited 2020 Jul 6]. https://www.worldbank.org/en/news/press-release/2019/07/24/world-bank-mobilizes-us300-million-to-finance-the-ebola-response-in-democratic-republic-of-congo.
- Gavi. The Vaccine Alliance. Gavi boosts funding for Ebola outbreak response. https://www.gavi.org/news/media-room/gavi-boosts-funding-ebola-outbreakresponse.
- Office of the United Nations Special Envoy on Ebola. Resources for Results V. 2015. https://ebolaresponse.un.org/sites/default/files/resources_for_results_v.pdf.
- World Bank. 2014-2015 West africa ebola crisis: impact update. World Bank Fiscal Report. 2016.
- World Health Organization. Funding. 2020. https://www.who.int/emergencies/diseases/ebola/drc-2019/funding
- Mauskopf J, Standaert B, Connolly MP, et al. Economic analysis of vaccination programs: an ISPOR good practices for outcomes research task force report. Value in Health. 2018;21(10):1133–1149. https://www.valueinhealthjournal.com/article/S1098-3015(18)33267-4/fulltext?_returnURL=https%3A//linkinghub.elsevier.com/retrieve/pii/S1098301518332674%3Fshowall%3Dtrue. doi:10.1016/j.jval.2018.08.005.
- WHO Guide on Standardization of Economic Evaluations of Immunization Programmes. 2023. https://www.who.int/publications-detail-redirect/WHO-IVB-19.10
- Clark DV, Kibuuka H, Millard M, et al. Long-term sequelae after ebola virus disease in Bundibugyo, Uganda: a retrospective cohort study. Lancet Infect Dis. 2015;15(8):905–912. doi:10.1016/S1473-3099(15)70152-0.
- Mohammed H, Vandy AO, Stretch R, et al. Sequelae and other conditions in ebola virus disease survivors, Sierra Leone, 2015. Emerg Infect Dis. 2017;23(1):66–73. doi:10.3201/eid2301.160631.
- Varkey JB, Shantha JG, Crozier I, et al. Persistence of ebola virus in ocular fluid during convalescence. N Engl J Med. 2015;372(25):2423–2427. doi:10.1056/NEJMoa1500306.
- Smith J, Lipsitch M, Almond JW. Vaccine production, distribution, access, and uptake. Lancet. 2011;378(9789):428–438. doi:10.1016/S0140-6736(11)60478-9.
- Hardt K, Bonanni P, King S, et al. Vaccine strategies: optimising outcomes. Vaccine. 2016;34(52):6691–6699. doi:10.1016/j.vaccine.2016.10.078.
- Jit M, Hutubessy R. Methodological challenges to economic evaluations of vaccines: is a common approach still possible? Appl Health Econ Health Policy. 2016;14(3):245–252. doi:10.1007/s40258-016-0224-7.
- Walker DG, Hutubessy R, Beutels P. WHO guide for standardisation of economic evaluations of immunization programmes. Vaccine. 2010;28(11):2356–2359. https://www.sciencedirect.com/science/article/pii/S0264410X09008986.
- Pitt C, Vassall A, Teerawattananon Y, et al. Foreword: health economic evaluations in low- and Middle-income countries: methodological issues and challenges for priority setting. Health Econ. 2016;25(Suppl 1):1–5. doi:10.1002/hec.3319.
- Griffiths UK, Legood R, Pitt C. Comparison of cconomic evaluation methods across low-income, Middle-income and high-income countries: what are the differences and why? Health Econ. 2016;25 (Suppl 1):29–41. doi:10.1002/hec.3312.