Abstract
Objective
To determine the economic impact of a minimally invasive temperature-controlled radiofrequency (TCRF) device for treating nasal airway obstruction (NAO).
Methods
A budget impact model was developed for two scenarios: a reference scenario of functional rhinoplasty surgery with concomitant septoplasty and inferior turbinate reduction (ITR) performed in the hospital outpatient department where TCRF is not an available treatment option and a new scenario consisting of in-office TCRF treatment of the nasal valve and ITR. A payor perspective was adopted with a hypothetical population plan size of one million members. Costs were estimated over a time horizon of 4 years. The eligible population included patients with severe/extreme NAO and nasal valve collapse (NVC) as the primary cause or significant contributor. Data inputs were sourced from targeted literature reviews. Uncertainty within the model structure and input parameters was assessed using one-way sensitivity analysis.
Results
The introduction of a TCRF device resulted in population-level cost savings of $20,015,123 and per-responder average cost savings of $3531 through a 4-year time horizon due to lower procedure costs and complication rates of the device relative to the surgical comparator. Results were robust when varying parameter values in sensitivity analyses, with cost savings being most sensitive to the prevalence of NAO and estimated response rates to functional rhinoplasty and TCRF.
Conclusions
In patients with severe/extreme NAO, with NVC as the primary or major contributor, introducing TCRF with ITR as a treatment option demonstrates the potential for significant cost savings over functional rhinoplasty with septoplasty and ITR.
PLAIN LANGUAGE SUMMARY
Nasal valve dysfunction is a common cause of nasal airway obstruction (NAO) that has a significant impact on heath and quality of life for affected individuals. Previously, patients were offered temporary measures or a type of surgery called functional rhinoplasty which is a highly complex surgery that can be costly, requires recovery time, and in rare cases, not be successful. Recently, a new minimally invasive treatment alternative for NAO called temperature-controlled radiofrequency (TCRF) that may be performed in a surgery center or a doctor’s office has become available. This paper provides the results of budget impact analysis performed to assess whether adding the TCRF procedure in place of surgery as a choice for patients with NAO will result in cost savings to an insurance payer with 1 million covered individuals in the United States over a period of 4 years. Results show that TCRF may result in an average of 9,416 fewer rhinoplasty surgeries, provide an average 4-year cost-savings of $3,531 for every patient that responds to TCRF treatment, and a savings of $20,015,123 over 4 years for the insurance provider. These potential cost savings over 4 years would likely be due to reduced procedure costs and complication rates compared to surgery.
Introduction
Nasal airway obstruction (NAO) is a common condition that presents with a feeling of fullness and congestion in the nasal cavity, resulting in a range of symptoms, such as difficulty breathing, headaches, fatigue, sleep disordered breathing, and daytime sleepinessCitation1–3. Causes of chronic NAO include the presence of structural abnormalities, such as nasal valve collapse (NVC), septal deviation (SD), and inferior turbinate hypertrophy that lead to the narrowing or collapse of the nasal airwayCitation1,Citation4. It is common for patients to present with a combination of multiple structural deformities, making correct diagnosis challenging and the key to successful treatmentCitation5. An incorrect or missed diagnosis of NAO caused by NVC can lead to persistent symptoms of NAO even after the patient proceeds to surgery if NVC is not addressedCitation6.
Treatment options for NAO, for which NVC is the primary cause or a significant contributor, aim to strengthen or stabilize the lateral nasal wall and open the nasal airway to increase airflowCitation7. First-line (1L) treatments for NAO include mechanical dilators (e.g. cones or nasal strips), which offer temporary relief and may not always be effective. Surgical procedures, such as functional rhinoplasty with septoplasty and inferior turbinate reduction (ITR), have been considered the standard of care to address NVCCitation7,Citation8. However, invasive surgical interventions are complex, costly, and carry a risk of complications and cosmetic changesCitation8,Citation9. Moreover, there is a non-trivial failure rate associated with surgery. such that symptoms are likely to persist, and revision surgery may be necessaryCitation10. For example, Spataro and colleagues reported septorhinoplasty revision rates from a large cohort of patients that ranged from 3% for primary septorhinoplasty to 11% for secondary septorhinoplasty procedures and others reference rhinoplasty revision rates of 5–15%Citation11,Citation12.
The Food and Drug Administration cleared a temperature-controlled radiofrequency (TCRF) device (VivAer®) in December 2017 to treat NAO by shrinking submucosal tissue, including cartilage in the internal nasal valve areaCitation13. TCRF can be performed in an ambulatory care or office-based setting following administration of topical or local anesthetics, representing an efficacious, less costly, minimally invasive treatment option for NAO resulting from NVC compared to surgery. TCRF treatment of NAO may be performed on the nasal valve alone or in conjunction with treatment of the inferior turbinate and/or septal swell body as they are important anatomic contributors to NAO. Clinical evidence has demonstrated that TCRF has a significant and sustained treatment effect for up to 4 years with no serious procedure-related complications reportedCitation14–18.
Although clinical studies have demonstrated the efficacy of TCRF and clinical outcomes for nasal airway obstruction symptom reduction have been demonstrated to be comparable to rhinoplasty surgeryCitation19, currently, there is no evidence that demonstrates the economic value of the device compared to surgery. This study aimed to estimate the budget impact of introducing TCRF as a treatment option for NAO in the US compared to a scenario where TCRF is not available.
Methods
Model overview
To explore the potential monetary savings of using the TCRF device as an alternative to functional rhinoplasty for NAO related to nasal valve dysfunction, a budget impact model was developed to estimate the cost impact of introducing TCRF into the market, specifically in a potential payer system, including the assessment of these potential impacts over time. This model evaluates two scenarios: a reference scenario of functional rhinoplasty (nasal valve repair) with septoplasty and inferior turbinate reduction (ITR) performed in the hospital outpatient department, which reflects current clinical practice where TCRF is not available as a treatment option. The new scenario reflects either treatment of the nasal valve and inferior turbinates with a TCRF device and an ITR as a concomitant procedure in-office setting or functional rhinoplasty with septoplasty, and ITR performed in the hospital outpatient department. Annual, total, and yearly average budget impact and budget impact by responder were considered primary outcomes of the model.
The budget impact model was developed following the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) good practice guidelines for budget impact models and economic modelingCitation20. The model was developed for the US and assumed a payor perspective, estimating costs over a 4-year time horizon for a hypothetical health insurance plan population of 1,000,000 members. Discounting was unnecessary since the budget holder’s interest is the actual impact expected for each yearCitation20. The full list of model assumptions is included in Table A1 within the Supplemental Materials.
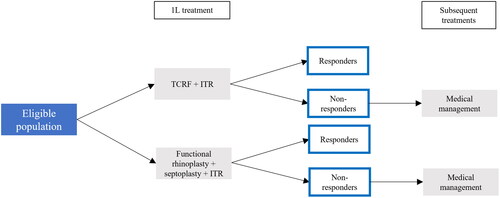
The budget impact was estimated using the decision tree model structure (). It was assumed that all patients treated in 1L had previously failed medical management, which would include the use of over-the-counter treatment options. The decision tree starts at the 1L, where TCRF became available as a treatment option in an office-based setting for eligible patients. If patients failed to respond to TCRF or the surgical comparator, patients were considered to incur continued baseline medical management costs throughout the time horizon. In alignment with the clinical trials, the model assumed no repeat TCRF administrations and consequently, this was not included as a subsequent treatment option. Costs relating to treatment administration, adverse events, and post-treatment costs were also included. See Table A2 for procedure cost inputs and adverse event incidence rates by treatment.
Model inputs
The model inputs consisted of epidemiological, treatment uptake, clinical, and cost inputs. Clinical inputs were designed in consultation with practicing Otolaryngologist–Head and Neck Surgeons. Cost inputs were designed in consultation with an Otolaryngology-Head and Neck Surgery billing expert. The eligible population () for treatment with TCRF was calculated from an initial plan population of 1,000,000 members, applying different population restrictions. These included the following criteria based on clinical study literature and clinical policy guidelines. Firstly, the eligible population included both patients newly diagnosed with NAO and those diagnosed with NAO before the first year of the model. The eligible population was also restricted to patients who have sought treatment and an Ear-Nose-Throat (ENT) physician visit. Additionally, NAO must have been classified using the NOSE Score, a validated patient-reported outcome measureCitation23. The eligible population was required to have severe/extreme NAO, defined as Nasal Obstruction Symptom Evaluation (NOSE) scores ≥55 (Supplemental Table A3). NVC must have been a significant contributory factor or the primary cause of NAO for patients to be eligible for treatment with TCRF. Not all eligible patients would have immediately considered TCRF as a treatment option. Thus, the eligible population was assumed to be restricted to 25% of patients receiving treatment each year based on the number of patients receiving surgical interventions each year. All eligible patients treated at 1L were assumed to have failed medical management before seeking an ENT physician visit.
Table 1. Demographic and epidemiology data.
Table 2. 1L treatment uptake inputs for reference and new scenario over 4 years after launch.
Treatment uptake inputs reflect the proportion of eligible patients receiving treatment in each year of the relevant scenario over the model’s time horizon (). TCRF with ITR was assumed to displace the surgical comparator (functional rhinoplasty with septoplasty and ITR) over the time horizon, capturing 40% of the market by Year 4. Considering that TCRF treatment of NAO is a relatively new and novel treatment option for NAO with limited historic market data, estimated uptake inputs were used in the model which were based on plausible estimates confirmed by experts in the field of rhinology with an aim of modeling uptake of the technology by a potential payer system. If treatment was unsuccessful, patients initiated medical management in subsequent treatment. The model did not include a revision of TCRF with ITR and functional rhinoplasty, as patients were assumed only to be treated with medical management as a subsequent treatment. This assumption was included to mitigate the evidence gap in the number of patients requiring revision treatment such that data requirements were reduced. See Table A4 in Supplemental Materials.
Within the model, successful treatment response to TCRF was defined as a ≥20% improvement in NOSE-scale score or improvement in at least 1 NOSE improvement scale severity category from baseline at 12 months and estimated to be 89.8%, as demonstrated in a previous study on TCRFCitation24. This TCRF treatment response rate is aligned with similar TCRF treated populations in different studiesCitation25. Treatment response for functional rhinoplasty with septoplasty was defined as an absolute reduction = in NOSE score of ≥30 points and estimated to be 75.0% based on a previous literature studyCitation26.
Mild and moderate adverse event categories were included in the model to reflect the different severity and cost. Mild adverse events were assumed to be treated in an outpatient setting, and more severe adverse events were assumed to be treated in a hospital setting. To date, no adverse events requiring treatment in a hospital setting have been reported in the TCRF treatment literatureCitation13–18,Citation24,Citation25. Adverse event frequencies for both mild and moderate severity adverse events are given in Table A5 in Supplemental Materials.
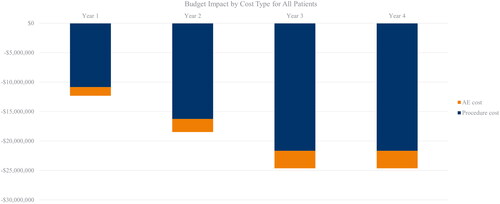
Given the payor perspective, the model included only costs incurred by the payor (e.g. co-payments and out of pocket expenses incurred by patients). Three categories of costs were included in the model: procedure costs relating to treatment administration, costs associated with medical resource use in the post-treatment period, and costs in treating adverse events. Details of the budget impacts by cost type are provided in Table A6 in Supplemental Materials. Prescription medication costs were included for medications likely to be reimbursed by payors. Procedure-related costs, such as anesthesia costs for surgical comparators, were included as these are a necessary cost incurred by payors and are not required for non-surgical interventions (e.g. TCRF).
The unit costs for each treatment option were sourced using Current Procedural Terminology (CPT) codes. Costs for TCRF treatment and medical management were based on in-office treatment setting costs, while surgical comparators were based on hospital outpatient department (HOPD) costs. An anesthesia cost of $449 was applied to the procedure costs of surgical comparators based on 2023 Medicare reimbursement rates for anesthesiologist time and medical direction during the procedure. An additional $100 of anesthesia drug cost was also included in the reported cost. See Table A2 in Supplemental Materials for the CPT codes used in the model.
After treatments were administered, it was expected that medication and resource use associated with monitoring (e.g. number of physician visits) were incurred within a 12-month post-treatment period. The costs associated with these activities included an ENT physician visit, pain relief medication, and antibiotics for the surgical comparator. Due to a lack of data availability, medical resource utilization (MRU) rates were assumed to be equivalent between responders and non-responders. See Supplemental Table A7 for post-treatment MRU by response status and Supplemental Table A8 for the average cost per responder by treatment. MRUs were also assumed to be zero starting the year after patients had achieved a response, as patients were considered to be asymptomatic and not incurring any additional treatment costs.
Sensitivity analysis
One-way sensitivity analyses were performed on the main input parameters by varying the values to assess their impact of parameter uncertainty on the budget impact results. Due to the lack of available well-defined confidence intervals for each input parameter, input parameters were varied by ±20%.
Results
According to the model’s estimation, the introduction of TCRF with ITR as a treatment for NAO resulting from NVC could lead to the avoidance of an average 2,354 functional rhinoplasty with septoplasty procedures annually over the 4-year time horizon in the hypothetical population ().
Table 3. Model results.
The model estimated 7,243 eligible patients for TCRF with ITR in Year 1, amounting to a yearly average budget impact across the 4-year time horizon of −$20,015,123 compared to the scenario where TCRF was not available as a treatment option (). Savings related to procedure costs reached a yearly average budget impact of −$17,609,786 ().
In the scenario where TCRF was available, the increase in the total number of 1L responders was estimated to reach 1,394 across the 4-year time horizon. The total budget impact by 1L responder was −$3,531 (), which showed the cost difference when introducing TCRF with ITR divided by the difference in the number of patients that responded to the treatment. The total number of surgeries estimated to be avoided due to the availability of TCRF across the 4-year time horizon was 9,416 ().
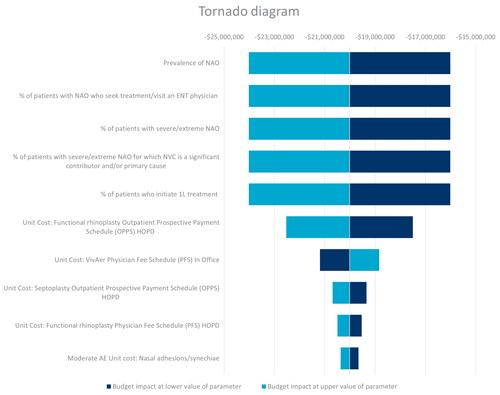
The one-way sensitivity analysis for the full population, shown in the Tornado diagram in , demonstrated that the budget impact results were primarily sensitive to changes in epidemiological inputs. Given that a standard scaling factor was applied to the inputs for the DSA, the epidemiology inputs were observed to have the same variation on the budget impact.
The yearly average budget impact was most sensitive to the prevalence of NAO. Varying this parameter by ±20% resulted in a total average yearly savings of $16,012,098 to $24,018,148 (Table A9 in Supplemental Material). In the sensitivity analysis for the budget impact per treatment responder (Figure A1 and Table A10 in Supplemental Material), the budget impact was most sensitive to the response rate for patients receiving functional rhinoplasty + septoplasty + ITR. Varying this parameter by ±20% resulted in a total average savings of $5,360 to $2,450.
Discussion
The results of this budget impact analysis suggested that introducing TCRF with ITR to treat NAO caused by NVC can provide substantial cost savings among a large population of eligible patients. A high proportion of the estimated cost savings in the model were attributed to the lower procedure costs of TCRF relative to surgical comparators and a significantly lower complication rate. The sensitivity analysis further showed that increasing the number of patients with NAO or eligible for TCRF treatment has the potential to increase overall population savings even more. In addition, lowering the estimated response rate to TCRF and increasing the cost of the procedure still resulted in considerable cost savings.
An important result to consider in this economic analysis comparing the two procedures is the inherent advantage of TCRF as a minimally invasive, office-based treatment for nasal valve dysfunction over invasive procedures, such as complex nasal valve repair performed as an ambulatory care surgical procedure. Patients with severe/extreme NAO due to NVC treated with TCRF have shown durable reductions in nasal obstruction symptoms, at least on par with functional rhinoplasty surgeryCitation19,Citation27,Citation28. Additionally, as a minimally invasive office-based treatment option, TCRF can be offered to patients with certain co-morbid conditions who may not be considered suitable surgical candidates, thus avoiding the need for pre-operative testing (e.g. laboratory tests, electrocardiogram) or a general anesthetic.
It is important to note that this model did not capture how the epidemiology of NAO may change over time and vary due to the introduction of TCRF. It is possible that introducing non-invasive treatment options, such as TCRF to the market could potentially increase the total number of patients seeking treatment by converting those patients who otherwise may not have considered a procedural intervention due to factors, such as patient preference or medical comorbidities precluding a general anesthetic. In addition to a reduction in procedure-related costs, a greater number of total patients seeking effective minimally invasive treatment for their NAO may also experience greater overall cost savings secondary leading to a reduction in the overall prevalence of untreated NAO within the population.
This budget impact model has several strengths and limitations. One of the strengths of this study was that a real-world estimate of the eligible patient population with severe NAO was derived based on publicly available data, providing a valid estimate of the number of patients who are likely to be directly affected by the availability of TCRF in the short term. An additional strength of the model was considering different administration settings of the treatments and the associated costs for each, allowing the analysis to reflect different administration settings. For example, costs for TCRF treatment and medical management were based on in-office treatment setting costs, while surgical comparators were based on HOPD costs.
The primary limitation of the model is the lack of available data for specific components of the budget impact model. The limited availability of existing data for new and emerging technologies poses an inevitable challenge, complicating projections regarding the economic impacts and other potential advantages of replacing conventional surgical procedures with new approaches. However, when the uncertainty of data was present due to the unavailability of data, the model used conservative assumptions that reduced the bias toward TCRF treatment. Data uncertainty was also investigated in the analysis through a one-way sensitivity analysis.
A limitation of the model based on the lack of available data was that all non-responders to procedural treatment were assumed to be treated with continued medical management, which may not be the case as patients may opt to undergo revision procedures with uncertainty around expected rates of subsequent response. However, it could be that patients who are non-responders to functional rhinoplasty and who go on to require revision rhinoplasty would incur significantly greater costs when compared to patients treated with TCRF with ITR. There is currently insufficient data to explore the projected costs of patients who undergo revision procedures.
An additional limitation was found in the treatment uptake inputs included in the model. Treatment uptake inputs were based on expert opinion on the treatment landscape projections and historical commercial data for the TCRF device. However, the uncertainty of TCRF with ITR uptake inputs was tested in the one-way sensitivity analysis, which resulted consistently in cost-saving budget impacts.
Lastly, the analysis was limited by a lack of available data for the medical resources used post-procedure. The assumption was made that MRU within a 12-month post-treatment period was equivalent between responders to TCRF and functional rhinoplasty, as well as non-responders. It is likely that when additional data reflecting changes in MRU is available, cost savings associated with post-procedural MRU will be higher for responders than estimated in this analysis.
Future areas for research include characterizing MRU associated with NAO for both untreated and treated patients, including responders and non-responders. Additionally, incorporating quality of life data would enable a cost-effectiveness analysis comparing treatment options. This would provide a more comprehensive understanding of the potential economic advantages of incorporating a minimally invasive TCRF option for the management of NAO into a health system, which may ultimately result in additional cost and quality of life advantages.
Conclusion
In patients with severe/extreme NAO, for which NVC is the primary or major contributor, the introduction of TCRF with ITR as a treatment option demonstrates the potential for significant cost savings over functional rhinoplasty with septoplasty and ITR as a result of lower treatment and complications costs.
Transparency
Declaration of funding
Aerin Medical funded this study and will sponsor the open access for the publication.
Declaration of financial/other relationships
DH, JB, and SW are employees of Aerin Medical. AH, SB, AF, RH, and GB are employees of Adelphi Values PROVE. GS is an independent consultant who supported this study and is contracted through Adelphi Values PROVE. MY is an employee of Stanford University. RO is an employee of SacENT and is a consultant for Aerin Medical.
Author contributions
All authors read and approved the final version of this manuscript. Concept and design: AH, SB, AF, GS, RH. Acquisition of data: DH. Analysis and interpretation of data: AH, SB, AF, GS, RH, GB, MY, DH, RO. Drafting of the manuscript: AH, SB, AF, GS, RH, GB. Critical revision of the paper for important intellectual content: MY, DH, JB, SW, RO. Administrative, technical, or logistic support: AH, SB, AF, GS, RH, GB. Supervision: DH.
Reviewer disclosures
Peer reviewers on this manuscript have received an honorarium from JME for their review work but have no other relevant financial relationships to disclose.
Supplemental Material
Download MS Word (143.3 KB)Acknowledgements
The authors thank Adeola Sanni, an independent consultant to Aerin Medical, for assistance with manuscript preparation.
Data availability statement
All data used in this study may be made available upon request.
References
- Mohamed S, Emmanuel N, Foden N. Nasal obstruction: a common presentation in primary care. Br J Gen Pract. 2019;69(689):628–629. doi: 10.3399/bjgp19X707057.
- Udaka T, Suzuki H, Kitamura T, et al. Relationships among nasal obstruction, daytime sleepiness, and quality of life. Laryngoscope. 2006;116(12):2129–2132. doi: 10.1097/01.mlg.0000239111.24094.a3.
- Rhee JS, Book DT, Burzynski M, et al. Quality of life assessment in nasal airway obstruction. Laryngoscope. 2003;113(7):1118–1122. doi: 10.1097/00005537-200307000-00004.
- Schuman TA, Senior BA. Treatment paradigm for nasal airway obstruction. Otolaryngol Clin North Am. 2018;51(5):873–882. doi: 10.1016/j.otc.2018.05.003.
- Clark DW, Del Signore AG, Raithatha R, et al. Nasal airway obstruction: prevalence and anatomic contributors. Ear Nose Throat J. 2018;97(6):173–176. doi: 10.1177/014556131809700615.
- Gagnieur P, Fieux M, Louis B, et al. Objective diagnosis of internal nasal valve collapse by four-phase rhinomanometry. Laryngoscope Investig Otolaryngol. 2022;7(2):388–394. doi: 10.1002/lio2.784.
- Yeung A, Hassouneh B, Kim DW. Outcome of nasal valve obstruction after functional and aesthetic-functional rhinoplasty. JAMA Facial Plast Surg. 2016;18(2):128–134. doi: 10.1001/jamafacial.2015.1854.
- Starr NC, Creel L, Harryman C, et al. Cost utility analysis of costal cartilage autografts and human cadaveric allografts in rhinoplasty. Ann Otol Rhinol Laryngol. 2022;131(10):1123–1129. doi: 10.1177/00034894211058115.
- Sharif-Askary B, Carlson AR, Van Noord MG, et al. Incidence of post-operative adverse events after rhinoplasty: a systematic review. Plast Reconstr Surg. 2019;145(3):669–684. doi: 10.1097/PRS.0000000000006561.
- Khosh MM, Jen A, Honrado C, et al. Nasal valve reconstruction: experience in 53 consecutive patients. Arch Facial Plast Surg. 2004;6(3):167–171. doi: 10.1001/archfaci.6.3.167.
- Spataro E, Piccirillo JF, Kallogjeri D, et al. Revision rates and risk factors of 175,842 patietns undergoing septorhinoplasty. JAMA Facial Plast Surg. 2016;18(3):212–219. doi: 10.1001/jamafacial.2015.2194.
- Bouaoud J, Loustau M, Belloc J-B, et al. Functional and aesthetic factors associated with vision rhinoplasty. Plast Reconstr Surg Glob Open. 2018;6(9):e1884. doi: 10.1097/GOX.0000000000001884.
- US Food and Drug Administration. 510(k) Premarket notification [cited 2023 Mar 15]. Available from: https://www.accessdata.fda.gov/cdrh_docs/pdf17/K172529.pdf
- Yao W, Ow R, Barham H. Temperature-controlled radiofrequency treatment of the nasal valve and nasal airway obstruction: early results of a prospective, multi-center study. J Otolaryngol Rhinol. 2021:104. doi: 10.23937/2572-4193.1510105.
- Ephrat M, Jacobowitz O, Driver M. Quality-of-life impact after in-office treatment of nasal valve obstruction with a radiofrequency device: 2-year results from a multicenter, prospective clinical trial. Int Forum Allergy Rhinol. 2021;11(4):755–765. doi: 10.1002/alr.22667.
- Silvers SL, Rosenthal JN, McDuffie CM, et al. Temperature-controlled radiofrequency device treatment of the nasal valve for nasal airway obstruction: a randomized controlled trial. Int Forum Allergy Rhinol. 2021;11(12):1676–1684. doi: 10.1002/alr.22861.
- Jacobowitz O, Driver M, Ephrat M. In-office treatment of nasal valve obstruction using a novel, bipolar radiofrequency device. Laryngoscope Investig Otolaryngol. 2019;4(2):211–217. doi: 10.1002/lio2.247.
- Brehmer D, Bodlaj R, Gerhards F. A prospective, non-randomized evaluation of a novel low energy radiofrequency treatment for nasal obstruction and snoring. Eur Arch Otorhinolaryngol. 2019;276(4):1039–1047. doi: 10.1007/s00405-018-05270-y.
- Han JK, Perkins J, Lerner D, et al. Comparison of nasal valve dysfunction treatment outcomes for temperature-controlled radiofrequency and functional rhinoplasty surgery: a systematic review and meta-analysis. Rhinology. 2024. doi: 10.4193/Rhin23.261.
- Sullivan SD, Mauskopf JA, Augustovski F, et al. Budget impact analysis-principles of good practice: report of the ISPOR 2012 budget impact analysis good practice II task force. Value Health. 2014;17(1):5–14. doi: 10.1016/j.jval.2013.08.2291.
- Wever CC. The nasal airway: a critical review. Facial Plast Surg. 2016;32(1):17–21. doi: 10.1055/s-0035-1570323.
- Raithatha R, Del Signore A. Prevalence and identification of nasalairway obstruction in patients presenting to otolaryngology clinics: results from a large descriptive practice survey. Ear NoseThroat J. 2023:1455613231196670.
- Stewart MG, Witsell DL, Smith TL, et al. Development and validation of the nasal obstruction symptom evaluation (NOSE) scale. Otolaryngol Head Neck Surg. 2004;130(2):157–163. doi: 10.1016/j.otohns.2003.09.016.
- Han JK, Silvers SL, Rosenthal JN, et al. Outcomes 12 months after temperature-controlled radiofrequency device treatment of the nasal valve for patients with nasal airway obstruction. JAMA Otolaryngol Head Neck Surg. 2022;148(10):940–946. doi: 10.1001/jamaoto.2022.2293.
- Casale M, Moffa A, Giorgi L, et al. Could the use of a new novel bipolar radiofrequency device (aerin) improve nasal valve collapse?A systematic review and meta-analysis. J Otolaryngol Head Neck Surg. 2023;52(1):42. doi: 10.1186/s40463-023-00644-7.
- Rhee JS, Sullivan CD, Frank DO, et al. A systematic review of patient-reported nasal obstruction scores: defining normative and symptomatic ranges in surgical patients. JAMA Facial Plast Surg.2014;16(3):219–225; quiz 232. doi: 10.1001/jamafacial.2013.2473.
- Zhao R, Chen K, Tang Y. Effects of functional rhinoplasty on nasal obstruction: a meta-analysis. Aesthetic Plast Surg. 2022;46(2):873–885. doi: 10.1007/s00266-021-02741-2.
- Floyd EM, Ho S, Patel P, et al. Systematic review and meta-analysis of studies evaluating functional rhinoplasty outcomes with the NOSE score. Otolaryngol Head Neck Surg. 2017;156(5):809–815. doi: 10.1177/0194599817691272.