Abstract
Objective
This analysis estimated the outcomes of triennial blood-based colorectal cancer (CRC) screening at various adherence, including perfect adherence, compared with triennial multi-target stool DNA (mt-sDNA) screening at the reported real-world adherence rate.
Methods
The validated CRC-AIM model simulated a US cohort of average-risk individuals receiving triennial screening with mt-sDNA or blood-based test from ages 45 to 75 years. Modeled specificity and sensitivity were based on reported data. Adherence was set at a real-world rate of 65.6% for mt-sDNA and at 65.6%, relative 10% incremental increases from 65.6%, or 100% for the blood-based test. Costs of mt-sDNA and the blood-based test were based on prices for clinically available tests ($508.87 and $895, respectively). Value-based pricing was estimated at a willingness-to-pay threshold of $100,000.
Results
Both tests resulted in life-years gained (LYG), reduced CRC cases, and reduced deaths versus no screening. With adherence for mt-sDNA set at 65.6% and for blood-based test set at 100%, mt-sDNA resulted in 30% more LYG, 52% more averted CRC cases, and 32% more averted CRC deaths. At reported sensitivity and specificity rates, mt-sDNA at 65.6% adherence dominates (is more effective and less costly) the blood-based test at any adherence. There was no price at which triennial screening with the blood-based test at any adherence was cost-effective compared with mt-sDNA at 65.6% adherence.
Conclusions
Triennial screening with mt-sDNA resulted in better clinical outcomes at a lower cost compared with the modeled blood-based test even at perfect adherence, supporting application of blood-based tests only as a secondary screening option.
PLAIN LANGUAGE SUMMARY
Blood-based colorectal cancer screening has lower diagnostic accuracy, lower clinical and health outcomes, and is more expensive than mt-sDNA, even with perfect blood-based screening participation. Although better than no screening at all, blood-based testing is unlikely to exceed performance of stool-based assessment unless a blood-based test is able to meaningfully detect precancerous growths.
Introduction
Clinical organizations suggest that average risk individuals aged 45–75 years should be screened with one of the many available colorectal cancer (CRC) screening optionsCitation1–3. The benefits of CRC screening with multiple modalities has been convincingly demonstrated in the US populationCitation4,Citation5. Adherence is a critical contributor to the clinical and economic impacts of average-risk CRC screeningCitation6–8. Not surprisingly, screening adherence has been less than perfect and varies across test- and interval-specific strategies because of barriers at multiple levelsCitation9–12. Among available recommended non-colonoscopic options, published real-world adherence to triennial multi-target stool DNA (mt-sDNA; Cologuard*) remains higher than any other guideline-endorsed screening strategyCitation1,Citation2,Citation13. Screening options that require minimal pre-test preparation and are broadly accessible may improve adherence, motivating efforts to develop blood-based screening tests that are accurate and acceptable. However, studies demonstrate mixed results with respect to the uptake and completion of blood-based CRC screening tests in several clinical scenariosCitation14–18. Blood-based CRC screening tests are not currently endorsed by CRC screening guidelinesCitation19, and their adherence is unknown. Previous simulation studies modeled comparative effectiveness of endorsed screening tests with a blood-based test assuming either perfect adherence to screeningCitation20,Citation21 or modeled assumed blood-test adherence ratesCitation7,Citation22. None of these studies evaluated the economic impact of differing adherence rates for blood-based screening or an estimated price range at which the blood-based tests are cost-effective compared with current stool-based tests.
In addition to adherence, the potential benefit-to-harm ratio associated with any CRC screening strategy is also dependent on test performance (sensitivity and specificity to detect adenomas and CRC)Citation6,Citation23. Blood-based tests have lower sensitivity to detect advanced neoplasia, particularly precancerous lesions, than mt-sDNACitation24,Citation25. The direct impact of adenoma and precancerous lesion detection, serving as an indicator of cancer prevention, has not been demonstrated across blood-based and stool-based CRC screening modalities. Accordingly, the objective of this analysis was to use a validated simulation model to estimate the clinical and economic outcomes of triennial blood-based CRC screening at various adherence rates – including perfect adherence – compared with triennial mt-sDNA screening at the reported real-world adherence rate.
Methods
CRC-AIM model
Microsimulation models are a means of estimating outcomes, and the impact of interventions on outcomes, in a simulated cohort of individuals as they transition from one health state to another. The Colorectal Cancer and Adenoma Incidence and Mortality Microsimulation Model (CRC-AIM) has been previously described, calibrated to CRC incidence in the USCitation26, cross-model validated to three CRC Cancer Intervention and Surveillance Modeling Network (CISNET) models, and externally validated to the United Kingdom Flexible Sigmoidoscopy Clinical trialCitation6,Citation27.
The model contains CRC natural history and screening components. The natural history component models the progression of CRC from the adenoma-carcinoma pathway in an unscreened population from birth until age 100 years or death. It matches the US incidence of CRCCitation27. The natural history dynamics of CRC-AIM include functions that estimate the risk of adenoma development by sex and age, location of the adenoma, adenoma growth rates, the probability of an adenoma to transition to preclinical cancer, the potential transition to symptomatic CRC, CRC growth, and CRC stage-specific survivalCitation27. The screening component of CRC-AIM contains assumptions related to CRC screening, namely, the performance characteristics of the CRC screening test (i.e. sensitivity, specificity, and associated complications), the frequency of the test, and patient adherence to the frequency of the testCitation6.
Outcomes in the current analysis were simulated using CRC-AIM version 2.0.3 in Python 3.8 for a US cohort of 2 million average-risk individuals that received screening with mt-sDNA or blood-based test every 3 years between the ages of 45 and 75 years.
Model assumptions
The assumed performance characteristics (specificity and sensitivity) for mt-sDNA were the same as those used by the US Preventive Services Task Force (USPSTF); characteristics for the ShieldFootnote** blood-based test were from results of a patient registrational studyCitation23,Citation25. Specificity, sensitivity for CRC, and sensitivity for large (≥10 mm) adenomas were 0.91, 0.94, and 0.42, respectively, for mt-sDNA and 0.90, 0.83, and 0.13, respectively, for the blood-based test (Supplemental Table S1).
Assumed adherence in the model for mt-sDNA was the published real-world adherence of 65.6%Citation13. Since real-world adherence to a blood-based CRC screening test is currently unknown, several adherence scenarios were used for the blood-based test, starting at the published adherence rate for mt-sDNA (65.6%), increasing by relative 10% increments from 65.6% (10% higher = actual 72.2%, 20% higher = actual 78.7%, 30% higher = actual 85.3%, or 40% higher = actual 91.8%) and ending at perfect (100%) adherence.
A diagnostic follow-up colonoscopy is needed after any positive, non-colonoscopy CRC screening test to search for and remove any precancerous adenomas or CRCCitation1,Citation2. The assumed performance characteristics for colonoscopy were the same as those used by the USPSTF (Supplemental Table S1)Citation23. Several complications from colonoscopy were modeled that were identical to those used by the USPSTF, where the risk of complications increases by ageCitation23.
In accordance with methods used by the USPSTF, adherence to a follow-up colonoscopy after a positive test was assumed to be 100% for both mt-sDNA and the blood-based testCitation23. A sensitivity analysis was performed where a conservative published real-world follow-up colonoscopy rate of 71.5%Citation28 was used for mt-sDNA and follow-up colonoscopy was assumed to be 100% for the blood-based test. Adherence to ongoing surveillance colonoscopy after discovery of advanced neoplasia was assumed to be 100% for both stool-based and blood-based tests.
The costs used in this analysis are expressed in US dollars. Cost inputs were $508.87 for mt-sDNA as reported in the Centers for Medicare & Medicaid Services (CMS) 2022 clinical laboratory fee schedule and $895 for the blood-based test as reported by the manufacturerCitation29,Citation30. Cost inputs for colonoscopy (with and without polypectomy), colonoscopy complications, and CRC medical costs were derived from published literature and are shown in Supplemental Table S2. The analysis was carried out from the payer perspective, wherein costs were valued based on reimbursement rates of commercial and Medicare beneficiaries, excluding any copayments and/or cost-sharing. Costs were inflated to December 2022 values. Utility inputs derived from published literature are also shown in Supplemental Table S2.
Outcomes and analysis
A lifetime horizon was chosen to assess the impact of screening per 1,000 individuals. Clinical outcomes of the analysis were life-years gained (LYG), number of CRC cases and deaths averted by screening, LYG per colonoscopy (represents the benefit-to-burden ratio), and LYG from adenoma or CRC detection compared with no screening. To determine the breakdown of LYG based on adenoma and CRC detection, each simulated individual with extended life-years was further evaluated. If the individual did not develop cancer as a result of screening, the additional life-years were linked to adenoma detection. Alternatively, if cancer was detected, the additional life-years were attributed to CRC detection. The economic value of the triennial blood-based test compared with triennial mt-sDNA was estimated by the incremental cost-effectiveness ratio (ICER), additional costs per case and death averted, and value-based pricing (VBP), which is the maximum price at which a willingness-to-pay (WTP) threshold is reached. The WTP for this analysis was set at $100,000 with secondary analyses set at a WTP of $50,000 and $150,000 per quality-adjusted life-years (QALYs). Details of VBP calculations are described in the Supplemental Methods. QALYs were calculated using the same general framework used by the USPSTF. All costs and QALYs were discounted at 3%Citation31.
Since there is a lack of data supporting the interval of blood-based CRC screening, a sensitivity analysis was conducted to estimate clinical outcomes and VBP of the blood-based test received at an interval of every 1 or 2 years. In addition, one-way deterministic sensitivity analyses where adherence, costs, and utilities were varied ±10% of their base case values were conducted. Finally, to show the robustness of economic outcomes of this analysis, probabilistic sensitivity analysis where all parameters were varied simultaneously over 500 iterations were conducted, and the ICERs were calculated accordingly.
Results
Estimated clinical outcomes
mt-sDNA resulted in greater LYG and reduced CRC cases and deaths compared with the blood-based test (). With adherence for mt-sDNA set at 65.6% and for blood-based test set at 100%, mt-sDNA resulted in 30% more LYG, 52% more averted CRC cases, and 32% more averted CRC deaths (). Compared with a triennial blood-based test at adherence rates ranging from 65.5% to relatively 40% higher, triennial mt-sDNA resulted in 31–41% more LYG and 12–14% more LYG per colonoscopy (the benefit-to-burden ratio; ), as well as 57–72% more averted CRC cases and 35–45% more averted CRC deaths (). Adenoma detection resulted in 83% of the LYG with mt-sDNA and 64–67% of the LYG with the blood-based test in the various adherence scenarios ().
Figure 1. (a) LYG and LYG per COL (benefit-to-burden ratio) and (b) CRC cases and deaths averted per 1,000 individuals. Results are for triennial mt-sDNA at published real-world adherence (65.6%) and a triennial blood-based test at adherence equal to mt-sDNA real-world adherence, increasing adherence relative to mt-sDNA real-world adherence, or at perfect (100%) adherence. Percentages over each column indicate the percent higher outcome with mt-sDNA relative to the blood-based test. Abbreviations: CRC, colorectal cancer; COL, colonoscopy; LYG, life-years gained; mt-sDNA, multi-target stool DNA.
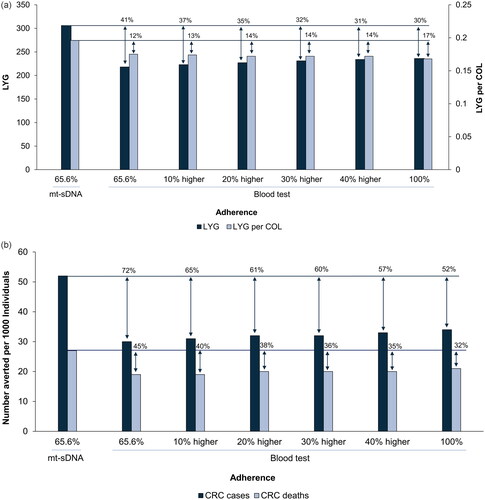
Figure 2. Percentage of LYG per 1,000 individuals resulting from detection of adenomas or CRC cases. Results are for triennial mt-sDNA at published real-world adherence (65.6%) and a triennial blood-based test at adherence equal to mt-sDNA real-world adherence, increasing adherence relative to mt-sDNA real-world adherence, or at perfect (100%) adherence. Abbreviations: CRC, colorectal cancer; LYG, life-years gained; mt-sDNA, multi-target stool DNA.
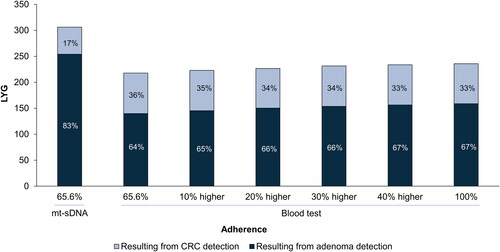
Table 1. Estimated health outcomes per 1,000 individuals screened for CRC from age 45–75 years.
Estimated economic value-based price
The ICERs were negative in all scenarios, indicating that the mt-sDNA test dominated (was more effective and less costly) blood-based testing at the list price of $895 at any adherence (Supplemental Figure S1). At a WTP threshold of $50,000, $100,000, or $150,000, there is no price at which the triennial blood-based test can be used cost-effectively instead of mt-sDNA ( and Supplemental Table S3). Among all scenarios, when comparing the additional cost per case and death averted between mt-sDNA and blood-based tests, the range was $185,877–$206,510 more with blood-based tests for cases averted and $277,162–$295,469 more for deaths averted.
Table 2. Estimated economic outcomes (discounted costs and QALYs).
Sensitivity analyses
Triennial mt-sDNA at published real-world adherence rates for screening and follow-up colonoscopy yielded better health outcomes and lower cost per patient screened when compared with blood-based testing with perfect adherence for screening and follow-up colonoscopy rates (data not shown). Triennial mt-sDNA at published real-world adherence resulted in higher LYG, higher LYG per colonoscopy (benefit-to-burden ratio), more averted CRC cases, and more averted CRC deaths than a blood-based test received every 2 years in all adherence scenarios (Supplemental Table S4). Only a blood-based test received every year and at 40% greater adherence than mt-sDNA or 100% adherence resulted in 2 and 7 higher LYG, respectively, than triennial mt-sDNA but at a trade-off of approximately 50% more (762 and 851, respectively) colonoscopies per 1,000 individuals (Supplemental Table S4). Depending on the adherence rate, there was an incremental cost of $145,492–$172,647 and $265,028–$281,458 for preventing an additional CRC case or death, respectively, with annual or biennial blood-based tests compared with triennial mt-sDNA.
At a WTP threshold of $100,000, a VBP of $21–$67 is estimated for the blood-based test if the frequency is every year and if adherence is at least relatively 20% higher than triennial mt-sDNA (Supplemental Table S5).
One-way sensitivity analyses found that adherence to screening and follow-up colonoscopy, along with the cost of blood test, had the greatest impact on the ICER (Supplemental Figure S2). The probabilistic sensitivity analysis found that the blood-based test was dominated (more costly and less effective) by mt-sDNA in 100% of the runs (Supplemental Figure S3).
Discussion
In this microsimulation study using the validated CRC-AIM modeling platform, triennial screening with either mt-sDNA or a blood-based test resulted in clinical benefits compared with no screening. When estimated outcomes from the two screening strategies were compared using published real-world adherence for mt-sDNA and perfect adherence for the blood-based test, mt-sDNA resulted in more LYG, more averted CRC cases and deaths, and lower aggregate medical expenditures per patient screened. A higher proportion of the LYG with mt-sDNA was attributed to effective CRC prevention (adenoma detection), as opposed to CRC detection, compared with the blood-based test. Thus, although a blood-based test results in positive health outcomes compared with no screening, it should only be considered as a screening option if a patient refuses or is unwilling to have a colonoscopy or stool-based test.
A blood-based CRC screening test may be appealing to some patients who are unable or unwilling to complete other effective endorsed strategies such as colonoscopy or stool-based tests. However, there are limited direct data comparing the adherence of stool-based and blood-based modalities in similar populations or in the same setting. The lack of payer coverage or recommended screening intervals for blood-based tests is attributed to the fact that most adherence studies focus on patients who opt for blood-based tests as a secondary choice after refusing the guideline-recommended screening methods. For example, a recent randomized trial by Coronado et al. found that offering a blood-based screening test increased CRC screening from 13% to 30% among adults who had declined prior CRC screeningCitation32. To account for the lack of adherence data for blood-based tests, the current analysis used a range of adherence starting with the published real-world adherence of 65.6% for mt-sDNACitation13 for direct comparison and increasing up to perfect (100%) adherence for the blood-based test. The model estimated that a blood-based test at 3-year intervals with perfect adherence produced inferior CRC outcomes and was more costly than mt-sDNA. Even when substantially reducing the price of the blood-based test to be equal to that of mt-sDNA, screening with a blood-based test was dominated (higher cost with lower effectiveness) compared with mt-sDNA. A previous microsimulation study estimated that perfect adherence to annual screening with a blood-based test would achieve similar numbers of CRC cases and deaths averted to triennial mt-sDNA but with a burden of 50% more colonoscopies and associated serious gastrointestinal eventsCitation7.
The sensitivity analysis revealed that estimated clinical outcomes with mt-sDNA remained superior to the blood-based test when follow-up colonoscopy rates were reduced to published real-world adherence rates and perfect follow-up colonoscopy rates were used for blood-based testing. The published real-world adherence rate of 71.5% for a follow-up colonoscopy after a positive mt-sDNA was conservative compared with the rate of 86% reported by Pickhardt et al.Citation33 Since there are no recommended intervals for blood-tests other than the CMS proposal for covering a test every 3 years, we further analyzed the outcomes of a blood-based test if offered every 2 years. This indicates that the lower sensitivity of the blood-based test to detect adenomas compared with mt-sDNA is the key factor behind the relatively poorer clinical outcomes, not the interval or adherence. The low performance is one of the stated reasons clinical organizations do not currently endorse blood-based CRC screening testsCitation2,Citation3.
The use of low sensitivity tests decreases the chances to prevent CRC since adenomas will go undetected and potentially progress to CRCCitation34, which increases the risk of CRC-related death. Previous analyses indicated that a triennial blood-based test may need 30% sensitivity to detect advanced adenomas and 100% adherence to achieve the LYG of triennial mt-sDNA at real-world adherenceCitation22. Lieberman and the American Gastroenterological Association CRC Workshop Panel proposed that the potential benchmark for an effective blood-based test would be an advanced adenoma sensitivity of 40–50%Citation35. Two other CRC screening modeling studies evaluated the outcomes of triennial blood-based testing using the minimum test performance thresholds defined by CMS (CRC sensitivity 74% and specificity 90%)Citation20,Citation21. Both modeling studies demonstrated that the triennial blood-based test at perfect adherence was more costly and less effective than triennial mt-sDNA or annual FIT at perfect adherence, primarily because of the inability of the blood-based test to detect advanced precancerous lesionsCitation20,Citation21. One model determined that a triennial blood-based test would require >90% CRC sensitivity, 80% advanced precancerous lesion sensitivity, 90% specificity, and cost less than $140 to be as cost-effective as annual FITCitation20. Thus, at present, blood-based CRC screening should not substitute for currently endorsed screening methods.
Limitations
The results of this study should be interpreted in the context of the assumptions. First, there are other noninvasive and invasive screening modalities that have been endorsed by US guidelines, but only one noninvasive stool-based test and no invasive tests were explored in this analysis. Second, this analysis only considered cross-sectional adherence rates; the impact of intermittent or longitudinal adherence on the cost-effectiveness of screening strategies was not explored. Third, while the inputs of this analysis, such as the cost of a blood-based test, were based on a limited number of resources, the model outcomes were tested through scenario and sensitivity analyses, and the results remained robust. Fourth, the costs of the blood-based test were assumed to be the same for commercial insurers and Medicare. It is possible that costs for blood-based testing may be reduced in the future, leading to greater cost-effectiveness. However, the model estimated that there is no price at which the triennial blood-based test would be considered cost-effective at a WTP threshold of $50,000–$150,000 when compared with triennial mt-sDNA. Importantly, until blood-based CRC screening tests are endorsed by the USPSTF, in contrast to stool-based testing, patients will likely be responsible for cost-sharing both for the initial screening test and for a follow-up colonoscopy after a positive test. Another limitation is that the analysis focused on the performance of a specific blood-based test. Emerging blood-based tests and biomarkers exhibit varying performance. Previous analyses underscored that the key determinant for the efficacy of blood-based tests lies in their capacity to prevent CRC cancers by detecting adenoma and pre-cancerous lesionsCitation22,Citation36. However, it is observed that most of the emerging blood-based tests currently fall short of detecting adenomas beyond serendipitous findingsCitation37. The performance and accuracy of a blood-based screening test may vary in real-world settings, compared to reported performance in clinical trials, and there is limited data to support the consistency of test performance for multiple rounds of screening. Finally, a next-generation mt-sDNA test has been found to have greater performance than the mt-sDNA performance used in the current analysis, but those performance values were not used in the current analysis because it is not commercially availableCitation38. It is anticipated that incorporating the enhanced performance characteristics of the next-generation mt-sDNA would result in similar or greater benefits versus a blood-based test compared with those demonstrated in the current analysis.
Conclusions
Access to multiple CRC screening options provides flexibility to accommodate patient preferences and drive greater engagement, consistent with the well-established perspective adopted by groups such as the USPSTF that the “best test is the one that gets done”Citation39. With the recent attention on blood-based CRC screening, appropriate positioning of these tests relative to other existing, endorsed options requires careful consideration. In this modeling analysis using a validated microsimulation platform, triennial CRC screening with a blood-based screening strategy compared favorably to no screening. However, when blood-based screening was compared with the noninvasive, stool-based mt-sDNA test, the estimated clinical and economic impacts were dominated by the latter option. These analyses emphasize the need for both effective early cancer detection and precancer detection to optimize programmatic CRC screening. Further, the analyses show that even when triennial mt-sDNA at a real-world adherence rate was compared with triennial blood-based screening with perfect adherence, the mt-sDNA strategy resulted in favorable CRC incidence, CRC mortality, LYG, colonoscopy utilization, and per-patient cost outcomes. Consequently, with the reported screening sensitivity for precancer detection, blood-based screening is projected to adversely impact the achievable benefits and burdens of CRC screening if these tests are used as a substitute for existing, higher performing test options. Thus, results from this study support application of blood-based tests only as a secondary option for patients who are not completing endorsed screening strategies, suggesting modification of the above statement to reflect that the “best test is the one that gets done appropriately”.
Transparency
Declaration of funding
Financial support for this study was provided through a contract with Exact Sciences Corporation. The funding agreement ensured the authors’ independence in designing the study, interpreting the data, writing, and publishing the report. Exact Sciences Corporation contributed to the study design, data analysis, interpretation of the data, and writing of the report.
Declaration of financial/other relationships
JBK has received research support from Exact Sciences through a contracted services agreement and is an inventor of Mayo Clinic intellectual property, licensed to Exact Sciences, for which he could receive royalties, paid to Mayo Clinic. AMF has been a consultant for AbbVie, Amgen, Centivo, Community Oncology Association, Covered California, EmblemHealth, Exact Sciences, Freedman Health, GRAIL, Harvard University, Health & Wellness Innovations, Health at Scale Technologies, MedZed, Penguin Pay, Risalto, Sempre Health, the State of Minnesota, U.S. Department of Defense, Virginia Center for Health Innovation, Wellth, and Zansors; has received research support from the Agency for Healthcare Research and Quality, Gary and Mary West Health Policy Center, Arnold Ventures, National Pharmaceutical Council, Patient-Centered Outcomes Research Institute, Pharmaceutical Research and Manufacturers of America, the Robert Wood Johnson Foundation, the State of Michigan, and the Centers for Medicare and Medicaid Services. DWE has a professional service agreement with Exact Sciences serving as an independent contractor to provide guidance on study design and analysis. ABO, VV, CE, and PL are employees of Exact Sciences Corporation.
Author contributions
JBK: conceptualization, writing – review and editing. AMF: conceptualization, writing – review and editing. DWE: conceptualization, writing – review and editing. ABO: conceptualization, methodology, supervision, visualization, writing – review and editing. VV: methodology, data curation, formal analysis, investigation, software, visualization of the study, writing – review and editing. CE: methodology, data curation, formal analysis, investigation, software, visualization of the study, writing – review and editing. PJL: conceptualization, methodology, writing – review and editing. All authors approved the final draft for submission.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Previous presentation
Portions of these data were presented at Digestive Disease Week (DDW) Annual Meeting, Chicago, IL, May 6–9, 2023.
Supplemental Material
Download MS Word (201.2 KB)Supplemental Material
Download MS Word (694.4 KB)Acknowledgements
Medical writing and editorial assistance were provided by Erin P. Scott, PhD, of Maple Health Group, LLC, funded by Exact Sciences Corporation.
Data availability statement
CRC-AIM demonstrates the approach by which existing CRC models can be reproduced from publicly available information and provides a ready opportunity for interested researchers to leverage the model for future collaborative projects or further adaptation and testing. To promote transparency and credibility of this new model, CRC-AIM’s formulas and parameters are available in the supplementary documents of an open-access publicationCitation27.
Notes
** Guardant Health, Inc., Redwood City, CA.
References
- Davidson KW, Barry MJ, Mangione CM, et al. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325(19):1965–1977. doi: 10.1001/jama.2021.6238.
- Wolf AMD, Fontham ETH, Church TR, et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin. 2018;68(4):250–281. doi: 10.3322/caac.21457.
- Shaukat A, Kahi CJ, Burke CA, et al. ACG clinical guidelines: colorectal cancer screening 2021. Am J Gastroenterol. 2021;116(3):458–479. doi: 10.14309/ajg.0000000000001122.
- Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116(3):544–573. doi: 10.1002/cncr.24760.
- Shaukat A, Mongin SJ, Geisser MS, et al. Long-term mortality after screening for colorectal cancer. N Engl J Med. 2013;369(12):1106–1114. doi: 10.1056/NEJMoa1300720.
- Piscitello A, Saoud L, Fendrick AM, et al. Estimating the impact of differential adherence on the comparative effectiveness of stool-based colorectal cancer screening using the CRC-AIM microsimulation model. PLoS One. 2020;15(12):e0244431. doi: 10.1371/journal.pone.0244431.
- D’Andrea E, Ahnen DJ, Sussman DA, et al. Quantifying the impact of adherence to screening strategies on colorectal cancer incidence and mortality. Cancer Med. 2020;9(2):824–836. doi: 10.1002/cam4.2735.
- Fisher DA, Karlitz JJ, Jeyakumar S, et al. Real-world cost-effectiveness of stool-based colorectal cancer screening in a Medicare population. J Med Econ. 2021;24(1):654–664. doi: 10.1080/13696998.2021.1922240.
- Jensen CD, Corley DA, Quinn VP, et al. Fecal immunochemical test program performance over 4 rounds of annual screening: a retrospective cohort study. Ann Intern Med. 2016;164(7):456–463. doi: 10.7326/M15-0983.
- Rastogi N, Xia Y, Inadomi JM, et al. Disparities in colorectal cancer screening in New York City: an analysis of the 2014 NYC Community Health Survey. Cancer Med. 2019;8(5):2572–2579. doi: 10.1002/cam4.2084.
- Ylitalo KR, Camp BG, Umstattd Meyer MR, et al. Barriers and facilitators of colorectal cancer screening in a Federally Qualified Health Center (FQHC). J Am Board Fam Med. 2019;32(2):180–190. doi: 10.3122/jabfm.2019.02.180205.
- Muthukrishnan M, Arnold LD, James AS. Patients’ self-reported barriers to Colon cancer screening in federally qualified health center settings. Prev Med Rep. 2019;15:100896. doi: 10.1016/j.pmedr.2019.100896.
- Miller-Wilson LA, Rutten LJF, Van Thomme J, et al. Cross-sectional adherence with the multi-target stool DNA test for colorectal cancer screening in a large, nationally insured cohort. Int J Colorectal Dis. 2021;36(11):2471–2480. doi: 10.1007/s00384-021-03956-0.
- Adler A, Geiger S, Keil A, et al. Improving compliance to colorectal cancer screening using blood and stool based tests in patients refusing screening colonoscopy in Germany. BMC Gastroenterol. 2014;14(1):183. doi: 10.1186/1471-230X-14-183.
- Liles EG, Coronado GD, Perrin N, et al. Uptake of a colorectal cancer screening blood test is higher than of a fecal test offered in clinic: a randomized trial. Cancer Treat Res Commun. 2017;10:27–31. doi: 10.1016/j.ctarc.2016.12.004.
- Liang PS, Zaman A, Kaminsky A, et al. Blood test increases colorectal cancer screening in persons who declined colonoscopy and fecal immunochemical test: a randomized controlled trial. Clin Gastroenterol Hepatol. 2023;21(11):2951–2957.e2.
- Symonds EL, Hughes D, Flight I, et al. A randomised controlled trial testing provision of fecal and blood test options on participation for colorectal cancer screening. Cancer Prev Res (Phila). 2019;12(9):631–640. doi: 10.1158/1940-6207.CAPR-19-0089.
- Young GP, Chen G, Wilson CJ, et al. “Rescue” of nonparticipants in colorectal cancer screening: a randomized controlled trial of three noninvasive test options. Cancer Prev Res (Phila). 2021;14(8):803–810. doi: 10.1158/1940-6207.CAPR-21-0080.
- Shaukat A, Levin TR. Current and future colorectal cancer screening strategies. Nat Rev Gastroenterol Hepatol. 2022;19(8):521–531. doi: 10.1038/s41575-022-00612-y.
- Ladabaum U, Mannalithara A, Weng Y, et al. Comparative effectiveness and cost-effectiveness of colorectal cancer screening with blood-based biomarkers (liquid biopsy) vs fecal tests or colonoscopy. Gastroenterology. 2024. doi: 10.1053/j.gastro.2024.03.011.
- van den Puttelaar R, Nascimento de Lima P, Knudsen AB, et al. Effectiveness and cost-effectiveness of colorectal cancer screening with a blood test that meets the Centers for Medicare & Medicaid services coverage decision. Gastroenterology. 2024. doi: 10.1053/j.gastro.2024.02.012.
- Fendrick AM, Vahdat V, Chen JV, et al. Comparison of simulated outcomes between stool- and blood-based colorectal cancer screening tests. Popul Health Manag. 2023;26(4):239–245. doi: 10.1089/pop.2023.0037.
- Knudsen AB, Rutter CM, Peterse EFP, et al. Colorectal cancer screening: an updated decision analysis for the U.S. Preventive Services Task Force. Rockville (MD): Agency for Healthcare Research and Quality; 2021 [cited 2023 Jul 12]. Available from: https://www.uspreventiveservicestaskforce.org/uspstf/document/final-modeling-report/colorectal-cancer-screening
- Imperiale TF, Ransohoff DF, Itzkowitz SH, et al. Multitarget stool DNA testing for colorectal-cancer screening. N Engl J Med. 2014;370(14):1287–1297. doi: 10.1056/NEJMoa1311194.
- Chung DC, Gray DM, Singh H, et al. A cell-free DNA blood-based test for colorectal cancer screening. N Engl J Med. 2024;390(11):973–983. doi: 10.1056/NEJMoa2304714.
- National Cancer Institute. Surveillance, Epidemiology, and End Results (SEER) program. National Cancer Institute; 2021 [cited 2023 Jul 18]. Available from: www.seer.cancer.gov
- Vahdat V, Alagoz O, Chen JV, et al. Calibration and validation of the colorectal cancer and adenoma incidence and mortality (CRC-AIM) microsimulation model using deep neural networks. Med Decis Making. 2023;43(6):719–736. doi: 10.1177/0272989X231184175.
- Cooper GS, Grimes A, Werner J, et al. Barriers to follow-up colonoscopy after positive FIT or multitarget stool DNA testing. J Am Board Fam Med. 2021;34(1):61–69. doi: 10.3122/jabfm.2021.01.200345.
- Clinical laboratory fee schedule. Baltimore (MD): Centers for Medicare & Medicaid Services; 2022 [cited 2023 Jun 23]. Available from: https://www.cms.gov/medicare/medicare-fee-for-service-payment/clinicallabfeesched
- Guardant Health Second Quarter 2022 Financial Results Call. Guardant Health; 2022 [cited 2023 Jun 23]. Available from: https://investors.guardanthealth.com/events-and-presentations/events/event-details/2022/Guardant-Health-Q2-2022-Earnings-Call/default.aspx
- Shepard DS. Cost-effectiveness in health and medicine. By M.R. Gold, J.E Siegel, L.B. Russell, and M.C. Weinstein (eds). New York: Oxford University Press, 1996. J Ment Health Policy Econ. 1999;2(2):91–92.
- Coronado GD, Jenkins CL, Shuster E, et al. Blood-based colorectal cancer screening in an integrated health system: a randomised trial of patient adherence. Gut. 2024;73(4):622–628. doi: 10.1136/gutjnl-2023-330980.
- Pickhardt PJ, Graffy PM, Weigman B, et al. Diagnostic performance of multitarget stool DNA and CT colonography for noninvasive colorectal cancer screening. Radiology. 2020;297(1):120–129. doi: 10.1148/radiol.2020201018.
- Rex DK, Ladabaum U, Anderson JC, et al. Does screening colonoscopy have a future in the United States?. Clin Gastroenterol Hepatol. 2023;21(12):3005–3010. doi: 10.1016/j.cgh.2023.05.034.
- Lieberman DA, Shaukat A, May FP, et al. Commentary: liquid biopsy for average-risk colorectal cancer screening. Clin Gastroenterol Hepatol. 2024. doi: 10.1016/j.cgh.2024.01.034.
- Kisiel J, Lieberman D, Fendrick A, et al. Estimated adenoma sensitivity threshold needed for blood-based colorectal cancer screening tests to be as effective as stool-based screening tests. Gastroenterology. 2023;164(6):S-983. doi: 10.1016/S0016-5085(23)03271-7.
- Hanna M, Dey N, Grady WM. Emerging tests for noninvasive colorectal cancer screening. Clin Gastroenterol Hepatol. 2023;21(3):604–616. doi: 10.1016/j.cgh.2022.12.008.
- Imperiale TF, Porter K, Zella J, et al. Next-Generation multitarget stool DNA test for colorectal cancer screening. N Engl J Med. 2024;390(11):984–993. doi: 10.1056/NEJMoa2310336.
- Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2016;315(23):2564–2575. doi: 10.1001/jama.2016.5989.

