Abstract
Aims
With recent European Union marketing authorization, tabelecleucel is the first off-the-shelf, allogeneic Epstein-Barr virus (EBV)-specific T-cell immunotherapy approved for the treatment of relapsed/refractory EBV-positive post-transplant lymphoproliferative disease (EBV+ PTLD). In the absence of a control arm, real-world evidence can provide a comparative benchmark for single-arm studies in ultra-rare populations. This study assessed the treatment effect of tabelecleucel in the single-arm phase 3 ALLELE study (NCT03394365) versus a treatment group from a multinational, multicenter retrospective chart review study (RS002) of patients with EBV+ PTLD.
Methods
In ALLELE, patients had disease relapsed/refractory to rituximab ± chemotherapy and received tabelecleucel 2x106 cells/kg on days 1, 8, and 15 in 35-day cycles. Patients in RS002 had disease relapsed/refractory to rituximab ± chemotherapy and received next line of systemic therapy between January 2000 and December 2018. Propensity score-based standardized mortality/morbidity ratio weighting was used to achieve balance between treatment and comparator arms. Kaplan-Meier estimators and Cox regression models were used to compare overall survival (OS) in the re-weighted sample.
Results
30 patients (n = 14 hematopoietic cell transplant [HCT], n = 16 solid organ transplant [SOT]) from ALLELE (data cutoff: November 2021) and 84 patients (n = 36 HCT, n = 48 SOT) from RS002 (data lock: January 2021) were included. Median time from diagnosis to first tabelecleucel dose (ALLELE) or start date of next line of systemic therapy (RS002) was 3.6 months. Tabelecleucel was associated with a substantial OS benefit compared with current treatment, with an unadjusted HR of 0.47 (95% confidence interval [CI] 0.25–0.88) and adjusted HR of 0.37 (95% CI 0.20–0.71) when using the start date of the next line of therapy as the index date. Sensitivity analyses yielded consistent results.
Conclusions
In this study of real-world data, tabelecleucel was associated with an OS benefit among patients with R/R EBV+ PTLD for whom there is high unmet need.
Introduction
Tabelecleucel is an off-the-shelf, allogeneic Epstein-Barr virus (EBV)-specific T-cell immunotherapy that was authorized under exceptional circumstances by the European Medicines Agency in 2022 as monotherapy for the treatment of adult and pediatric patients 2 years of age and older with relapsed/refractory (R/R) EBV-positive (EBV+) post-transplant lymphoproliferative disease (PTLD) who have received at least one prior therapy (for solid organ transplant [SOT], prior therapy includes chemotherapy unless inappropriate)Citation1, based on ongoing results from the open-label, single-arm, phase 3 ALLELE trialCitation2. In the ALLELE trial, tabelecleucel showed no evidence of safety concerns seen with autologous chimeric antigen receptor T-cell therapies used to treat other hematological malignancies. There were no reports of graft-versus-host disease (GvHD), SOT rejection, or fatal treatment-emergent serious adverse events assessed as related to tabelecleucel, and there were no reports of cytokine release syndrome or immune effector cell-associated neurotoxicity syndrome eventsCitation2. Due to the rarity of EBV+ PTLD, there is an ongoing commitment for the European Medicines Agency to be provided with any new information regarding the safety and efficacy for tabelecleucel, an annual update on the ALLELE trial and a post-authorization safety study.
PTLD is a well-recognized complication of both allogeneic hematopoietic cell transplant (HCT) and SOT that is associated with EBV infection of B cells, either due to reactivation of the virus after transplantation or from primary EBV infection in patients who were EBV seronegative prior to transplantCitation3, and is one of the most common malignancies in these post-transplant, immunocompromised, T-cell-deficient patients following SOTCitation4.
PTLD is an important source of morbidity and mortality in patients who have undergone allogeneic HCT. Nearly all cases of PTLD following HCT are EBV+ Citation3. T-cell depletion regimens, used to reduce the incidence of GvHD, increase the risk of PTLDCitation3,Citation5. The incidence of PTLD in the first year following HCT is estimated to be 1.1–1.7%Citation6,Citation7, with a median time to PTLD following HCT of 3 monthsCitation8. Rituximab is recommended for the treatment of EBV+ PTLD following HCTCitation9. However, from the time of failure of rituximab, survival is poor, as demonstrated by the dismal median overall survival (OS) of 0.7 monthsCitation8.
PTLD also constitutes an often-lethal malignant complication in patients who develop the disease after SOTCitation10. In the SOT setting, patients have a lifelong risk of developing PTLD corresponding to their cumulative, lifelong immunosuppressionCitation11; the occurrence of PTLD is largely dependent on the transplanted organ, the type and degree of immunosuppression, and patient characteristicsCitation3,Citation10. The proportion of patients with PTLD following SOT that is EBV+ is 47–52%Citation12,Citation13. Median time to PTLD following SOT is 1.7 years in patients with EBV+ PTLDCitation14. Rituximab and chemotherapy are recommended for the treatment of EBV+ PTLD following SOTCitation15. However, from the time of failure of rituximab and chemotherapy, survival is also poor, as demonstrated by the median OS of 4.1 monthsCitation14.
In the absence of a control arm in ALLELE, we conducted a comparative study using an external control arm drawn from real-world data. Such real-world evidence can be an important source of information for comparative effectiveness, particularly for treatments of rare diseases with high unmet needs, or those with breakthrough designations, where placebo-controlled trials may be infeasible or unethicalCitation16.
Methods
Study design
An external comparator arm to the single-arm phase 3 ALLELE single-arm study (NCT03394365) was developed using real-world data from the descriptive, multinational, multicenter, retrospective RS002 chart review study that included patients with EBV+ PTLD following HCT after failure of rituximab or following SOT after failure of rituximab plus chemotherapy. Data were collected from 29 centers in Europe (specifically Austria, Belgium, France, Germany, Italy, Spain, and Sweden) and North America (Canada and the United States).
A comparative analysis was then conducted, in which propensity score (PS)-based standardized mortality ratio weighting (SMRW) was used to achieve balance between the ALLELE (treatment) and RS002 (comparator) arms, and OS associated with tabelecleucel was estimated.
Study oversight
ALLELE and RS002 were approved by the independent ethics committee, research ethics board, or institutional review board at each center and complies with the Declaration of Helsinki, the Harmonised Tripartite Guideline for Good Clinical Practice from the International Conference on Harmonisation, and local laws. All patients who participated in ALLELE provided written informed consent.
Endpoints
OS was the endpoint evaluated for comparison, which was defined as the time from the index date to the date of death. Cause of death was recorded by the physicians in the case report form. Censoring occurred by loss to follow-up, or administrative end of data (or cutoff date), whichever came first.
OS was assessed using the start date of the next line of therapy as the index date (i.e. the date of the first dose of tabelecleucel for patients in ALLELE and the date of the start of the next line of systemic therapy following failure of rituximab after HCT or rituximab plus chemotherapy after SOT for patients in RS002), unless otherwise stated.
Statistics
Characteristics of study participants at the time of PTLD diagnosis and at the start of the next line of systemic therapy (for RS002), transplant characteristics, and time-related variables are presented. All continuous variables are summarized using the number of patients with an observation (n), median, 25th percentile (quartile 1) and 75th percentile (quartile 3), minimum, and maximum. All categorical variables are summarized using frequencies and percentages.
Baseline characteristics of patients in the external control arm were compared with those of patients enrolled in ALLELE.
Potential confounding, resulting from differences in prognostic factors between the treatment groups, was addressed using PS weighting based on SMRWsCitation17. This approach gives a weight of 1 for all patients who received tabelecleucel, and gives the comparator patients a weight of PS/(1 − PS). Thus, the SMRW method reweights the comparator patients to be representative of the treated patients. Under specific assumptions, such as no unmeasured confounding (conditional exchangeability), SMRW estimators result in an estimate of the average treatment effect among a population of patients represented by those who received tabelecleucel. SMRW makes more efficient use of the data than PS-based matching that discards patients who are not matchedCitation17.
A PS is defined as the conditional probability of being treated with tabelecleucel based on prespecified confounding factorsCitation18–20. PS methods are meant to mimic the process that occurs in a randomized controlled trial (RCT) by balancing covariates at an index date (a treatment decision point), analogous to the date of randomization in an RCTCitation21.
Based on literature review, the following prognostic factors were associated with OS and were identified as potentially important confounders (related to both the treatment decision and the outcome): age at PTLD diagnosis; response to initial treatment with rituximab; multi-site bone marrow involvement; lactate dehydrogenase (LDH) level; transplant organ type; PTLD stage; Eastern Cooperative Oncology Group (ECOG) performance status; sex; time from transplant to PTLD diagnosis; reduction of immune suppression at PTLD diagnosis; co-morbidities at PTLD diagnosis; and the International Prognostic Index incorporating the following five factors (each factor was worth 1 point): age ≥60 years; elevated LDH level; Ann Arbor Stage III or IV disease; ECOG performance status ≥2; and two or more extranodal sites. The final variables were determined according to the literature, data availability, and clinical relevance, and were included in a logistic regression model to estimate PS: age risk at the start of the next line of systemic therapy (high [≥60 years] vs. low [<60 years]); sex (female vs. male); LDH risk (high [≥250 U/L], low [<250 U/L], or missing); early onset of PTLD (yes [≤100 days for HCT or ≤2 years for SOT] vs. no [>100 days for HCT or >2 years for SOT]); transplant type (HCT vs. SOT); extranodal sites of PTLD (yes vs. no); number of lines of prior therapies (≥2 vs. 1); and time (months) from PTLD diagnosis to initial relapse/refractory date.
The distribution of the time-to-event endpoint (i.e. OS) was summarized using both unweighted and PS-weighted Kaplan-Meier estimators along with their corresponding 95% confidence interval (CI). The difference in OS was compared between the external comparator arm (patients from RS002) and the treatment arm (patients from ALLELE) by using unweighted or weighted log-rank tests. The OS benefit of tabelecleucel compared with current treatment was quantified using a hazard ratio (HR) with 95% CI estimated with both unweighted or weighted Cox proportional hazards regression models using a robust “sandwich” variance estimator to appropriately account for SMRWsCitation22. In the survival analysis, data for the comparator arm are shown for the first 36 months to align with the follow-up time in ALLELE.
Data sharing statement
Patient-level data will not be made available, as ALLELE is an ongoing study. This may be reassessed upon study completion and on a case-by-case basis.
Results
Patient demographics
As of November 5, 2021, a total of 30 patients with EBV+ PTLD from ALLELE were included in this analysis: 14 patients (46.7%) following HCT had disease that was R/R to rituximab and 16 patients (53.3%) following SOT had disease that was R/R to rituximab plus chemotherapy, prior to study entry. A total of 84 patients with EBV+ PTLD from the comparator arm (RS002) were included in the analysis: 36 patients (42.9%) following HCT with disease that was R/R to rituximab and 48 patients (57.1%) following SOT with disease that was R/R to rituximab plus chemotherapy.
Demographic and baseline characteristics are summarized in . At the time of first dose of tabelecleucel, patients in ALLELE had a median age of 41.8 years and 15 of 30 (50.0%) were female. For patients selected for RS002, the median age at the time of the next line of therapy was 44.1 years and 27 of 84 (32.1%) were female.
Table 1. Demographic and baseline characteristics.
The onset of PTLD occurred early (≤100 days from the time of HCT or ≤2 years from the time of SOT) for 40.0% of patients in ALLELE and for 52.4% of patients in RS002; PTLD had spread to extranodal sites in 73.3% and 66.7% of patients in ALLELE and RS002, respectively. One prior systemic therapy before the index date was reported for 53.3% and 65.5% of patients in ALLELE and RS002, respectively, and two or more prior therapies were reported for 46.7% and 34.5% of patients in ALLELE and RS002.
The median time from transplant to PTLD diagnosis (interquartile range [IQR]) was 7.4 months (3.8, 66.9) for patients in ALLELE and 6.5 months (3.0, 79.2) for patients in RS002 (). The median time from diagnosis to failure (i.e. relapse or refractory) of PTLD therapy (IQR) was 2.0 months (0.9, 3.6) for patients in ALLELE and 3.1 months (0.8, 8.2) for patients in RS002. The median time from diagnosis to either the first dose of tabelecleucel in ALLELE or the start date of the next line of systemic therapy in RS002 (IQR) was 3.6 months (2.0, 13.0) and 3.6 months (1.1, 9.6), respectively.
Table 2. Time-related variables.
Baseline comparability evaluation
For evaluation of baseline comparability, PS was estimated, PS-based weights (SMRW) were defined, and the covariate balance between patients in ALLELE and RS002 was assessed before and after PS adjustment.
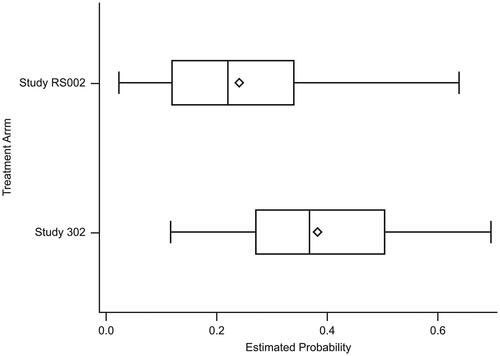
The distribution of PS estimated from the logistic regression model showed sufficient agreement between RS002 (median = 0.221; IQR: 0.119, 0.340) and ALLELE (median = 0.369; IQR: 0.271, 0.504) (see ). The PS overlapped for 81.6% of the total patients included in the analysis.
Figure 1. Boxplot of the estimated conditional probability of receiving treatment between the treatment arm and the external comparator arm. The PS overlapped for 81.6% of the total patients included in the analysis. The edges of the box indicate the IQR, values between Q1 and Q3. The diamond indicates the mean, the line inside the box indicates the median. The whiskers represent the minimum and maximum observations within a range from Q1 − 1.5*IQR to Q3 + 1.5*IQR.
Study RS002: estimated conditional probability of being treated: mean = 0.241; median = 0.221; IQR = 0.119, 0.340; minimum, maximum = 0.023, 0.639.
ALLELE: estimated conditional probability of being treated: mean = 0.383; median = 0.369; IQR = 0.271, 0.504; minimum, maximum = 0.117, 0.697.
IQR, Interquartile range; PS, Performance score; Q, Quartile.
The PSs were then used to estimate weights in the SMRW method; the balance of each covariate was evaluated in both pre- and post-weighting scenarios. Based on the standardized mean difference, the post weighting balance for the baseline covariates was achieved ().
Table 3. Comparison of baseline covariates before and after weighting.
Overall survival
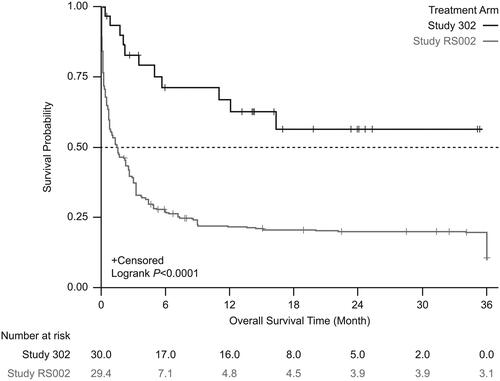
From the start date of next line therapy, unadjusted Kaplan-Meier analysis showed significantly lower mortality in patients treated with tabelecleucel than in patients receiving current treatment (). This result was supported by analyses using the SMRW method (). In the unadjusted Cox proportional hazards regression model with robust variance estimate, tabelecleucel demonstrated significant OS benefit compared with current treatment, with an HR of 0.47 (95% CI 0.25–0.88) (). This result was strengthened after adjustment using the SMRW method, with an HR of 0.37 (95% CI 0.20–0.71) ().
Figure 2. Kaplan-Meier survival estimates between the treatment arm and the external comparator arm (unadjusted). Patients who received tabelecleucel had significantly longer overall survival than patients who received current treatment. The index date is defined as the date of the first dose of tabelecleucel in ALLELE and the date of the next line of systemic therapy in RS002.
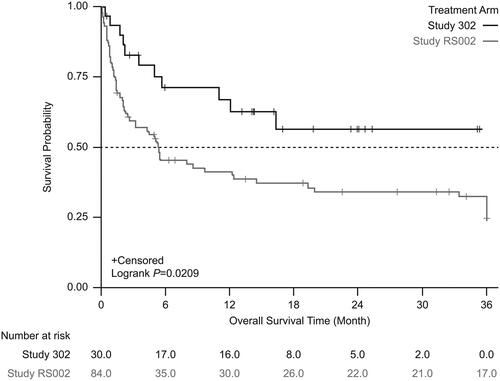
Figure 3. Kaplan-Meier survival estimates between the treatment arm and the external comparator arm (adjusted: standardized mortality ratio weighting). Patients who received tabelecleucel had significantly longer overall survival than patients who received current treatment. The index date is defined as the date of the first dose of tabelecleucel in ALLELE and the date of the next line of systemic therapy in RS002.
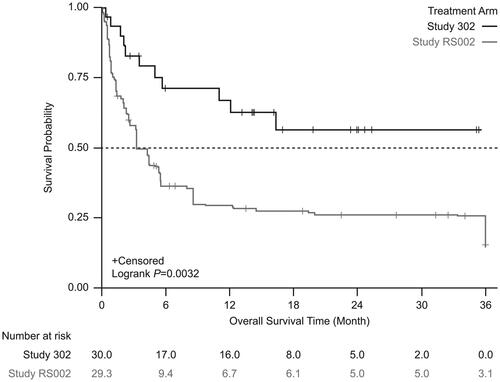
Table 4. OS between the treatment arm and the external comparator arm.
In sensitivity analyses performed using the date that disease was R/R to rituximab (HCT) or rituximab and chemotherapy (SOT) in RS002 (N = 165), unadjusted Kaplan-Meier analysis showed significantly lower mortality in patients treated with tabelecleucel than in patients receiving current treatment (). This result was supported by analyses using the SMRW method (). In the unadjusted Cox proportional hazards regression model with robust variance estimate, tabelecleucel also demonstrated significant OS benefit compared with current treatment, with an HR of 0.29 (95% CI 0.17–0.51). This association was strengthened after adjustment using the SMRW method, with an HR of 0.27 (95% CI 0.15–0.48).
Figure 4. Kaplan-Meier survival estimates between the treatment arm and the external comparator arm (unadjusted). Patients who received tabelecleucel had significantly longer overall survival than patients who received current treatment. The index date is defined as the date of the first dose of tabelecleucel in ALLELE and the date that disease was relapsed/refractory to rituximab ± chemotherapy in RS002.
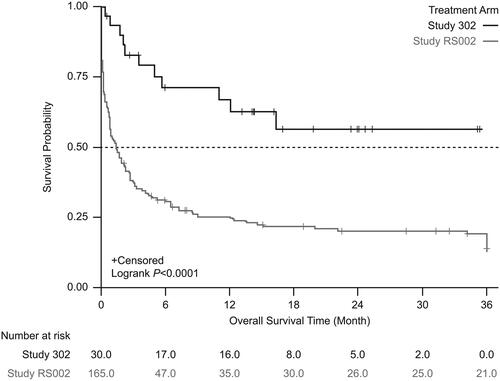
Figure 5. Kaplan-Meier survival estimates between the treatment arm and the external comparator arm (adjusted: standardized mortality ratio weighting). Patients who received tabelecleucel had significantly longer overall survival than patients who received current treatment. The index date is defined as the date of the first dose of tabelecleucel in ALLELE and the date that disease was relapsed/refractory to rituximab ± chemotherapy in RS002.
The results of both unadjusted and adjusted models were generally consistent and robust, with the SMRW method producing more precise CI estimates.
Discussion
For ultra-rare diseases with poor prognoses and limited treatment options, single-arm trials are usually conducted to support conditional approval by regulatory agencies. Identifying an external comparator using real-world data can provide useful information about the effect of the treatment under study. To comprehensively evaluate outcomes associated with current treatment in patients with R/R EBV+ PTLD in a real-world setting, a rigorous retrospective chart review was undertaken, which reported dismal median OS in these patients (0.7 months post-HCT after rituximab ± chemotherapy failure; 4.1 months post-SOT after rituximab + chemotherapy failure)Citation8,Citation14. This chart review was one of the largest studies reporting outcomes in patients with EBV+ PTLD post-HCT and -SOT after failure of rituximab or rituximab plus chemotherapy, respectively.
In the current comparative analysis, tabelecleucel was shown to have significant OS benefit compared with current treatment when using the start date of the next line of therapy as the index date, with unadjusted and adjusted HRs of 0.47 (95% CI 0.25–0.88) and 0.37 (HR = 0.37; 95% CI 0.20–0.71), respectively. We are not aware of any other reports in the literature comparing EBV-specific T-cell immunotherapy with current treatment in patients with EBV+ PTLD post-HCT or SOT after failure of rituximab or rituximab plus chemotherapy, respectively.
A variety of measures were used to collect information on patients from RS002 that closely resembled the ALLELE population. Appropriate methods to adjust for differences in important prognostic factors were applied to attain covariate balance between the treatment and comparator arms. SMRW was selected as the PS-based weighting strategy that we used, as it reweights patients in the comparator arm to be representative of the treated patients, which makes efficient use of the data and results in an estimated treatment effect that generalizes to a well-defined populationCitation20. OS was chosen as the endpoint for the comparative analysis as it can be assessed accurately in a real-world setting. In contrast, response rate data obtained from chart review in a real-world setting has limitations, including no standardized modalities and timepoints for evaluating response to treatment, temporal changes in treatment and technology, variable evaluation frequencies, and variability in physicians’ practices. Since the index date was defined as the start date of the next line of therapy, the estimated OS benefit of tabelecleucel may be conservative. In the European Union, where tabelecleucel is approvedCitation1, patients may receive treatment earlier than in the clinical trial setting. Therefore, an estimated OS benefit of tabelecleucel using the R/R date as the index date is also included.
Several potential limitations in this comparator analysis exist. Use of observational data is often associated with various biases such as selection bias, immortal person-time bias, survival bias, and confounding bias. To address those biases, careful consideration was given to selection of the index date, identification of important prognostic factors, minimization of missing data, and statistical control of observed confounding. Treatment information from RS002 was retrospectively collected spanning over 20 years, thus making it less contemporaneous. However, the treatment landscape has not changed meaningfully over the same period of time; therefore, this should not considerably impact the findings. Despite all the measures implemented to overcome biases, some associated with observational data may remain (e.g. bias due to unmeasured confounders). In this analysis, all patients in the HCT cohort were post-rituximab and all patients in the SOT cohort were post-rituximab + chemotherapy. In the future, there may be an opportunity to study the population at earlier stages of EBV+ PTLD treatment.
Conclusions
This study uses real-world observational data to create a comparator group for a single-arm clinical trial in a population with an ultra-rare disease. Tabelecleucel was found to be associated with OS benefit compared with current treatment in patients with R/R EBV+ PTLD for whom there is poor survival and no other approved PTLD treatment options. These findings contextualize the efficacy benefit of tabelecleucel.
Transparency
Declaration of financial/other interests
AB, NGB, AM, and BX are employees of Atara Biotherapeutics. BM and MT are employees of Pierre Fabre Group. HZ has acted in an advisory/consultancy role or received speaker honoraria from Atara Biotherapeutics, Pierre Fabre, and Roche; received travel, accommodation, and expenses from Janssen and Atara Biotherapeutics. MAB serves on scientific advisory committees for the American Academy of Allergy, Asthma & Immunology, Amgen, Atara Biotherapeutics, the Brigham and Women’s Hospital, ExstoBio, Gilead/Kite, Intercept, National Institute of Diabetes and Digestive and Kidney Diseases, Regeneron, and Vertex; and owns equity in AccompanyHealth and Target RWE.
Author contributions
All authors contributed to data interpretation and the writing, reviewing, and amending of the manuscript; the first draft was prepared by the academic authors and a medical writer funded by Atara Biotherapeutics. All the authors made the decision to submit the manuscript for publication and vouch for the accuracy and completeness of the data and for the fidelity of the study to the protocol.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Previous presentations
Portions of this research were presented in poster format at the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) Europe 2023 (Copenhagen, Denmark; November 12–15, 2023).
Acknowledgements
Samy Suissa is acknowledged for his participation on the study steering committee, and for his contribution to the study design and analysis. Mohamad Mohty is acknowledged for providing clinical expertise. Medical writing assistance was provided by Lee Blackburn, AMICULUM Ltd, funded by Atara Biotherapeutics.
Additional information
Funding
References
- Ebvallo (tabelecleucel) Summary of Product Characteristics. [Internet]. Pierre Fabre; 2022 [cited 2023 Jun 16]. Available from: https://www.ema.europa.eu/en/documents/product-information/ebvallo-epar-product-information_en.pdf
- Mahadeo KM, Baiocchi R, Beitinjaneh A, et al. Tabelecleucel for allogeneic haematopoietic stem-cell or solid organ transplant recipients with Epstein-Barr virus-positive post-transplant lymphoproliferative disease after failure of rituximab or rituximab and chemotherapy (ALLELE): a phase 3, multicentre, open-label trial. Lancet Oncol. 2024;25(3):376–387. doi:10.1016/s1470-2045(23)00649-6.
- Dierickx D, Habermann TM. Post-transplantation lymphoproliferative disorders in adults. N Engl J Med. 2018;378(6):549–562. doi:10.1056/NEJMra1702693.
- Engels EA, Pfeiffer RM, Fraumeni JF, Jr., et al. Spectrum of cancer risk among US solid organ transplant recipients. JAMA. 2011;306(17):1891–1901. doi:10.1001/jama.2011.1592.
- Landgren O, Gilbert ES, Rizzo JD, et al. Risk factors for lymphoproliferative disorders after allogeneic hematopoietic cell transplantation. Blood. 2009;113(20):4992–5001. doi:10.1182/blood-2008-09-178046.
- Dierickx D, Tousseyn T, Sagaert X, et al. Single-center analysis of biopsy-confirmed posttransplant lymphoproliferative disorder: incidence, clinicopathological characteristics and prognostic factors. Leuk Lymphoma. 2013;54(11):2433–2440. doi:10.3109/10428194.2013.780655.
- García-Cadenas I, Yáñez L, Jarque I, et al. Frequency, characteristics, and outcome of PTLD after allo-SCT: a multicenter study from the Spanish group of blood and marrow transplantation (GETH). Eur J Haematol. 2019;102(6):465–471. doi:10.1111/ejh.13226.
- Socié G, Barba P, Barlev A, et al. Outcomes for patients with EBV-positive PTLD post-allogeneic HCT after failure of rituximab-containing therapy. Bone Marrow Transplant. 2024;59(1):52–58. doi:10.1038/s41409-023-02127-9.
- Styczynski J, van der Velden W, Fox CP, et al. Management of Epstein-Barr virus infections and post-transplant lymphoproliferative disorders in patients after allogeneic hematopoietic stem cell transplantation: Sixth European Conference on Infections in Leukemia (ECIL-6) guidelines. Haematologica. 2016;101(7):803–811. doi:10.3324/haematol.2016.144428.
- Nijland ML, Kersten MJ, Pals ST, et al. Epstein-Barr virus-positive posttransplant lymphoproliferative disease after solid organ transplantation: pathogenesis, clinical manifestations, diagnosis, and management. Transplant Direct. 2016;2(1):e48. doi:10.1097/txd.0000000000000557.
- Baker A, Frauca Remacha E, Torres Canizales J, et al. Current practices on diagnosis, prevention and treatment of post-transplant lymphoproliferative disorder in pediatric patients after solid organ transplantation: results of ERN TransplantChild Healthcare Working Group Survey. Children (Basel). 2021;8(8):661. doi:10.3390/children8080661.
- Jagadeesh D, Tsai DE, Wei W, et al. Post-transplant lymphoproliferative disorder (PTLD) after solid organ transplant (SOT): a multicenter real world analysis (RWA) of 877 patients (pts) treated in the modern era. J Clin Oncol. 2020;38(15_suppl):e20026. doi:10.1200/JCO.2020.38.15_suppl.e20026.
- Trappe RU, Dierickx D, Zimmermann H, et al. Response to rituximab induction is a predictive marker in B-cell post-transplant lymphoproliferative disorder and allows successful stratification into rituximab or R-CHOP consolidation in an international, prospective, multicenter phase II trial. J Clin Oncol. 2017;35(5):536–543. doi:10.1200/jco.2016.69.3564.
- Dharnidharka V, Thirumalai D, Jaeger U, et al. Clinical outcomes of solid organ transplant patients with Epstein-Barr virus-driven (EBV+) post-transplant lymphoproliferative disorder (PTLD) who fail rituximab plus chemotherapy: a multinational, retrospective chart review study. Blood. 2021;138(Suppl 1):2528–2528. doi:10.1182/blood-2021-147307.
- Allen UD, Preiksaitis JK. Post-transplant lymphoproliferative disorders, Epstein-Barr virus infection, and disease in solid organ transplantation: guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 2019;33(9):e13652. doi:10.1111/ctr.13652.
- Khozin S, Blumenthal GM, Pazdur R. Real-world data for clinical evidence generation in oncology. J Natl Cancer Inst. 2017;109(11):djx187. doi:10.1093/jnci/djx187.
- Sato T, Matsuyama Y. Marginal structural models as a tool for standardization. Epidemiology. 2003;14(6):680–686. doi:10.1097/01.EDE.0000081989.82616.7d.
- Rosenbaum PR, Rubin DB. The Central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41–55. doi:10.1093/biomet/70.1.41.
- Rosenbaum PR, Rubin DB. Reducing bias in observational studies using subclassification on the propensity score. J Am Stat Assoc. 1984;79(387):516–524. doi:10.1080/01621459.1984.10478078.
- Brookhart MA, Wyss R, Layton JB, et al. Propensity score methods for confounding control in nonexperimental research. Circ Cardiovasc Qual Outcomes. 2013;6(5):604–611. doi:10.1161/circoutcomes.113.000359.
- Brookhart MA. Counterpoint: the treatment decision design. Am J Epidemiol. 2015;182(10):840–845. doi:10.1093/aje/kwv214.
- Lin DY, Wei LJ. The robust inference for the Cox proportional hazards model. J Am Stat Assoc. 1989;84(408):1074–1078. doi:10.1080/01621459.1989.10478874.
