Abstract
Background
One of the most prevalent conditions in Western societies is gastroesophageal reflux disease (GERD). In Switzerland, the standard treatment for GERD is proton pump inhibitor (PPI)-based medical management, but surgical options such as Nissen fundoplication and magnetic sphincter augmentation (MSA) are available. RefluxStop is a novel device that offers an alternative solution. The purpose of this report is to evaluate the cost-effectiveness of RefluxStop compared to PPIs and existing surgical treatments.
Methods
A model (Markov) was developed using the Swiss healthcare payer perspective with a lifetime horizon, 1-month cycle length, and a 3% annual discount rate for costs and benefits. Adverse events specific to treatment arms were incorporated, and benefits were measured in quality-adjusted life-years (QALYs). Clinical efficacy data for RefluxStop was obtained from its CE mark study, and comparator treatments were based on published literature. Deterministic and probabilistic sensitivity analyses were used to explore uncertainty. Since there are no head-to-head studies between RefluxStop and PPI therapy, Nissen fundoplication, or MSA, a limitation of this study is the use of naïve, indirect comparison of clinical effectiveness between the studied treatment options.
Results
Higher QALYs and lower costs were provided by RefluxStop compared to Nissen fundoplication and the MSA system. The incremental cost-effectiveness ratio (ICER) for RefluxStop was CHF 2,116 in comparison to PPI-based medical management. At a cost-effectiveness threshold of CHF 100,000 per QALY gained, the probability of RefluxStop being cost-effective was high, with probabilities of 100%, 97%, and 100% against PPI-based medical management, Nissen fundoplication, and MSA, respectively. The robustness of the analysis was provided by deterministic and probabilistic sensitivity analyses.
Conclusion
This cost-effectiveness analysis demonstrates that there is a high likelihood of RefluxStop being a cost-effective treatment modality in adults with GERD when compared with other treatment options available in Switzerland.
PLAIN LANGUAGE SUMMARY
Gastroesophageal reflux disease (GERD) is one of the most prevalent conditions in Western societies. Standard treatment in Switzerland entails proton pump inhibitor (PPI)-based medical management or surgical options (i.e., Nissen fundoplication and magnetic sphincter augmentation [MSA]) in selected cases. RefluxStop is a new technology indicated for the surgical treatment of GERD that restores the normal anatomy of the anti-reflux barrier. The clinical benefits and monetary costs of RefluxStop must be weighed against available treatment options to determine the role of this new technology in Switzerland. Cost-effectiveness analyses compare the relative costs and clinical outcomes of disease management when pursuing different paths in the patient journey landscape, as measured by quality-adjusted life-years (QALYs). In the present study, RefluxStop in comparison to Nissen fundoplication, and MSA, provided higher QALYs and lower costs. Against PPI therapy, the costs were slightly higher but the QALYs were also higher, generating a favourable Incremental cost-effectiveness ratio. Furthermore, at the cost-effectiveness threshold of CHF 100,000 per QALY gained, RefluxStop was highly likely to be cost-effective in comparison to PPI therapy, Nissen fundoplication, and MSA with probabilities of 100%, 97%, and 100%, respectively. Ultimately, this cost-effectiveness analysis showed that RefluxStop has a high likelihood of cost-effectiveness as a GERD treatment in Switzerland against other treatment options, with results being robust even with uncertainties considered in additional sensitivity analyses.
Introduction
Gastroesophageal reflux disease (GERD) is a common ailment that is denoted by retrograde movement of gastric contents in the alimentary tractCitation1. Common manifestations include heartburn, bloating, chest pain, regurgitation, and nocturnal episodes causing sleep disturbancesCitation1,Citation2. Chronic disease that is left untreated may lead to a significant complication called Barrett’s esophagus, an important precursor lesion for esophageal carcinomaCitation2,Citation3. GERD is common worldwide, with a global pooled prevalence estimated at 13.8%, affecting approximately 1.03 billion peopleCitation3,Citation4.
Currently, no Swiss clinical practice guidelines exist for GERD and dyspepsia in adultsCitation5. As a result, for the purpose of this study we have adopted the latest National Institute of Health and Care Excellence (NICE) guidelines from the United Kingdom for prescribers to follow. These guidelines indicate that proton pump inhibitor (PPI)-based medical management is the first-line management strategy for adults with GERD despite persistent symptoms being reported in most patients receiving PPI therapy everydayCitation6,Citation7. Patients not willing to pursue long-term medical therapy or exhibiting intolerance should be considered for laparoscopic antireflux surgery, which includes laparoscopic Nissen fundoplication and magnetic sphincter augmentation (MSA)Citation6,Citation8–10.
RefluxStop (Implantica, Zug, Switzerland) is a new medical device indicated for management of patients with GERD via implantationCitation11. The premise of this new technology is restructuring of the antireflux barrier by restoring the normal anatomy and orientation of the lower esophageal sphincter (LES), gastroesophageal flap valve, and angle of His without compression of the food passagewayCitation11. The purpose of this report was to evaluate the cost-effectiveness of RefluxStop in comparison to PPI-based medical management, laparoscopic Nissen fundoplication, and MSA in adults with GERD in Switzerland. The results may aid decision-making for optimization of clinical benefits with the economic burden and resources of the healthcare system.
Methods
A cost-effectiveness analysis was performed to compare RefluxStop with PPI therapy, Nissen fundoplication, and MSA in GERD patients. A lifetime horizon was used to assess clinical outcomes and costs from the analytic perspective of the Swiss statutory health insurance position. Direct costs were solely included without incorporation of societal costs, such as the lost productivity in the employment realm. Quality-adjusted life-years (QALYs)Citation12 were used to quantify clinical outcomes. Consistent with recent Swiss economic evaluations, all costs and QALYs had a 3% per year discount appliedCitation12,Citation13. Other model attributes include a cycle length of 1 month, half cycle correction, and events occurring in the analysis were presumed to take place at the halfway point of each month.
Model structure
A state transition (Markov) model was employed as the framework in our analysis to evaluate the cost-effectiveness of RefluxStop. This involved a patient cohort progressing through a sequence of mutually exclusive health states that reflect the patient journey of management choices assessed in this study (i.e. RefluxStop, PPIs, Nissen fundoplication, and MSA). This model is an adaptation of a recent cost-effectiveness analysis of RefluxStop in the United Kingdom, applied to Swiss settingsCitation14. Variance in the model structure was present for medical () and surgical () options, reflecting the distinction of these modalities. For both medical and surgical options, irresponsiveness to standard and high-dose PPI therapy, primary surgery and reoperation, progression of Barrett’s esophagus and esophageal carcinoma, and death were considered in the health states. Medication- and surgery-related adverse events (AEs) were also included in the model structure.
Figure 1. Model structure applied to PPI-based medical management (A) and surgical treatment options (B).
Abbreviations: C. Diff, Clostridium difficile; CKD, chronic kidney disease; PPIs, proton pump inhibitors; reop, reoperation.
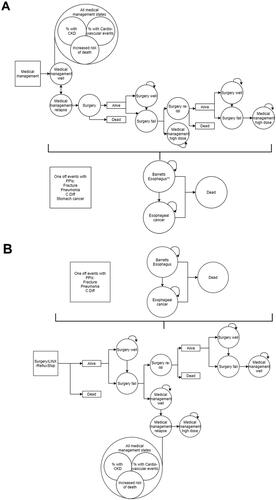
All patients were presumed to be managed with PPIs as a first-line therapy in the medical therapy arm of the study (i.e. entered the model in the “Medical management well” state) with the application of a monthly GERD relapse risk. Subjects were given twice the dose of PPIs (designated as “Medical management relapse” state) in the occurrence of symptom relapse. This state could lead to subsequent reversal to the standard dose of PPIs (i.e. “Medical management well” state) or progress to surgical management, restricted to Nissen fundoplication in this model arm, as per the NICE recommendations for GERD careCitation6. Postoperatively, health states were described as successful (i.e. “Surgery well”) or not successful (i.e. “Surgery fail”) outcomes. Following unsuccessful surgery, subjects required either reoperation (i.e. “Surgery re-op”) or were presumed to continue lifetime PPI therapy at a double dose (i.e. “Medical management high dose”). In the case of reoperation failure, subjects would receive lifelong double dose PPI therapy. Nissen fundoplication, MSA, and RefluxStop were included in the surgical arms of the analysis, as depicted in , in which patients were included in the model at primary operation. The initial procedure was categorized as successful (i.e. “Surgery well”) or unsuccessful (i.e. “Surgery fail”). In the case of initial surgical failure, patients were either subject to reoperation (i.e. “Surgery re-op”) or treatment with standard dose PPIs (i.e. “Medical management well”). For those receiving subsequent medical therapy, a monthly likelihood of disease relapse was applied, and patients were presumed to be switched to lifelong PPI therapy at twice the dose (i.e. “Medical management high dose”). Patients requiring a second operation were assumed to undergo the same procedure as their primary surgery in Nissen fundoplication and RefluxStop treatment arms; however, reoperation for patients who initially underwent MSA were presumed to undergo Nissen fundoplication. Those experiencing failure of reoperation in each of the surgical treatment arms were presumed to be treated with PPI therapy of a standard dose (i.e. “Medical management well”).
All patients carried a risk of Barrett’s esophagus at 0.083% per month. Only patients with established Barrett’s esophagus were subject to an additional risk of progression to esophageal adenocarcinoma, at a rate of 0.06% per month. Furthermore, mortality of the general population for Switzerland and specific health states associated with mortality were included as described in the text.
Model inputs
Clinical parameters
A theoretical group of 1,000 patients with a lower age limit of 52 years, composed of 56% males, was followed by the model; this was consistent with the RefluxStop multicenter trialCitation11. Similar demographic characteristics had been broadly employed by trials of GERD treatments, such as the UK REFLUX trialCitation15 and a US study assessing medical and surgical treatments for those with chronic GERDCitation16. summarizes the clinical parameters that informed the model.
Table 1. Clinical and quality-of-life inputs used in the model.
Efficacy and safety results reported in the RefluxStop trial were sourced to inform the modelCitation11 with additional use of 3-year follow-up data yet to be publishedCitation19. Recent publications were used to source efficacy inputs for PPI-based medical management, Nissen fundoplication, and MSA. To reflect reporting of each surgical intervention from the included studies, the monthly likelihood of surgical failure was divided into short- and long-term periods: (1) up to 1-year postoperatively; and (2) greater than 1-year postoperatively. Postoperative AEs and intraoperative complications were included for all surgical modalities modeled in this study regardless of whether an initial procedure or reoperation, and a recent review on outcomes following Nissen fundoplication informed the events examinedCitation18. Long-term PPI use was associated with a risk of AEs in the medical management group, such as Clostridium difficile (C.Diff) infectionCitation41, osteoporotic injuryCitation42, gastric carcinomaCitation43, chronic kidney disease (CKD)Citation44, community-acquired pneumoniaCitation45, and cardiovascular (CV) eventsCitation46. In our study, fractures, pneumonia, gastric cancer, and C.Diff infection were presumed as solitary events without repetition, and CKD and CV events were modeled with the proportion in state method (i.e. a certain proportion of those in states for medical management were considered to experience CKD and CV events at any given time). With this methodology, the mortality rate attributed to CKD and CV events were equivalent to the rate at which those managed with PPI therapy experienced the same long-term events.
Long-term sequelae associated with GERD included Barrett’s esophagus and esophageal carcinoma, as previously mentionedCitation2,Citation3. Using the model for appraising the NICE recommendations on dyspepsia and GERD, the likelihood of developing Barrett’s esophagus was 0.083% per monthCitation21, and was applied to all treatment groups of this analysis. Similarly applied to all groups, the rate of esophageal carcinoma in those with Barrett’s esophagus was 0.06% per month, obtained from a published meta-analysisCitation47.
The model also considered excess mortality within each model arm attributable to surgery, esophageal carcinoma, and AEs associated with long-term PPI therapy. Pertaining to surgery, an exceedingly low risk of intraoperative mortality (0.05%) was added to primary procedures across all surgical model arms, and reoperations were considered to carry twice this risk (0.1%). Based on a recent systematic review, the model applied a relative mortality risk of 1.57 to subjects included in medical management states, meant to reflect the increased risk of death associated with AEs of long-term PPI useCitation23. Thus, estimates of mortality did not favor any of the model arms.
Utility decrements were used to delineate Health-Related Quality of Life (HRQoL). For those with successful surgery, utility decrements were rooted on the percentage of patients with persistent dysphagia as an AE related to surgery. A 1-month utility decrement of 0.24 was included following all index surgeries and reoperations in all surgical arms of the model, founded on the impact of laparoscopic cholecystectomy on quality-of-lifeCitation17 as a representation of a conservative approach. For those receiving PPI therapy, utility decrements were rooted on a cost-effectiveness analysis of PPI-based medical management and laparoscopic Nissen fundoplication for reflux disease, obtained from the REFLUX trialCitation15,Citation17. This trial also informed another analysis for the utility decrement related to Nissen fundoplication that was unsuccessful, applied in our model for both MSA and RefluxStopCitation15. Utility decrements were similarly applied for patients with Barrett’s esophagus and esophageal carcinoma. Since the population norms in Switzerland were not available, an average obtained from neighboring nations (i.e. Germany, France, and Italy) was used assuming a close representation due to demographic, socioeconomic, and cultural considerations of these adjacent countriesCitation35. elaborates on the model inputs pertaining to HRQoL.
Cost inputs
The Swiss DRG and published literature were used to inform costsCitation48 whenever possible. To account for inflation, a web-based tool was utilized for cost conversion in data from older literature sourcesCitation49,Citation50. summarizes the key cost inputs applied in the analysis.
Table 2. Key cost inputs applied in the model.
The costs related to PPI therapy (i.e. omeprazole, pantoprazole, rabeprazole, lansoprazole, and esomeprazole) and surgery were considered in our analysis. The cost of surgical treatments was comprised of only procedural costs for Nissen fundoplication. Since all three antireflux surgeries involve similar laparoscopic techniques, the procedural costs of Nissen fundoplication were applied to the insertion consideration of other surgical interventions (i.e. MSA and RefluxStop). However, as MSA is likely of shorter duration, a reduced procedural cost by a margin of 25% was used for scenario analysis, with a subsequent insignificant change in results. Other costs related to the device cost and training costs were applied to MSA and RefluxStop only.
In addition to the initial diagnostic and treatments costs, a monthly management cost was applied for subjects with Barrett’s esophagus, consistent with the patient care pathway outlined in a cost-effectiveness analysis of Barrett’s esophagus managed with endoscopic therapyCitation40. Diagnostic endoscopy was assumed to be employed for all patients, and the 16.6% with dysplasia were presumed to be treated via endoscopic mucosal ablation, radiofrequency ablation, and subsequent concomitant PPI therapy and endoscopic monitoring every 2 yearsCitation40. Those that did not develop dysplasia incurred costs of PPI therapy and endoscopic monitoring onlyCitation40. In cases where progression to esophageal carcinoma occurred, the care pathway supporting NICE recommendations for dyspepsia and GERD was assumedCitation21. The esophageal carcinoma health state incurred costs including initial diagnostic testing and treatment costs, monthly costs of PPI therapy, and the cost of terminal care for those succumbing to illness.
Monthly costs were incurred by PPI-related AEs and were applied to patients that had CKD or a CV event. One-off costs related to solitary events such as osteoporotic injury, pneumonia, and C.Diff infection were included. With regards to gastric cancer, a lifetime cost attributable to chemotherapy and supportive care was applied.
Costs of AEs related to operation included conversion to open surgery, esophageal dilation, major surgical complications, and removal of a device. Intraoperative injuries, such as those to the spleen, liver, or gastrointestinal tract, were presumed to be part of the procedure cost itself.
Economic analysis
Lifetime estimates for total costs, life-years (LYs), life expectancy, and QALY per patient were generated for each treatment arm and between-arm incremental differences were extrapolated. Incremental measures generated to compare treatment arms included: (1) incremental cost-effectiveness ratio (ICER), representing the cost per QALY gained; (2) incremental net health benefit (NHB), assessing the impact on health in the population; and (3) incremental net monetary benefit (NMB), indicating the monetary value of an intervention. A Swiss cost-effectiveness threshold of CHF 100,000 per QALY gained was based on a recent publication of cost-effectiveness and used to calculate NMB and NHBCitation13. Positive incremental NMB or NHB values indicated treatment benefit to the healthcare services at the threshold designated for country-specific cost-effectiveness, with increasing magnitudes of NHB or NMB indicative of increased benefit.
To evaluate the inherent uncertainty related to our results, deterministic sensitivity analyses were performed. In these additional assessments, model inputs were independently manipulated with documentation of the analysis results to delineate the inputs with the most effect. Parameters were varied using ranges based on reported confidence intervals (CIs) and, when not available, conjecture ensued. Model inputs associated with uncertainty were simultaneously sampled from potential distributions in probabilistic sensitivity analyses, with a total of 1,000 model iterations performed, each utilizing a different set of inputs. CIs were used wherever possible to derive standard errors used in probabilistic value generation. The standard error was assumed to be 10% of the mean value in cases of unprocurable information. Additionally, due to the availability of clinical data (on file) for 3-year follow-up from two centers in Switzerland, we conducted a scenario analysis with 5.26% PPI use, a 5% reoperation rate, and endoscopic dilatation (4.4%) in the model clinical parameters.
Results
Base case results
Patients in the RefluxStop arm experienced 1.04–2.97 more QALYs compared to those in comparator arms (). The estimate for life expectancy in our analysis was higher in the RefluxStop group compared to all other arms, showcasing favorable differences of 0.73–1.73 years (). RefluxStop compared favorably against Nissen fundoplication and MSA with regards to the number of surgeries required per patient, treatment failures, endoscopic dilation of postoperative dysphagia, and surgical removals (applicable to MSA only), as depicted in .
Table 3. Cost-effectiveness outcomes estimated in the base case analysis, per patient.
Table 4. Clinical outcome estimates in the base-case analysis (per 1,000 patients unless otherwise stated).
With CHF 100,000 per QALY gained as the Swiss cost-effectiveness threshold, RefluxStop was cost-effective compared to all treatment options evaluated in the model (). The ICER of RefluxStop against PPI-based medical management was CHF 2,116. Furthermore, RefluxStop was dominant against Nissen fundoplication and MSA in terms of lower costs and higher effectiveness. RefluxStop demonstrated positive NHB (2.91 compared to PPI therapy, 1.04 compared to Nissen fundoplication, and 2.84 compared to MSA) and NMB (CHF 290,785 against PPI-based medical management, CHF 104,433 against Nissen fundoplication, and CHF 283,854 against MSA) values compared with all assessed treatments.
The costs associated with RefluxStop were somewhat balanced by the lower costs of PPIs (savings of CHF 7,022, CHF 2,351, and CHF 7,090 vs. medical management, Nissen fundoplication, and MSA, respectively) and treatment of PPI-related events (savings of CHF 1,072, CHF 306, and CHF 921 vs. medical management, Nissen fundoplication, and MSA, respectively). In comparison to the other surgical options, lifetime savings favored RefluxStop in terms of surgical outcomes and complications (respectively vs. Nissen fundoplication and MSA: savings of CHF 197 and CHF 45 for intraoperative events and complications requiring surgery; CHF 3,831 and CHF 1,731 for endoscopic dilation; and, against MSA only, a saving of CHF 130 related to surgical device removal).
Sensitivity analyses
Model inputs with the most influence in deterministic sensitivity analyses were the probability of treatment failure with RefluxStop, the probability of medical relapse followed by surgery, the stable medical management utility decrement, and the medical management high-dose utility decrement. Model results remained consistent following variation in individual input parameters. No parameters, with the exception of the monthly surgical failure rate with RefluxStop in comparison to Nissen fundoplication, skewed the results when inputs were varied individually. Cost-effectiveness in the long-term was predominantly unaffected by exploration of various cost estimates and risk of AEs. Even with reduction of procedural cost for MSA by a margin of 25% (CHF 11,198 instead of CHF 13,998), RefluxStop maintained dominance for ICER.
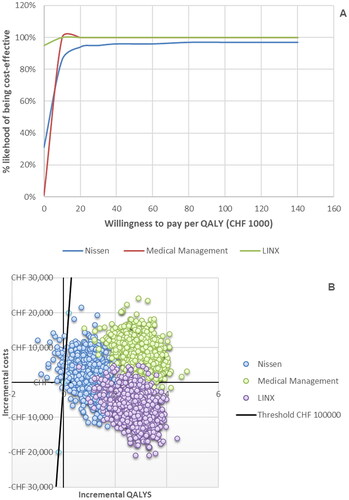
In the probabilistic analysis, RefluxStop demonstrated a high probability of relative cost-effectiveness, with probabilities of 100%, 97%, and 100% compared to PPI-based medical management, Nissen fundoplication, and MSA, respectively, using the Swiss cost-effectiveness threshold of CHF 100,000. Most probabilistic iterations exhibited the favorable cost-effectiveness for RefluxStop below the threshold (). In probabilistic analysis with 1,000 iterations, the mean ICERs were CHF 1,930 per QALY when compared to PPI-based medical management, CHF 2,724 per QALY when compared to Nissen fundoplication, and CHF –2,581 (dominant) per QALY when compared to MSA.
Figure 2. Results of the probabilistic sensitivity analyses against all three comparators presented as cost-effectiveness acceptability curves (A) and as a cost-effectiveness plane showing the spread of the individual iterations (B).
The black line in (B) indicates the Swiss cost-effectiveness threshold of CHF 100,000 per QALY. The points lying to the right of this line indicate iterations in which RefluxStop was cost-effective vs the assessed comparator (marked by the color of the individual points) and the points lying to the left of the black line indicate those iterations in which RefluxStop was not cost-effective.
Abbreviations: QALY, quality-adjusted life year.
In a scenario analysis with Swiss-specific clinical parameters for PPI use, reoperation rates and endoscopic dilation, the ICERs were CHF 4,565 (vs PPI based medical management), CHF 8,570 (vs Nissen Fundoplication), and dominant against MSA. The analysis only changed the direction of results for Nissen fundoplication, which was dominant in the base case analysis. The ICERs were all well below the cost-effectiveness threshold CHF 100,000, making RefluxStop a cost-effective option.
Discussion
Clinical guidance for management of GERD and dyspepsia in adults does not currently exist in SwitzerlandCitation5. NICE guidance indicates that first-line management of GERD in adults is PPI therapy, with H2-receptor antagonists offered adjunctively in cases of intolerance or inadequate responseCitation6. When medical management is inadequate or in patients not wanting long-term medical treatment, anti-reflux surgery via laparoscopic Nissen fundoplication is recommendedCitation6. Recently, NICE found sufficient evidence to support the use of MSA (LINX system) as an alternative procedure in patients with PPI-refractory GERDCitation8–10. However, several other novel procedures (i.e. RefluxStop, endoscopic transoral incisionless fundoplication, medigus ultrasonic surgical endostapler, mucosal resection techniques, and radiofrequency ablation) have been developed using alternative mechanisms of reinstating the antireflux barrierCitation1,Citation11,Citation67,Citation68.
With an estimated 993,000 adults in Switzerland suffering from GERD, the total costs of reflux disease for the Swiss population is estimated at CHF 230 million per year, with direct medical care costs dominated by medication (i.e. PPIs), accounting for CHF 180 million per year of the total GERD management expenditureCitation69. Considering that 11.8% of Swiss adults with GERD use prescription medication on a regular basis, one may presume that the lack of disease management incurs additional costs of complications and morbidity that contribute to an additional burden for the total healthcare expenditure in SwitzerlandCitation69. As such, emphasis on cost-effective modalities for disease management that provide optimal efficacy in relation to minimal cost is necessary to effectively reduce the impact of GERD on Switzerland’s healthcare system.
In our study, the predicted positive impact of RefluxStop on longevity, that persisted with consideration of quality-of-life, was demonstrated by the incremental gains in QALYs and LYs as compared with other assessed treatment modalities. In addition, a lower estimation for surgical complications with RefluxStop compared to laparoscopic Nissen fundoplication and MSA were appreciated.
Our analysis shows that there is a high likelihood that RefluxStop is a cost-effective treatment for reflux patients in comparison to the currently available options in Switzerland. The base case ICER estimates were significantly below the Swiss CHF 100,000 per QALY threshold, with additional analyses suggesting robustness of estimates by variation of model parameters, especially regarding cost reduction of the LINX device. Thus, the balance between RefluxStop’s health benefits and its higher cost were appreciated in this study. Based on favorable NHB and NMB values, RefluxStop is the intervention that provides health and monetary benefits to the Swiss healthcare system relative to comparators.
With the known treatment effect of RefluxStopCitation11 in conjunction with the results of this report, the cost-effectiveness of RefluxStop from the Swiss payer perspective is apparent and supports broader implementation. Unfortunately, surgical innovation is difficult to disseminate and necessitates broader frameworks beyond evidence synthesis. For instance, training considerations are important for the espousal of novel surgical treatmentsCitation70. As such, specific training considerations are required for working with RefluxStop, such as positioning the device above the LES and building a suitable device platform via approximation of the gastric fundal wall to the esophagusCitation11.
A notable strength of this study is the derivation of clinical parameters for the model from RefluxStop’s recent 3-year dataCitation19 in addition to the previously published 1-year resultsCitation11. Although this data may be viewed with caution due to the single-arm nature of the study, use of randomized controlled trials (RCTs) to evaluate surgical interventions is an established challenge with much less RCT-derived evidence synthesized for surgical treatments as compared to medical therapyCitation71. A limitation of this study was obtaining clinical data for comparators from published literature, with heterogeneous population demographics and clinical outcome measures as compared to the RefluxStop CE mark trial. For instance, the definitions for treatment failure following surgery differed between studies that informed the model. Thus, this model resembles an indirect, naïve comparison. This should be considered when interpreting the results, but extensive sensitivity analysis and additional scenario analysis with real-world Swiss data may provide useful insights to address these limitations. Health economic models should be contextually appreciated to show an “average” patient care scenario, as reflection of the broader clinical care pathway cannot be adequately achieved for individual patients by any model. The necessity of assumptions to inform absent data is another frequent issue in cost-effectiveness modeling. Uncertainty in results is caused by these limitations; however, variation of inputs in the sensitivity analysis contributed a significant balance to our study by the strength of estimates in the model. Another limitation of the present study is the variation of base rates for reimbursement between Swiss cantons, and this analysis used the base rate of Bern canton for estimation of costs. However, review of these base rates for different cantons revealed marginal differences that likely do not impact the results or conclusions of the study significantly. To further delineate the future role of RefluxStop in GERD management, an RCT comparing RefluxStop with laparoscopic Nissen fundoplication is currently in development and expected to add evidence supporting the use of RefluxStop in patients with GERD.
Conclusions
The results of this analysis demonstrate a high likelihood of RefluxStop being a cost-effective treatment modality for adults with GERD in comparison to current management options available in Switzerland.
Transparency
Declaration of funding
No funding was received to produce this article.
Declaration of financial/other interests
SH and SM are employed by a consultancy company that was commissioned by Implantica to develop the model. MK is an employee of Implantica. No author has any personal conflicts to declare.
Author contributions
SH, MK, and SM designed and developed the model. YB and JZ contributed to the design of the model and validated the results from a clinical perspective. All authors contributed to the manuscript.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Previous presentations
The data described in the manuscript was partially presented at ISPOR Europe 2023.
References
- Antunes C, Aleem A, Curtis SA. Gastroesophageal reflux disease. StatPearls. Treasure Island (FL): StatPearls Publishing; 2022.
- Chen J, Brady P. Gastroesophageal reflux disease: pathophysiology, diagnosis, and treatment. Gastroenterol Nurs. 2019;42(1):20–28. doi: 10.1097/SGA.0000000000000359.
- Choi KKH, Sanagapalli S. Barrett’s esophagus: review of natural history and comparative efficacy of endoscopic and surgical therapies. World J Gastrointest Oncol. 2022;14(3):568–586. doi: 10.4251/wjgo.v14.i3.568.
- Nirwan JS, Hasan SS, Babar ZU, et al. Global prevalence and risk factors of Gastro-oesophageal Reflux Disease (GORD): Systematic review with meta-analysis. Sci Rep. 2020;10(1):5814. doi: 10.1038/s41598-020-62795-1.
- Lenoir C, El Biali M, Luthy C, et al. Snapshot of proton pump inhibitors prescriptions in a tertiary care hospital in Switzerland: less is more? Int J Clin Pharm. 2019;41(6):1634–1641. doi: 10.1007/s11096-019-00929-w.
- National Institute for Health and Care Excellence. Clinical guideline [CG184]: gastro-oesophageal reflux disease and dyspepsia in adults: investigation and management. London (UK): ational Institute for Health and Care Excellence (NICE); 2019.
- Delshad SD, Almario CV, Chey WD, et al. Prevalence of gastroesophageal reflux disease and proton pump inhibitor-refractory symptoms. Gastroenterology. 2020;158(5):1250–1261.e2. doi: 10.1053/j.gastro.2019.12.014.
- National Institute for Health and Care Excellence: Guidelines. Interventional procedures guidance [IPG749]: laparoscopic insertion of a magnetic ring for gastroesophageal reflux disease. London (UK): National Institute for Health and Care Excellence (NICE); 2023.
- Bonavina L, Horbach T, Schoppmann SF, et al. Three-year clinical experience with magnetic sphincter augmentation and laparoscopic fundoplication. Surg Endosc. 2021;35(7):3449–3458. doi: 10.1007/s00464-020-07792-1.
- Skubleny D, Switzer NJ, Dang J, et al. LINX(®) magnetic esophageal sphincter augmentation versus Nissen fundoplication for gastroesophageal reflux disease: a systematic review and meta-analysis. Surg Endosc. 2017;31(8):3078–3084. doi: 10.1007/s00464-016-5370-3.
- Bjelović M, Harsányi L, Altorjay Á, et al. Non-active implantable device treating acid reflux with a new dynamic treatment approach: 1-year results: RefluxStop™ device; a new method in acid reflux surgery obtaining CE mark. BMC Surg. 2020;20(1):159. doi: 10.1186/s12893-020-00794-9.
- Barbier M, Durno N, Bennison C, et al. Cost-effectiveness and budget impact of venetoclax in combination with rituximab in relapsed/refractory chronic lymphocytic leukemia in Switzerland. Eur J Health Econ. 2022;23(5):837–846. doi: 10.1007/s10198-021-01398-7.
- Panje CM, Lupatsch JE, Barbier M, et al. A cost-effectiveness analysis of consolidation immunotherapy with durvalumab in stage III NSCLC responding to definitive radiochemotherapy in Switzerland. Ann Oncol. 2020;31(4):501–506. doi: 10.1016/j.annonc.2020.01.007.
- Harper S, Grodzicki L, Mealing S, et al. Cost-effectiveness of a novel, non-active implantable device as a treatment for refractory gastro-esophageal reflux disease. J Med Econ. 2023;26(1):603–613. doi: 10.1080/13696998.2023.2201063.
- Grant A, Wileman S, Ramsay C, et al. The effectiveness and cost-effectiveness of minimal access surgery amongst people with gastro-oesophageal reflux disease – a UK collaborative study. The REFLUX trial. Health Technol Assess. 2008;12(31):1–181, iii–iv. doi: 10.3310/hta12310.
- Spechler SJ, Hunter JG, Jones KM, et al. Randomized trial of medical versus surgical treatment for refractory heartburn. N Engl J Med. 2019;381(16):1513–1523. doi: 10.1056/NEJMoa1811424.
- Bojke L, Hornby E, Sculpher M, REFLUX Trial Team. A comparison of the cost effectiveness of pharmacotherapy or surgery (laparoscopic fundoplication) in the treatment of GORD. Pharmacoeconomics. 2007;25(10):829–841. doi: 10.2165/00019053-200725100-00003.
- Karolinska Institute. Literature review and analysis: laparoscopic Nissen fundoplication. [Unpublished]. 2017 (Version KILNF001).
- Implantica. RefluxStop CE Mark Trial – 3-year data; 2022 Data on file. [Unpublished but currently in peer-review].
- Food and Drug Administration (FDA). Summary of Safety and Effectiveness Data (SSED) for LINX reflux management system; 2012 [cited 2023 Apr 6]. Available from: https://www.accessdata.fda.gov/cdrh_docs/pdf10/p100049b.pdf.
- National Institute for Health and Care Excellence (NICE). Dyspepsia and gastro-oesophageal reflux disease. Appendix H: Full Health Economics Report; 2014. https://www.nice.org.uk/guidance/cg184/evidence/appendix-h-full-health-economics-report-pdf-6955335252
- WHO CVD Risk Chart Working Group. World Health Organization cardiovascular disease risk charts: revised models to estimate risk in 21 global regions. Lancet Glob Health. 2019;7(10):e1332–e1345.
- Shiraev TP, Bullen A. Proton pump inhibitors and cardiovascular events: a systematic review. Heart Lung Circ. 2018;27(4):443–450. doi: 10.1016/j.hlc.2017.10.020.
- Collins G, Altman D. Predicting the risk of chronic kidney disease in the UK: an evaluation of QKidney® scores using a primary care database. Br J Gen Pract. 2012;62(597):e243–e250. doi: 10.3399/bjgp12X636065.
- Hussain S, Singh A, Habib A, et al. Proton pump inhibitors use and risk of chronic kidney disease: evidence-based meta-analysis of observational studies. Clin Epidemiol Global Health. 2019;7(1):46–52. doi: 10.1016/j.cegh.2017.12.008.
- Kanis JA, Johnell O, Oden A, et al. FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int. 2008;19(4):385–397. doi: 10.1007/s00198-007-0543-5.
- Zhou B, Huang Y, Li H, et al. Proton-pump inhibitors and risk of fractures: an update meta-analysis. Osteoporos Int. 2016;27(1):339–347. doi: 10.1007/s00198-015-3365-x.
- Sun X, Douiri A, Gulliford M. Pneumonia incidence trends in UK primary care from 2002 to 2017: population-based cohort study. Epidemiol Infect. 2019;147:e263. doi: 10.1017/S0950268819001559.
- Lambert AA, Lam JO, Paik JJ, et al. Risk of community-acquired pneumonia with outpatient proton-pump inhibitor therapy: a systematic review and meta-analysis. PLoS One. 2015;10(6):e0128004. doi: 10.1371/journal.pone.0128004.
- Public Health England. Clostridium difficile infection: mandatory surveillance 2017/18; 2018. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/724368/CDI_summary_2018.pdf
- Janarthanan S, Ditah I, Adler DG, et al. Clostridium difficile-associated diarrhea and proton pump inhibitor therapy: a meta-analysis. Am J Gastroenterol. 2012;107(7):1001–1010. doi: 10.1038/ajg.2012.179.
- Brusselaers N, Wahlin K, Engstrand L, et al. Maintenance therapy with proton pump inhibitors and risk of gastric cancer: a nationwide population-based cohort study in Sweden. BMJ Open. 2017;7(10):e017739. doi: 10.1136/bmjopen-2017-017739.
- National Institute for Health and Care Excellence. Interventional procedures guidance [IPG585]: laparoscopic insertion of a magnetic titanium ring for gastro-oesophageal reflux disease; 2017. Available from: https://www.nice.org.uk/guidance/ipg585
- Alicuben ET, Bell RCW, Jobe BA, et al. Worldwide experience with erosion of the magnetic sphincter augmentation device. J Gastrointest Surg. 2018;22(8):1442–1447. doi: 10.1007/s11605-018-3775-0.
- Szende A, Janssen B, Cabases J, editors. Self-reported population health: an international perspective based on EQ-5D. Dordrecht (Netherlands): Springer; 2014.
- Ainslie WG, Catton JA, Davides D, et al. Micropuncture cholecystectomy vs conventional laparoscopic cholecystectomy: a randomized controlled trial. Surg Endosc. 2003;17(5):766–772. doi: 10.1007/s00464-002-8568-5.
- Bouvy JC, Ebbers HC, Schellekens H, et al. The cost-effectiveness of periodic safety update reports for biologicals in Europe. Clin Pharmacol Ther. 2013;93(5):433–442. doi: 10.1038/clpt.2013.13.
- Ayazi S, Zheng P, Zaidi AH, et al. Magnetic Sphincter augmentation and postoperative dysphagia: characterization, clinical risk factors, and management. J Gastrointest Surg. 2020;24(1):39–49. doi: 10.1007/s11605-019-04331-9.
- Tsai C, Steffen R, Kessler U, et al. Postoperative dysphagia following magnetic sphincter augmentation for gastroesophageal reflux disease. Surg Laparosc Endosc Percutan Tech. 2020;30(4):322–326. doi: 10.1097/SLE.0000000000000785.
- Pollit V, Graham D, Leonard C, et al. A cost-effectiveness analysis of endoscopic eradication therapy for management of dysplasia arising in patients with Barrett’s oesophagus in the United Kingdom. Curr Med Res Opin. 2019;35(5):805–815.
- Trifan A, Stanciu C, Girleanu I, et al. Proton pump inhibitors therapy and risk of Clostridium difficile infection: systematic review and meta-analysis. World J Gastroenterol. 2017;23(35):6500–6515. doi: 10.3748/wjg.v23.i35.6500.
- Nassar Y, Richter S. Proton-pump inhibitor use and fracture risk: an updated systematic review and meta-analysis. J Bone Metab. 2018;25(3):141–151. doi: 10.11005/jbm.2018.25.3.141.
- Poly TN, Lin MC, Syed-Abdul S, et al. Proton pump inhibitor use and risk of gastric cancer: current evidence from epidemiological studies and critical appraisal. Cancers (Basel). 2022;14(13):3052. doi: 10.3390/cancers14133052.
- Nochaiwong S, Ruengorn C, Awiphan R, et al. The association between proton pump inhibitor use and the risk of adverse kidney outcomes: a systematic review and meta-analysis. Nephrol Dial Transplant. 2018;33(2):331–342. doi: 10.1093/ndt/gfw470.
- Nguyen PA, Islam M, Galvin CJ, et al. Meta-analysis of proton pump inhibitors induced risk of community-acquired pneumonia. Int J Qual Health Care. 2020;32(5):292–299. doi: 10.1093/intqhc/mzaa041.
- Batchelor R, Kumar R, Gilmartin-Thomas JFM, et al. Systematic review with meta-analysis: risk of adverse cardiovascular events with proton pump inhibitors independent of clopidogrel. Aliment Pharmacol Ther. 2018;48(8):780–796. doi: 10.1111/apt.14955.
- Yousef F, Cardwell C, Cantwell MM, et al. The incidence of esophageal cancer and high-grade dysplasia in Barrett’s esophagus: a systematic review and meta-analysis. Am J Epidemiol. 2008;168(3):237–249. doi: 10.1093/aje/kwn121.
- Swiss DRG. [cited 2023 Apr 6]. Available from: https://www.swissdrg.org/de/grouper/grouper/online-grouper.
- Jones K, Burns A. Unit costs of health and social care 2021. Kent (UK): Personal Social Services Research Unit (PSSRU); 2021.
- Shemilt I, Thomas J, Morciano M. A web-based tool for adjusting costs to a specific target currency and price year. Evid Policy J Res Debate Pract. 2010;6(1):51–59. doi: 10.1332/174426410X482999.
- Swiss Federal Office. Spezialitätenliste. [Available from: https://www.spezialitaetenliste.ch/ShowPreparations.aspx.
- Buyukkaramikli N, Abraham K, Vroling H, et al. Treatment of non-erosive gastroesophageal reflux disease patients with proton pump inhibitors. 2020 [cited 2023 May 10]. Available from: https://www.bag.admin.ch/dam/bag/en/dokumente/kuv-leistungen/leistungen-und-tarife/hta/berichte/h0025ppir-hta-report.pdf.download.pdf/h0025ppir-hta-report.pdf.
- Palser TR, Ceney A, Navarro A, et al. Variation in laparoscopic anti-reflux surgery across England: a 5-year review. Surg Endosc. 2018;32(7):3208–3214. doi: 10.1007/s00464-018-6038-y.
- Kerr M, Bray B, Medcalf J, et al. Estimating the financial cost of chronic kidney disease to the NHS in England. Nephrol Dial Transplant. 2012;27 Suppl 3(Suppl 3):iii73–iii80. doi: 10.1093/ndt/gfs269.
- Gandjour A, Kleinschmit F, Lauterbach KW, INTERCARE International Investigators. International Comparison of Costs and Quality in Health Care. European comparison of costs and quality in the treatment of acute myocardial infarction (2000-2001). Eur Heart J. 2002;23(11):858–868. doi: 10.1053/euhj.2001.3080.
- Blach S, Schaetti C, Bruggmann P, et al. Cost-effectiveness analysis of strategies to manage the disease burden of hepatitis C virus in Switzerland. Swiss Med Wkly. 2019;149:w20026. doi: 10.4414/smw.2019.20026.
- Fischer B, Serra A, Telser H. Cost-effectiveness of treating patients with chronic kidney disease and prior hyperkalemia with renin-angiotensin-aldosterone system inhibitor and patiromer: a Swiss public healthcare perspective. Adv Ther. 2022;39(6):2717–2730. doi: 10.1007/s12325-022-02123-3.
- Sandoz MS, Ess SM, Keusch GW, et al. Prevalence and direct medical costs of end-stage renal disease in patients with type 2 diabetes mellitus in Switzerland for 2001. Swiss Med Wkly. 2004;134(31-32):448–458. doi: 10.4414/smw.2004.10682.
- Kidneyfailurerisk.com. The kidney failure risk equation. [cited 2023 Apr 6]. Available from: https://kidneyfailurerisk.com/.
- Wieser S, Rüthemann I, De Boni S, et al. Cost of acute coronary syndrome in Switzerland in 2008. Swiss Med Wkly. 2012;142:w13655. doi: 10.4414/smw.2012.13655.
- Svedbom A, Hernlund E, Ivergård M, et al. Osteoporosis in the European Union: a compendium of country-specific reports. Arch Osteoporos. 2013;8(1):137. doi: 10.1007/s11657-013-0137-0.
- Keitel K, Alcoba G, Lacroix L, et al. Observed costs and health care use of children in a prospective cohort study on community-acquired pneumonia in Geneva, Switzerland. Swiss Med Wkly. 2014;144:w13925. doi: 10.4414/smw.2014.13925.
- National Institute for Health and Care Excellence. Quality standard [QS110]: pneumonia in adults; 2016. Available from: https://www.nice.org.uk/guidance/qs110
- Pillai N, Dusheiko M, Maillard MH, et al. The evolution of health care utilisation and costs for inflammatory bowel disease over ten years. J Crohns Colitis. 2019;13(6):744–754. doi: 10.1093/ecco-jcc/jjz003.
- National Institute for Health and Care Excellence (NICE). Technology appraisal guidance [TA378]: Remucirumab for treating advanced gastric cancer or gastro-oesophageal junction adenocarcinoma previously treated with chemotherapy. 2016. Available from: https://www.nice.org.uk/guidance/ta378
- Laudicella M, Walsh B, Munasinghe A, et al. Impact of laparoscopic versus open surgery on hospital costs for colon cancer: a population-based retrospective cohort study. BMJ Open. 2016;6(11):e012977. doi: 10.1136/bmjopen-2016-012977.
- Manzo CA, Asti E, Bonavina L. Hiatal hernia, lower esophageal sphincter and their combined effect on the natural history of gastroesophageal reflux disease: implications for surgical therapy. Ann Laparosc Endosc Surg. 2021;6:44–44. doi: 10.21037/ales-20-26.
- Rettura F, Bronzini F, Campigotto M, et al. Refractory gastroesophageal reflux disease: a management update. Front Med (Lausanne). 2021;8:765061. doi: 10.3389/fmed.2021.765061.
- Schwenkglenks M, Marbet UA, Szucs TD. Epidemiology and costs of gastroesophageal reflux disease in Switzerland: a population-based study. Soz Praventivmed. 2004;49(1):51–61. doi: 10.1007/s00038-003-2090-y.
- The Royal College of Surgeons of England. From innovation to adoption. Successfully spreading surgical innovation; 2014. Available from: https://www.rcseng.ac.uk/-/media/files/rcs/library-and-publications/non-journal-publications/rcs_innovation_to_adoption_2014_web-(1).pdf
- McCulloch P, Taylor I, Sasako M, et al. Randomised trials in surgery: problems and possible solutions. BMJ. 2002;324(7351):1448–1451. doi: 10.1136/bmj.324.7351.1448.
