Abstract
Aims
To describe healthcare resource utilization (HCRU) and associated costs after initiation of injectable glucagon-like peptide-1 receptor agonist (GLP-1 RA) therapy by adult patients with type 2 diabetes (T2D) in the prospective, observational, 24-month TROPHIES study in France, Germany, and Italy.
Materials and methods
HCRU data for cost calculations were collected by treating physicians during patient interviews at baseline and follow-up visits approximately 6, 12, 18, and 24 months after GLP-1 RA initiation with once-weekly dulaglutide or once-daily liraglutide. Costs were evaluated from the national healthcare system (third-party payer) perspective and updated to 2018 prices.
Results
In total, 2,005 patients were eligible for the HCRU analysis (1,014 dulaglutide; 991 liraglutide). Baseline patient characteristics were generally similar between treatment groups and countries. The largest proportions of patients using ≥2 oral glucose-lowering medications (GLMs) at baseline (42.9–43.4%) and month 24 (44.0–45.1%) and using another injectable GLM at month 24 (15.3–23.2%) were in France. Mean numbers of primary and secondary healthcare contacts during each assessment period were highest in France (range = 4.0–10.7) and Germany (range = 2.9–5.7), respectively. The greatest proportions (≥60%) of mean annualized costs per patient comprised medication costs. Mean annualized HCRU costs per patient varied by treatment cohort and country: the highest levels were in the liraglutide cohort in France (€909) and the dulaglutide cohort in Germany (€883).
Limitations
Limitations included exclusion of patients using insulin at GLP-1 RA initiation and collection of HCRU data by physician, not via patient-completed diaries.
Conclusions
Real-world HCRU and costs associated with the treatment of adults with T2D with two GLP-1 RAs in TROPHIES emphasize the need to avoid generalization with respect to HCRU and costs associated with a particular therapy when estimating the impact of a new treatment in a country-specific setting.
PLAIN LANGUAGE SUMMARY
Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) have become frequent treatments of hyperglycemia in type-2 diabetes (T2D). Not all types of clinical study provide information about the cost of these treatments or the effects they might have on use of other medicines and equipment to control T2D or the need for visits to a doctor or nurse and different types of treatment in hospital. This study collected this information during the regular care of adults in France, Germany, or Italy who were prescribed either dulaglutide or liraglutide (both types of GLP-1 RAs) by their family doctor or a specialist in T2D. There were differences in costs and the need for other medicines and medical services between people using either dulaglutide or liraglutide and for people who were using the same GLP-1 RA in each of the three countries. The information from this study could be used to more accurately understand the overall costs and medical care needed when patients use dulaglutide or liraglutide in France, Germany, or Italy.
Introduction
Diabetes is a chronic condition with a high economic cost in Europe. One in 11 adults (aged 20–79 years) in Europe has diabetes (total ∼61 million adults)Citation1 and more than 95% of people with diabetes have type 2 diabetes (T2D)Citation2. Diabetes-related expenditure in Europe totaled USD 189 billion in 2021, with Europe having the second highest average cost (after the North America and Caribbean International Diabetes Federation region) per adult with diabetes – USD 3,086 per annumCitation1. However, despite the considerable financial burden of diabetes, and specifically T2D, cost data on the real-world management of T2D are rarely collected in prospective research studies.
Due to homogenous patient populations and strictly adhered to treatment regimens, only limited evaluations of resource use and the cost of T2D therapy can be conducted in randomized clinical trials. Conversely, real-world evidence from large, well-designed, prospective, observational studies in T2D can provide information fundamental for healthcare decision makers, such as estimates of resource use and cost in more diverse patient populations within specific countries and/or healthcare systems. This type of research is particularly important in T2D because of its chronic and complex nature and the large, heterogeneous population affected by the disease.
T2D is a progressive disease and maintaining glycemic control over the course of the disease often requires treatment intensification by switching from oral glucose-lowering medications (GLMs) to injectable therapy. Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) are typically the first injectable therapy recommended for T2D, offering glycemic control in addition to other health benefits, such as weight reduction and potential cardiovascular disease (CVD) preventionCitation3,Citation4.
Several GLP-1 RAs are available, each displaying a different profile with regards to duration of action and ease of dosing, efficacy, tolerability, and immunogenicityCitation4–6. Two such GLP-1 RAs, dulaglutide (Trulicity®, Eli Lilly and Company USA; approved in the European Union in 2014)Citation7 and liraglutide (Victoza®, Novo Nordisk, Denmark; approved in the European Union in 2009)Citation8, have demonstrated positive outcomes in clinical trialsCitation9–13. The multinational, prospective, real-world TROPHIES study, conducted between 2017 and 2021, sought primarily to estimate the time spent on the first GLP-1 RA until a significant treatment change due to treatment- or diabetes-related factors in patients initiating their first injectable treatment for T2D with these two commonly prescribed GLP-1 RAs (at the time the study was designed and started)Citation14,Citation15 over 24 months in France, Germany, and ItalyCitation16,Citation17. The TROPHIES study also offered an excellent opportunity to evaluate the healthcare resource use (HCRU) and costs associated with GLP-1 RA treatment in real-world clinical practice in these three countries. In the current environment, in which increasing numbers of people with T2D are being treated with GLP-1 RAsCitation18–21, HCRU and cost data are key inputs for cost-effectiveness evaluations in specific countries.
Our objective is to describe the HCRU and associated costs during the 24 months after initiation of injectable GLP-1 RA therapy in the prospective, observational TROPHIES study of adult patients with T2D in France, Germany, and Italy.
Methods
Design and patients
As reported elsewhere, TROPHIES was a non-interventional, multinational, 24-month study, initiated to investigate the use of dulaglutide and liraglutide in routine clinical practice in adults with T2D (Supplementary Figure S1)Citation16. Participant enrolment took place between July 2017 and May 2019, and the last patient visit occurred during June 2021. Patients aged ≥18 years were eligible to participate if they were naïve to injectable treatment for T2D (except for short-term [≤4 weeks] use of insulin for acute conditions or insulin use during pregnancy) and were scheduled to begin their first injectable GLM with either dulaglutide once weekly or liraglutide once daily (based on physician decision) in one of three large European countries: France, Germany, and Italy. Patients initiating treatment with a GLP-1 RA in combination with insulin (defined as the initiation of insulin within the first 30 days of GLP-1 RA treatment initiation) or those being treated with an investigational drug or procedure were excluded.
Patients were enrolled at general practitioner or specialist healthcare practitioner (HCP) sites across France, Germany, and Italy. Physicians prescribed and dosed dulaglutide and liraglutide according to the approved label in their respective country. The recommended dose for dulaglutide at the time of the study was 0.75 mg once weekly as monotherapy and 1.5 mg once weekly when used as add-on therapy. The 0.75 mg once-weekly dose could be considered a starting dose in add-on therapy for potentially vulnerable patientsCitation22. The liraglutide starting dose was 0.6 mg per day. After at least 1 week, the dose was increased to 1.2 mg per day and could be further increased to 1.8 mg per day for patients expected to benefit from a dose increase and based on clinical responseCitation8.
Data relating to study objectives were collected at baseline (from medical records over the previous 6-month period) and during subsequent routine clinical care visits at approximately 6, 12, 18, and 24 months (± 45 days) after GLP-1 RA initiation (see Supplementary Figure S1). Although visits were planned at ± 45 days, they often fell outside this window in routine practice; for the purposes of the analyses, windows of ± 91 days were used. Baseline data collected included demographic and clinical characteristics (e.g. diabetes duration, previous glycated hemoglobin [HbA1c] level, concomitant diseases, and previous treatment). Electronic case report forms were used for data entry.
The study was conducted in accordance with the ethical principles of the Declaration of HelsinkiCitation23 and the applicable laws and regulations of the three countries. Appropriate local bodies approved the study. All patients analyzed provided authorization for the use and disclosure of their personal health information covering the collection and release of data regarding treatment and its outcomes for the entire study period.
Study endpoints
TROPHIES collected data on clinical outcomes, persistence, treatment patterns, patient-reported outcomes, HCRU, and associated costs. The primary endpoint was the duration of treatment with the patients’ first GLP-1 RA (dulaglutide or liraglutide) without a significant treatment change due to treatment- or diabetes-related factors (defined as either intensification or discontinuation of dulaglutide or liraglutide initiated at baseline). Primary endpoint results at 24 months, as well as interim and final clinical, treatment pattern and patient-reported outcomes, have been published in detail elsewhereCitation17,Citation24,Citation25.
Medication and healthcare resource use
The collection of medication and HCRU data for inclusion in cost calculations was a secondary objective of the TROPHIES study. These data, collected using standardized case report forms during patient interviews with the treating physician at baseline (for the previous 6 months) and all follow-up visits (i.e. for the period since the last visit), are listed below and aggregated as shown.
Medication
GLMs and doses (i.e. index GLP-1 RA, oral antidiabetic drugs [i.e. alpha glucosidase inhibitor, biguanide, dipeptidyl peptidase-IV [DPP-IV] inhibitor, meglitinide, sodium-glucose cotransporter-2 [SGLT-2] inhibitor, sulfonylurea, thiazolidinedione] and other injectable antidiabetic drugs [insulin or non-index GLP-1 RA]).
HCRU
Number of contacts with primary care HCPs (i.e. visits or phone calls with primary care doctor and practice- or hospital-based visits, home visits or phone calls with primary care nurse).
Number of visits with secondary care specialist HCPs – cardiologist, dentist, dermatologist, diabetologist/endocrinologist/internal medicine, dietician, nephrologist, neurologist, ophthalmologist, podiatrist, psychiatrist, psychotherapist, and specialist nurse.
Number of contacts with diabetes educator/specialized staff (i.e. practice- or hospital-based visits, home visits or phone calls) and whether or not the patient required training on how to use the GLP-1 RA device at each visit.
Proportion of patients with hospital admissions (including daytime hospitalizations, overnight non-intensive care hospitalizations, and overnight intensive care hospitalizations) related to diabetes (e.g. hypoglycemia) or short- or long-term diabetic complications (e.g. cardiovascular) and number of days of hospitalization for each.
Number of emergency room (ER) visits, and whether or not the patient was transported by ambulance and, if so, how many times.
Number of days spent caring and number of days of work missed by primary caregiver (not HCP); and proportion of patients with missed days at work and number of days of work missed by patients.
Proportion of patients using self-monitored blood glucose (SMBG) devices and average number of SMBG device tests performed per week.
Costs
Information on the sources and handling of direct medical and resource-use unit costs in TROPHIES has been published in detail elsewhereCitation26. In summary, costs were evaluated from the national healthcare system (third-party payer) perspective and updated to 2018 prices. All prices were taken from published sources in Euros (€). Resource-use costs included ER visit costs, hospitalization costs, and other medical costs (i.e. visits to specialists and visits to/of primary care doctors/nurses). Medication costs, which included index GLP-1 RA drug (dulaglutide or liraglutide) costs, oral diabetic drug costs, and other injectable medication costs (either insulin or non-index GLP-1 RA), were based on local 2018 list prices in each country. Direct non-medical and indirect costs were not assessed in this study.
For each country, costs per patient were calculated by applying local unit costs (from the healthcare system perspective) to each resource used. Several cost calculation assumptions were made. All medications were assumed to be generic formulations, where available (except dulaglutide and liraglutide), and public prices were used. Minimally priced presentations were used if different packages were available. Combination medication was assigned a cost for the total daily dose of the lowest priced medication. Hospitalization costs were valued based on cost per episode for each type of admission.
Sample size and statistical measures
The sample size calculation for TROPHIES has been reported in detail elsewhereCitation16. A sample size of 350 patients per country in each treatment cohort was considered sufficient to provide good precision for estimating the median time to the first significant treatment change for each index GLP-1 RA. All patients who fulfilled the inclusion criteria were included in the analyses. Analyses were performed using all data up to the point of the last data collection for patients who were lost to follow-up or who withdrew from the study. Descriptive statistics were planned for the main analyses; thus, no formal statistical comparisons of HCRU or cost data between treatment cohorts were conducted. No imputation of missing data was performed. Baseline data were reported as mean (standard deviation) for continuous variables and absolute and relative frequencies for categorical variables. Descriptive statistics were calculated for the study variables: mean (standard deviation) and/or median (interquartile range) for continuous variables; and absolute and relative frequencies for categorical variables.
All reported costs are post-baseline only (i.e. costs related to resource use data collected at baseline, which refer to the 6-month pre-index GLP-1 RA initiation period, were excluded), and covered the actual follow-up time for each patient (i.e. from index GLP-1 RA start date to study completion [24 months], or to study discontinuation for any reason). Reported annualized medication and resource-use costs per country reflect the whole 24-month study duration but are standardized to yearly costs (i.e. the cost for a patient who completed the 24-month study has been divided by 2, the cost for a patient who discontinued at 18 months has been divided by 1.5, and the cost for a patient who discontinued at 6 months has been multiplied by 2). A sensitivity analysis was conducted on mean annualized resource-use costs per country in which hospitalization costs were Winsorized at the 1st and 99th percentiles to limit the impact of spurious outliers. Data was Winsorized by attributing the 1st percentile cost when the actual cost was lower in value than the 1st percentile estimate and, similarly, attributing the 99th percentile cost when the actual cost was higher than the 99th percentile estimateCitation27. All statistical analyses were conducted using SAS 9.4 (The SAS Institute, Cary, NC).
Results
In total, 2,207 patients entered the TROPHIES study, and 2,005 patients were eligible for the HCRU analysis, of whom 1,014 initiated dulaglutide and 991 initiated liraglutide. The remaining 202 patients were considered ineligible for various reasons, such as not meeting eligibility criteria, not providing informed consent, missing source documents, or lack of principal investigator’s signature on case report form. Most patients were enrolled at specialist HCP sites (651 in France, 665 in Germany, and 639 in Italy) rather than at general practitioner sites (28 in France, 20 in Germany, and two in Italy). Of the 2,005 patients included in the HCRU analysis, 481 (24.0%) discontinued the study, the main reasons being “lost to follow-up” (224 of 481, 46.6%) and “patient decision” (143 of 481, 29.7%). The primary endpoint and clinical results of TROPHIES, as well as additional details regarding the reasons for discontinuation of initial injectable therapy have been reported elsewhereCitation17.
Baseline demographic and clinical characteristics
Overall, the characteristics and employment status of patients prescribed dulaglutide (N = 1,014) or liraglutide (N = 991) at baseline were generally similar between treatment groups (Supplementary Table S1). One notable between-cohort difference was the numerically higher proportion of liraglutide patients presenting with ≥1 macrovascular condition at baseline compared with dulaglutide patients (25.1% and 13.8%, respectively); this difference was driven by the patients from France (36.4% and 10.7%, respectively). Conversely, a slightly higher proportion of dulaglutide patients had ≥1 microvascular condition at baseline compared with liraglutide patients (20.0% and 18.8%, respectively); this difference was mainly driven by the patients from Germany (26.6% and 17.8%, respectively). By country, mean age was slightly higher in the Italian cohort, and mean BMI, mean weight, and the proportion of patients with hypertension were slightly higher in the German cohort, compared with the other country cohorts.
Clinical outcomes (HbA1c and weight)
Although the final 24-month clinical results of TROPHIES have been reported elsewhereCitation17, it should be noted that both GLP-1 RAs were effective, resulting in clinically meaningful and sustained HbA1c reductions, and meaningful and sustained weight reductions from baseline to 24 months.
Healthcare resource utilization
Glucose-lowering medications and monitoring
The mean once-weekly dose of dulaglutide over the 24-month study duration was similar between countries (range = 1.40–1.44 mg per week), as was the mean once-daily dose of liraglutide (range = 1.21–1.26 mg per day) (). Most patients in the study were using at least one oral antidiabetic drug at baseline (92.3% in the dulaglutide cohort and 90.5% in the liraglutide cohort) and at month 24 (92.2% and 90.4%, respectively). Of note, the smallest proportions of patients using ≥2 oral antidiabetic drugs at baseline and month 24 were in Italy (24.0% and 27.8%, respectively, in the dulaglutide cohort; 22.2% and 24.7%, respectively, in the liraglutide cohort); whereas the largest proportions were in France (43.4% and 44.0% of dulaglutide patients, respectively; 42.9% and 45.1% of liraglutide patients, respectively). Overall, 9.3% of patients in the dulaglutide cohort and 14.5% of patients in the liraglutide cohort were using ≥1 other injectable antidiabetic drug (i.e. insulin or another GLP-1 RA) at month 24. The largest proportions of patients using another injectable antidiabetic drug at month 24 were in France (15.3% of dulaglutide patients and 23.2% of liraglutide patients); whereas the smallest proportions were in Italy (4.5% and 7.5%, respectively). Insulin, specifically, was used by 8.4% and 11.0% of patients treated with dulaglutide and liraglutide, respectively, at month 24. The smallest proportions of patients using insulin at month 24 were recorded in Italy (4.5% and 4.7% of dulaglutide- and liraglutide-treated patients, respectively) and the largest proportions were recorded in France (12.7% and 15.5%, respectively). Information on the utilization of each oral antidiabetic drug is presented in Supplementary Table S2. Data on the proportion of patients who used an SMBG device, and the number of test strips used per week is described in Supplementary Table S3.
Table 1. Mean index glucagon-like peptide-1 receptor agonist doses and proportions of patients using oral antidiabetic drugs and/or other injectable antidiabetic drugs overall and per country.
Healthcare contacts
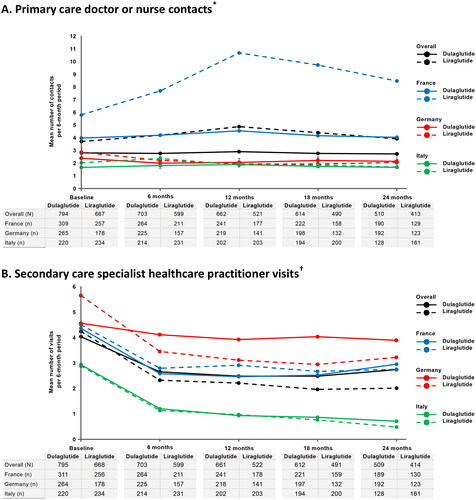
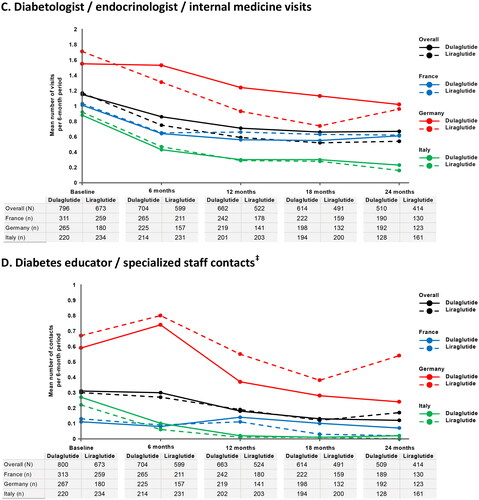
The mean numbers of primary care doctor or nurse contacts were higher in France in both GLP-1 RA cohorts during each 6-month assessment period (range = 4.0–10.7 contacts) compared with Germany (range = 1.9–2.9 contacts) and Italy (range = 1.7–2.4 contacts), with patients in Italy having fewer primary care contacts on average than patients in Germany during all 6-month assessment periods, except in the liraglutide cohort at months 6 and 12 ( and ). The mean numbers of secondary care specialist HCP visits were highest in Germany (range = 2.9–5.7 visits) and lowest in Italy (range = 0.5–2.9 visits) in both treatment cohorts during each 6-month assessment period (). Notably, there was a trend in both GLP-1 RA cohorts towards a reduction in the mean number of secondary care specialist HCP visits from baseline to 6 months before the number of visits stabilized. Following the same trend, patients in Germany had the highest mean numbers of diabetologist/endocrinologist/internal medicine visits during all 6-month assessment periods in both cohorts (range = 0.7–1.7 visits) and patients in Italy had the lowest (range = 0.2–0.9 visits) (). The mean numbers of diabetes educator/specialized staff contacts were also highest in Germany in both GLP-1 RA cohorts during each 6-month assessment period (range = 0.2–0.8 contacts) (). Information on the number of diabetes educator/specialized staff visits at which training on the use of the index GLP-1 RA device was required is presented in Supplementary Table S4.
Figure 1. Mean number of healthcare contacts per 6-month period overall and by country during treatment with dulaglutide or liraglutide in clinical practice. (a) Primary care doctor or nurse contacts*. (b) Secondary care specialist healthcare practitioner visits†. (c) Diabetologist/endocrinologist/internal medicine visits. (d) Diabetes educator/specialized staff contacts‡.
* Primary care contacts include visits or phone calls with primary care doctor and practice- or hospital-based visits, home visits or phone calls with primary care nurse.
†Secondary care specialist HCP visits include cardiologist, dentist, dermatologist, diabetologist/endocrinologist/internal medicine, dietician, nephrologist, neurologist, ophthalmologist, psychiatrist, psychotherapist, podiatrist, and specialist nurse.
‡Diabetes educator/specialized staff contacts include practice- or hospital-based visits, home visits, or phone calls.
N/n, number of patients providing data by cohort in overall study and by country, respectively (i.e. means calculated for the proportion of patients with non-missing data).
Table 2. Non-medication resource use in patients with type 2 diabetes mellitus receiving dulaglutide or liraglutide overall and per country.
Hospital admissions and emergency room visits
Overall, the proportions of patients with hospital admissions declined from baseline over the course of the study in both GLP-1 RA cohorts, from 9.4% and 14.1% at baseline in the dulaglutide and liraglutide cohorts, respectively, to 7.9% and 7.0% at 24 months, respectively (). Notably, the proportions of patients with hospital admissions were higher in France in both treatment cohorts during all assessment periods, compared with Germany and Italy. Overall, the mean number of days of hospitalization related to diabetes or short- or long-term diabetic complications remained stable between baseline and the 24-month post-baseline study visit in both treatment cohorts, although there were considerable differences between individual countries. Additional data on hospital admissions, by category of hospitalization (i.e. daytime, overnight non-intensive care, and overnight intensive care hospitalizations), and ER visits, including whether or not the patient was transported by ambulance and, if so, how many times, are presented in Supplementary Table S5.
Primary caregiver time and missed time at work by caregiver and patient
Primary caregiver time spent caring and missed time at work were minimal in both treatment cohorts across the duration of the 24-month study (). Overall, the proportions of patients who missed days at work were generally low across all time periods in both treatment cohorts (range = 3.8–7.5% per 6-month period). Notably, the proportions were lowest in Italy (range = 0.5–4.5% per 6-month period). The mean number of days of work missed by patients overall during the study varied by assessment period and cohort, ranging from 0 to 4.7 days per 6-month period. The number of workdays missed also tended to be lower in Italy (range = 0–0.4 days per 6-month period). Data on employment status at baseline are shown in Supplementary Table S1.
Costs
A summary of mean annualized direct medical and resource-use costs per patient by country is presented in and Supplementary Table S6). The greatest proportions (≥60%) of mean annualized costs per patient were made up of medication costs (i.e. index GLP-1 RA drug [dulaglutide or liraglutide], oral diabetic drug cost, and other injectable diabetic medication cost [either insulin or GLP-1 RA]) in each treatment cohort across all three countries. Mean annualized medication costs in both treatment cohorts were highest in Germany (€1,588 in the dulaglutide cohort and €1,643 in the liraglutide cohort). Mean annualized medication costs were similar in the dulaglutide cohort in France and Italy (€1,209 and €1,254, respectively) but were lower in Italy (€1,036) compared with France in the liraglutide cohort (€1,355). Mean annualized index GLP-1 RA costs made up the majority of medication costs in all three countries.
Figure 2. Mean annualized cost (€) per patient by country.
Index GLP-1 RA cost = dulaglutide or liraglutide; other injectable medication cost = either insulin or non-index GLP-1 RA. Other costs include visits to specialists and visits to/of primary care doctors/nurses.
In France, 59 and 74 patients receiving dulaglutide and liraglutide, respectively, had a non-zero cost for other injectable medication cost; 19 and 26 patients receiving dulaglutide and liraglutide, respectively, had a non-zero cost for ER visit(s) cost; and 80 and 58 patients receiving dulaglutide and liraglutide, respectively, had a non-zero cost for hospitalization(s) cost.
In Germany, 28 and 52 patients receiving dulaglutide and liraglutide, respectively, had a non-zero cost for other injectable medication cost; 12 and six patients receiving dulaglutide and liraglutide, respectively, had a non-zero cost for ER visit(s) cost; and 42 and 30 patients receiving dulaglutide and liraglutide, respectively, had a non-zero cost for hospitalization(s) cost.
In Italy, 13 and 26 patients receiving dulaglutide and liraglutide, respectively, had a non-zero cost for other injectable medication cost; 16 and 22 patients receiving dulaglutide and liraglutide, respectively, had a non-zero cost for ER visit(s) cost; and 14 and 31 patients receiving dulaglutide and liraglutide, respectively, had a non-zero cost for hospitalization(s) cost.
Costs are expressed in reference to 2018 public prices (in €).
GLP-1 RA medication 2018 (€) list prices were as follows: dulaglutide (4 pens) = France €86, Germany €100, and Italy €97; liraglutide (2 x 18 mg, i.e. 1.2 mg/day) = France €97, Germany €112, and Italy €79.
Abbreviations. ER, emergency room; GLP-1 RA, glucagon-like peptide-1 receptor agonist; OAD, oral antidiabetic drug.
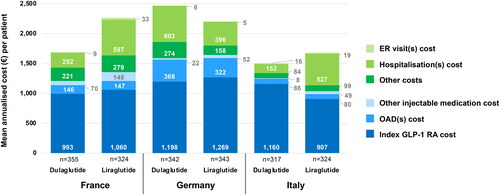
Mean annualized non-medication resource-use costs per patient varied by treatment cohort and by country, with the highest levels in the liraglutide cohort in France (€909) and the dulaglutide cohort in Germany (€883) and the lowest levels in dulaglutide cohorts in Italy (€252) and France (€482) ( and Supplementary Table S6). Hospital admissions made up the greatest proportions of mean annualized resource use costs across all three countries, with ER visits making up the smallest proportions. The greatest variation in mean annualized resource-use costs between countries was seen in hospitalization costs. The results of the Winsorized cost sensitivity analysis supported those of the main cost analysis and are presented in Supplementary Figure S2.
Discussion
We have described the HCRU and costs of treatment in clinical practice from the prospective, 24-month, real-world TROPHIES study of adult patients with T2D in France, Germany, and Italy who started their first injectable GLP-1 RA with either once-weekly dulaglutide or once-daily liraglutide. Prior prospective data on real-world HCRU and costs associated with the use of GLP-1 RAs in T2D are limited. The prospective, 24-month CHOICE study compared resource use and costs of an early GLP-1 RA (exenatide) with insulin treatment, determining that much of the higher cost of GLP-1 RA treatment, compared with insulin, was compensated for by lower HCRU costsCitation28. More recently, a comparative cohort study based on linked prospective healthcare databases compared HCRU and costs for an SGLT-2 inhibitor (empagliflozin) with GLP-1 RAs in the Danish population, finding that lower average healthcare costs for the SGLT-2 inhibitor were driven by lower drug costsCitation29. The TROPHIES study comprehensively assessed the HCRU and costs associated with the treatment of T2D with two commonly prescribed GLP-1 RAs in real-world clinical practice in multiple European healthcare settings over a 24-month follow-up period. Moreover, TROPHIES provided rare and valuable insights into resource utilization aspects not typically evaluated in prospective, observational studies, such as caregiver time and missed time at work by patients and/or caregivers.
In terms of medication, the average doses of dulaglutide or liraglutide taken by patients over the duration of the 24-month study were consistent across the three countries, and most patients were using at least one oral antidiabetic drug at baseline, regardless of country. Differences between countries became apparent when evaluating the proportions of patients using two or more antidiabetic drugs at baseline and study endpoint, or using at least one other injectable drug (i.e. insulin or another GLP-1 RA), and insulin specifically, at study endpoint, with patients in France being more likely to fall into these categories than those in Italy. It may be hypothesized that the use of more GLMs, including insulin, at study endpoint in the French cohort is a consequence of the higher baseline HbA1c values observed in France, resulting in the need for more intensified treatment to achieve glycemic control. This finding reflects our knowledge of common T2D management practice in Italy, where physicians only add a GLP-1 RA to metformin, the standard of care in Italy at the time of TROPHIES initiation, when metformin alone does not sufficiently control the disease. This real-world, longitudinal, country-specific data on the use of other GLMs in conjunction with GLP-1 RAs is important for healthcare decision-makers, particularly when considering a cost-effectiveness analysis within a particular healthcare system. We identified several other factors that may have influenced treatment choices in individual countries, for example, the numerically larger proportion of liraglutide (25.1%) than dulaglutide (13.8%) patients who presented with ≥1 macrovascular condition mainly driven by France was likely a result of the French Diabetes Society position statement that recommended the administration of liraglutide to patients with T2D who required secondary cardiovascular disease prevention on the basis of the outcomes of the LEADER study published during the patient enrolment periodCitation30,Citation31. Furthermore, SGLT-2 inhibitors, the other class of antidiabetic medication known to offer cardiovascular protection in T2D, were not available in France at the time of TROPHIES design and initiation. In addition, the longer mean duration of diabetes at baseline in the dulaglutide cohort compared with the liraglutide cohort in Germany (7.4 vs. 6.4 years)Citation17 may explain the higher prevalence of microvascular complications observed in the dulaglutide cohort at baseline. Furthermore, physicians in Germany may have been initiating GLP-1 RA treatment earlier than French or Italian physicians because of the weight loss potential with these agents, given that German patients had a high mean weight and BMICitation32. In fact, German T2D management guidelines at the time of TROPHIES initiation recommended using GLP-1 RAs in conjunction with oral GLMs (preferably metformin) in patients with substantial weight problemsCitation33. Acknowledging the minor differences between the patient populations in each country, as well as differences in national recommendations and local prescribing habits, TROPHIES provides a rare multi-country view of treatment patterns from a single study implemented with a consistent design across countries.
Differences between countries were also observed with respect to healthcare contacts. Higher mean numbers of primary care doctor or nurse contacts were seen throughout the study in France than in Germany or Italy, regardless of treatment cohort. The low numbers of primary care contacts observed in Italy were likely the result of prescribing regulations associated with GLP-1 RAs that prohibited primary care prescribing at the time of the studyCitation34, which were relaxed in 2022Citation35. The number of primary care contacts in the dulaglutide cohort in France are aligned with a large French population-based survey study of people treated for diabetes (ENTRED) conducted in 2006/2007Citation36. The higher number of primary care contacts observed in the liraglutide cohort in France was potentially driven by the higher level of cardiovascular risk seen in this cohort at baseline. It should be noted that primary care visits were generally more frequent in the liraglutide cohorts in all countries, compared with the dulaglutide cohorts, likely due to up-titration consultations for liraglutide, whereas the majority (≥80.0%) of patients in the dulaglutide cohort initiated treatment at 1.5 mg once weekly across countries, thereby not requiring up-titration visitsCitation17. The proportions of patients with hospital admissions (all categories) were also higher in France in both treatment cohorts throughout the study than in Germany or Italy. Hospital admissions in France were mainly daytime hospitalizations, which reflects common diabetes management practice in France, where patients undergo annual full assessments of their condition, including disease education, during hospital day visits. These results are aligned with recent publications highlighting the trend towards reduced numbers of patients with diabetes requiring hospitalization for acute complications in ItalyCitation37,Citation38. In contrast, the mean numbers of secondary care specialist HCP visits, diabetologist/endocrinologist/internal medicine visits, and diabetes educator/specialized staff contacts visits were highest in Germany, probably reflecting common clinical practice in Germany, in which secondary care specialists (i.e. diabetologist/endocrinologist/internal medicine) primarily prescribe/initiate injectable diabetes therapiesCitation39, and high levels of participation in a T2D disease management program, which encourages regular HCP visits, with family care physicians often referring their disease management program patients to diabetologists/endocrinologists. In Italy, most patients with T2D are referred to specialist diabetes centers prior to initiation of more advanced therapies, such as GLP-1 RAs; these patients are seen on average 1.5 times per year for the purpose of reviewing their treatment planCitation40. Interestingly, across all countries but more significantly so in France and Italy, reductions in the mean number of secondary care specialist HCP visits from baseline to 6 months were observed before the number of visits stabilized, likely due to the need for more frequent visits early after initiation of a new treatment. Overall, it can be surmised that differences in resource use between countries were more likely to be related to different regulations and patient pathways in France, Germany, and Italy than to the use of one or other GLP-1 RA in this study.
Estimating the total societal cost of a disease raises several challenges, such as presenteeism (i.e. the act of showing up for work without being productive), and the requirements for large sample sizes and long-term follow-up periods. However, caregiver time and missed time at work, both for carers and patients, was minimal across treatment cohorts and countries in TROPHIES, suggesting that the overall societal cost of the disease associated with decreased work productivity is also minimal. However, it should be noted that the average age of the TROPHIES cohort was just under 60 years of age at baseline; therefore, it is likely that a significant proportion of the cohort were retired. Given that the prevalence of T2D among adolescents and young adults is risingCitation41, caregiver time and missed time at work may become a more important issue in the future.
In TROPHIES, medication costs, and specifically index GLP-1 RA costs, made up the largest proportions of annualized costs across all three countries; however, as expected, due to between-country price differences, there were variations in medication costs between countries. The highest medication costs were estimated in both treatment cohorts in Germany, perhaps because of the notably higher rates of persistence with GLP-1 RA treatment reported for Germany (data not reported), as well as the higher GLP-1 RA prices in Germany, compared to France and Italy.
Resource-use costs largely remained constant in the TROPHIES study after the initiation of GLP-1 RA treatment, likely reflecting standard follow-up procedures for patients with T2D. There was no indication of an increase in resource-use costs over the 24-month follow-up period or a peak in any treatment cohort in any country, suggesting stability on treatment.
Unsurprisingly, resource-use costs varied by treatment cohort and by country in TROPHIES, primarily driven by differences between countries in hospital admission costs, which made up the largest proportion of resource-use costs in all three countries. It is recognized in the literatureCitation26 that prices for non-medication resource use vary widely between countries in Europe. The highest mean annualized non-medication resource-use costs per patient found in the liraglutide cohort in France could be related to the clinical characteristics or increased cardiovascular risk profile of patients in this cohort, or other non-identified factors. Overall, these findings from the TROPHIES study highlight the need to avoid generalization with respect to HCRU and costs associated with a particular therapy when estimating the impact of a new treatment in a specific country setting, as also concluded based on the earlier findings of the CHOICE studyCitation28.
Limitations
TROPHIES was a non-randomized, observational study; therefore, causal inference cannot be made in the unadjusted analyses since there were differences in patient characteristics (measured and unmeasured confounders) other than the index treatment group that may have influenced outcomes. Moreover, the treatments explored (dulaglutide and liraglutide) were selected as they were the most prescribed GLP-1 RAs at the time the study was designed and started; however, interpretation of the results should take into consideration other treatments that were approved and marketed during the study. Patients using insulin when initiating dulaglutide or liraglutide were excluded from the study despite combination GLP-1 RA/insulin therapy being common practice to improve glycemic control and mitigate the risk of hypoglycemia and weight gain, as per treatment guidelinesCitation3; therefore, the TROPHIES patient population may not fully represent all patients initiating GLP-1 RAs in the real world. Furthermore, differences in GLP-1 RA prescribing regulations between countries in conjunction with difficulties enrolling patients at some types of sites, for example GP prescribers, may also have resulted in TROPHIES patient populations in each country that were not fully representative of the real-world patient population in that country. HCRU data were collected during patient interviews by the physician rather than via patient-completed resource-use diaries or directly from medical records across settings and may therefore not be fully complete. Although the frequency of patient contacts and costs were not driven by the study protocol, the potential effect of study participation bias on HCRU and cost cannot be ruled out. Furthermore, use of available unit costs from a variety of sources derived using various approaches introduce some uncertainty into cost estimates. Therefore, whilst there are likely differences in direct costs between countries due to differences in healthcare systems and assigned tariffs (e.g. for GLP-1 RAs and hospitalizations), methodological differences in deriving unit costs may also contribute to differencesCitation42. Finally, the 24-month follow-up period of the TROPHIES study may not have been long enough to capture the treatment costs of long-term complications of T2D or understand the impact of weight loss observed with GLP-1 RA treatment on HCRU and cost of treatment.
Conclusions
T2D imposes a substantial financial burden on the healthcare systems of Europe, given the high prevalence of the disease documented. However, HCRU data from prospective studies on the use of GLP-1 RAs in adults with T2D to inform cost analyses are limited. The TROPHIES study provides unique and valuable real-world HCRU and cost data in three key European countries. Real-world resource utilization and costs associated with the treatment of adults with T2D with two GLP-1 RAs in TROPHIES emphasize the need to avoid generalization with respect to HCRU and costs associated with a particular therapy when estimating the impact of a new treatment in a specific country setting.
Transparency
Declaration of funding
This work was funded by Eli Lilly and Company.
Declaration of financial/other interests
KB, AD, EH, MOF, MY, HS, AB, and LEGP are full-time employees and minor stock holders of Eli Lilly and Company. JL works as a consultant for Eli Lilly and Company. BG has served as: an advisory panel/board member for Abbott, AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Gilead, GSK, Intercept, Medtronic, MSD, Novartis, Novo Nordisk, Roche Diagnostics, and Sanofi; a clinical investigator for Abbott, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, BMS, Eli Lilly, GSK, Insulet, Janssen, Medtronic, MSD, Novartis, Novo Nordisk, Roche Diagnostics, and Sanofi; research support for Eli Lilly, Medtronic, Novo Nordisk, Sanofi and Vitalaire. FG has served as: an advisor for AstraZeneca; a research investigator for Eli Lilly; a speaker for AstraZeneca and Eli Lilly; a consultant for Abbott, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Merck Sharp & Dohme, Novo Nordisk, Roche Diabetes Care, and Sanofi; and has received grants from Eli Lilly, Lifescan, and Roche Diabetes Care. MF has received research support, consulting fees, speaker’s fees, and travel support from Eli Lilly; and is on the advisory boards for Eli Lilly, Boehringer Ingelheim, and Berlin Chemie AG.
Author contributions
AB, AD, BG, MF, MY, and EH were involved with the analysis and interpretation of the data for the work. KSB and LEGP were involved with the conception and design of the work, and the interpretation of the data. FG, HS, and MOF were involved with the interpretation of the data for the work. JL was involved with the analysis of the data for the work. All authors provided critical revision of the manuscript for important intellectual content and have participated sufficiently in the work to agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors have given their final approval of the manuscript to be published.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Previous presentations
Giorgino F, et al. The Real-World Observational Prospective Study of Health Outcomes with Dulaglutide & Liraglutide in Type 2 Diabetes Patients (TROPHIES): Final 24-month primary endpoint analysis. Poster 746-P presented at the American Diabetes Association (ADA) 82nd Scientific Sessions; New Orleans, LA; 3–7 June, 2022.
Füchtenbusch M, et al. The Real-World Observational Prospective Study of Health Outcomes with Dulaglutide & Liraglutide in Type 2 Diabetes Patients (TROPHIES): Final 24-month patient-reported outcomes. Poster 738-P presented at the American Diabetes Association (ADA) 82nd Scientific Sessions; New Orleans, LA; 3–7 June, 2022.
Guerci B, et al. The Real-World Observational Prospective Study of Health Outcomes with Dulaglutide & Liraglutide in Type 2 Diabetes Patients (TROPHIES): Final 24-month clinical characteristics and treatment pattern analyses. Poster 726-P presented at the American Diabetes Association (ADA) 82nd Scientific Sessions; New Orleans, LA; 3–7 June, 2022.
Supplemental Material
Download MS Word (206.9 KB)Acknowledgements
The authors would like to acknowledge Kirsi Norrbacka (former employee of Eli Lilly and Company) for the study design, Fangyu Wang and Hanbo Qiu (Eli Lilly and Company) for statistical programming, and Sue Williamson and Ioannis Nikas (Rx Communications, Mold, UK) for medical writing assistance with the preparation of this manuscript, funded by Eli Lilly and Company.
References
- IDF Diabetes Atlas 10th edition. Diabetes in Europe – 2021. International Diabetes Federation; 2021 [cited 2024 May 20]. Available from: https://diabetesatlas.org/atlas/tenth-edition/
- World Health Organization. Diabetes Fact Sheet; 2022 Sep 16 [cited 2023 Nov 9]. Available from: https://www.who.int/news-room/fact-sheets/detail/diabetes
- Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycaemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022;45(11):2753–2786. doi: 10.2337/dci22-0034.
- Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368(9548):1696–1705. doi: 10.1016/S0140-6736(06)69705-5.
- Trujillo JM, Nuffer W, Ellis SL. GLP-1 receptor agonists: a review of head-to-head clinical studies. Ther Adv Endocrinol Metab. 2015;6(1):19–28. doi: 10.1177/2042018814559725.
- Nauck MA, Quast DR, Wefers J, et al. GLP-1 receptor agonists in the treatment of type 2 diabetes – state-of-the-art. Mol Metab. 2021;46:101102. doi: 10.1016/j.molmet.2020.101102.
- European Medicines Agency. Trulicity: summary of product characteristics; 2021 Jul [cited 2023 Nov 9]. Available from: https://www.ema.europa.eu/en/documents/product-information/trulicity-epar-product-information_en.pdf
- European Medicines Agency. Victoza: summary of product characteristics; 2021 Feb [cited 2023 Nov 9]. Available from: https://www.ema.europa.eu/en/documents/product-information/victoza-epar-product-information_en.pdf
- Feinglos MN, Saad MF, Pi-Sunyer FX, et al. Effects of liraglutide (NN2211), a long-acting GLP-1 analogue, on glycaemic control and bodyweight in subjects with type 2 diabetes. Diabet Med. 2005;22(8):1016–1023. doi: 10.1111/j.1464-5491.2005.01567.x.
- Barrington P, Chien JY, Tibaldi F, et al. LY2189265, a long-acting glucagon-like peptide-1 analogue, showed a dose-dependent effect on insulin secretion in healthy subjects. Diabet Obes Metab. 2011;13(5):434–438. doi: 10.1111/j.1463-1326.2011.01365.x.
- Dungan KM, Povedano ST, Forst T, et al. Once-weekly dulaglutide versus once-daily liraglutide in metformin-treated patients with type 2 diabetes (AWARD-6): a randomised, open-label, phase 3, non-inferiority trial. Lancet. 2014;384(9951):1349–1357. doi: 10.1016/S0140-6736(14)60976-4.
- Anderson JE, Thieu VT, Boye KS, et al. Dulaglutide in the treatment of adult type 2 diabetes: a perspective for primary care providers. Postgrad Med. 2016;128(8):810–821. doi: 10.1080/00325481.2016.1218260.
- Gentilella R, Romera I, Nicolay C, et al. Change in HbA1c across the baseline HbA1c range in type 2 diabetes patients receiving once-weekly dulaglutide versus other incretin agents. Diabetes Ther. 2019;10(3):1113–1125. doi: 10.1007/s13300-019-0625-3.
- Divino V, DeKoven M, Khan FA, et al. GLP-1 RA treatment patterns among type 2 diabetes patients in five European countries. Diabetes Ther. 2017;8(1):115–128. doi: 10.1007/s13300-016-0224-5.
- Divino V, Boye KS, Lebrec J, et al. GLP-1 RA treatment and dosing patterns among type 2 diabetes patients in six countries: a retrospective analysis of pharmacy claims data. Diabetes Ther. 2019;10(3):1067–1088. doi: 10.1007/s13300-019-0615-5.
- García-Pérez L-E, Boye KS, Rosilio M, et al. The Real-world Observational Prospective study of Health outcomes with dulaglutIde and liraglutide in typE 2 diabeteS patients (TROPHIES): design and baseline characteristics. Diabetes Ther. 2021;12(7):1929–1946. doi: 10.1007/s13300-021-01076-0.
- Giorgino F, Guerci B, Füchtenbusch M, et al. The real-world observational prospective study of health outcomes with dulaglutide and liraglutide in patients with type 2 diabetes (TROPHIES): final, 24-month analysis of time to first significant treatment change, treatment persistence, and clinical outcomes. Diabetes Obes Metab. 2023;25(12):3465–3477. doi: 10.1111/dom.15244.
- Farmer RE, Beard I, Raza SI, et al. Prescribing in type 2 diabetes patients with and without cardiovascular disease history: a descriptive analysis in the UK CPRD. Clin Ther. 2021;43(2):320–335. doi: 10.1016/j.clinthera.2020.12.015.
- Funck KA-O, Knudsen JA-O, Hansen TA-O, et al. Real-world use of cardioprotective glucose-lowering drugs in patients with type 2 diabetes and cardiovascular disease: a Danish nationwide cohort study, 2012 to 2019. Diabetes Obes Metab. 2020;23(2):520–529. doi: 10.1111/dom.14245.
- van den Heuvel JM, Farzan N, van Hoek M, et al. Mining treatment patterns of glucose-lowering medications for type 2 diabetes in the Netherlands. BMJ Open Diabetes Res Care. 2020;8(1):e000767. doi: 10.1136/bmjdrc-2019-000767.
- Eberly RA, Yang L, Essien UR, et al. Racial, ethnic, and socioeconomic inequalities in glucagon-like peptide-1 receptor agonist use among patients with diabetes in the US. JAMA Health Forum. 2021;2(12):e214182. doi: 10.1001/jamahealthforum.2021.4182.
- Eli Lilly and Company Limited. Trulicity: summary of product characteristics; 2016 [cited 2023 Nov 9]. Available from: https://www.medicines.org.uk/emc/product/7482/smpc
- World Medical Association. Declaration of Helsinki. Recommendations guiding physicians in biomedical research involving human subjects. JAMA. 1997;277(11):925–926.
- Guerci B, Giorgino F, Sapin H, et al. The real-world observational prospective study of health outcomes with dulaglutide and liraglutide in patients with type 2 diabetes (TROPHIES): patient disposition, clinical characteristics and treatment persistence at 12 months. Diabetes Obes Metab. 2022;24(12):2373–2382. doi: 10.1111/dom.14823.
- Boye KS, Lebrec J, Dib A, et al. The real-world observational prospective study of health outcomes with dulaglutide and liraglutide in type 2 diabetes patients (TROPHIES): final patient-reported outcomes at 24 months. Diabetes Obes Metab. 2023;25(12):3453–3464. doi: 10.1111/dom.15145.
- Pöhlmann J, Norrbacka J, Boye KS, et al. Costs and where to find them: identifying unit costs for health economic evaluations of diabetes in France, Germany and Italy. Eur J Health Econ. 2020;21(8):1179–1196. doi: 10.1007/s10198-020-01229-1.
- Weichle T, Hynes DM, Durazo-Arvizu R, et al. Impact of alternative approaches to assess outlying and influential observations on health care costs. Springerplus. 2013;2(1):614. doi: 10.1186/2193-1801-2-614.
- Kiiskinen U, Matthaei S, Reaney M, et al. Resource use and costs of exenatide bid or insulin in clinical practice: the European CHOICE study. Clinicoecon Outcomes Res. 2013;5:355–367. doi: 10.2147/CEOR.S44060.
- Thomsen RW, Christensen LWB, Kahlert J, et al. Healthcare resource utilization and costs for empagliflozin versus glucagon-like peptide-1 receptor agonists in routine clinical care in Denmark. Diabetes Ther. 2022;13(11-12):1891–1906. doi: 10.1007/s13300-022-01323-y.
- Darmon P, Bauduceau B, Bordier L, et al. Prise de position de la Société Francophone du Diabéte (SFD) sur la prise en charge médicamenteuse de l’hyperglycémie du patient diabétique de type 2. Méd Mal Métabol. 2017;11:577–593.
- Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311–322. doi: 10.1056/NEJMoa1603827.
- Nauck MA, Meier JJ, Cavender MA, et al. Cardiovascular actions and clinical outcomes with glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Circulation. 2017;136(9):849–870. doi: 10.1161/CIRCULATIONAHA.117.028136.
- Nationale VersorgungsLeitlinien. Type 2 diabetes. 2013. [cited 2024 Feb 29]. Available from: https://extranet.who.int/ncdccs/Data/DEU_D1_Type%202%20Diabetes.pdf
- Gazzetta Ufficiale della Republica Italiana. Serie generale. Anno 157° – Numero 45. Roma – Mercoledì, 2016 feb 24.
- AIFA (Italian Medicines Agency). Nota 100; 2022 [updated 2023 Jan 27, cited 2023 Nov 9]. Available from: https://www.aifa.gov.it/en/nota-100
- Druet C, Bourdel-Marchasson I, Weill A, et al. Type 2 diabetes in France: epidemiology, trends of medical care, social and economic burden. Presse Med. 2013;42(5):830–838. doi: 10.1016/j.lpm.2013.02.312.
- Instituto Superiore di Sanità. EpiCcentro – L’epidemiologia per la sanità pubblica. Epidemiologia delle ospedalizzazioni per diabete in Italia; 2020 dic 22 [cited 2023 Nov 9]. Available from: https://www.epicentro.iss.it/diabete/epidemiologia-ospedalizzazioni-per-diabete-in-italia
- Giacomozzi C, Villa M, Lombardo F, et al. Studio descrittivo sull’andamento delle ospedalizzazioni con diabete in Italia nel periodo 2010-2018. Boll Epidemiol. 2021;2(1):8–15.
- Mount J, Boye KS, Hennies N, et al. Obesity and glycemic control in people with type 2 diabetes in Germany: a retrospective cohort analysis. Poster P036 presented at Diabetes Kongress 2023; 2023 May 17–20; Berlin, Germany. doi: 10.1055/s-0043-1767898.
- Annali Associazione Medici Diabetologie (AMD). Diabete di tipo 2; 2022 [CITED 2023 Nov 9]. Available from: https://aemmedi.it/wp-content/uploads/2023/05/Annali_2022_diabete2-prot.pdf
- Lascar N, Brown J, Pattison H, et al. Type 2 diabetes in adolescents and young adults. Lancet. 2018;6(1):69–80.
- García-Mochón L, Špacírová Z, Espín J. Costing methodologies in European economic evaluation guidelines: commonalities and divergences. Eur J Health Econ. 2022;23(6):979–991. doi: 10.1007/s10198-021-01414-w.